High Dose of Acute Normobaric Hypoxia Does Not Adversely Affect Sprint Interval Training, Cognitive Performance and Heart Rate Variability in Males and Females
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
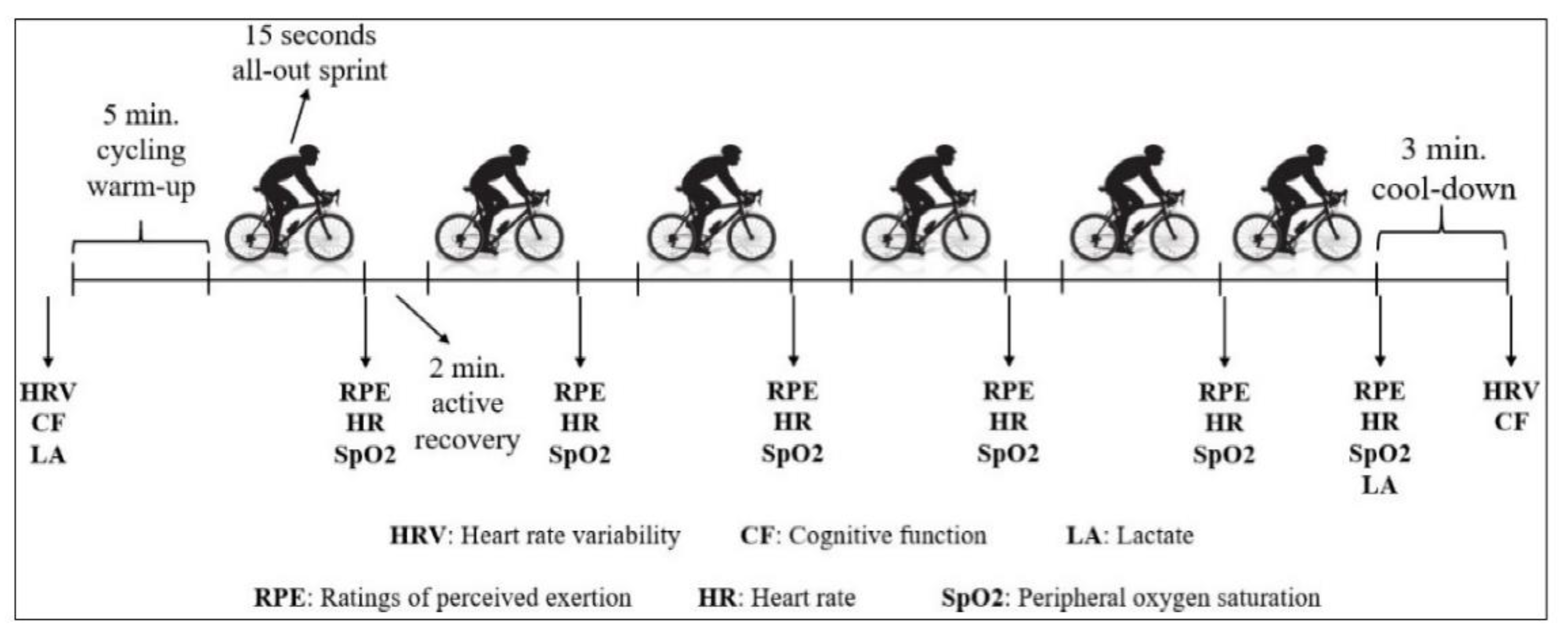
2.2. Study Design
2.3. SIT Protocol and Computed Performance Indices
2.4. Heart Rate Variability
2.5. Cognitive Performance
2.6. Statistics
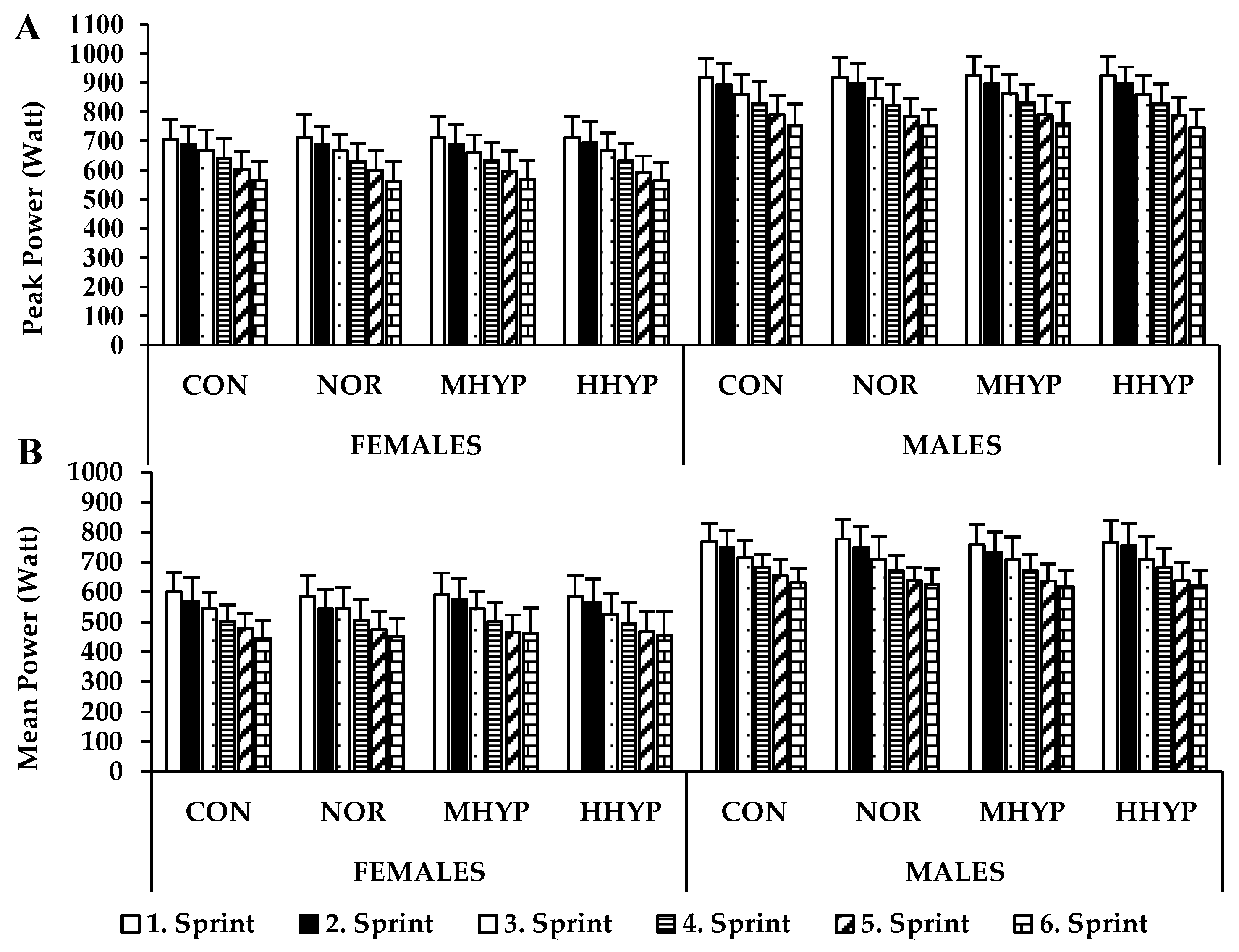
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamagishi, T.; Babraj, J. Effects of reduced-volume of sprint interval training and the time course of physiological and performance adaptations. Scand. J. Med. Sci. Sport. 2017, 27, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Hughes, S.C.; Heigenhauser, G.J.; Bradwell, S.N.; Gibala, M.J. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J. Appl. Physiol. 2005, 98, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.A.; Chen, Z.-P.; Murphy, K.T.; Aughey, R.; McKenna, M.; Kemp, B.E.; Hawley, J.A. Intensified exercise training does not alter AMPK signaling in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E737–E743. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Heigenhauser, G.J.; Gibala, M.J. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J. Appl. Physiol. 2006, 100, 2041–2047. [Google Scholar] [CrossRef]
- Edge, J.; Bishop, D.; Goodman, C. Effects of chronic NaHCO3 ingestion during interval training on changes to muscle buffer capacity, metabolism, and short-term endurance performance. J. Appl. Physiol. 2006, 101, 918–925. [Google Scholar] [CrossRef]
- Vogt, M.; Puntschart, A.; Geiser, J.; Zuleger, C.; Billeter, R.; Hoppeler, H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J. Appl. Physiol. 2001, 91, 173–182. [Google Scholar] [CrossRef]
- Schmutz, S.; Däpp, C.; Wittwer, M.; Durieux, A.C.; Mueller, M.; Weinstein, F.; Vogt, M.; Hoppeler, H.; Flück, M. A hypoxia complement differentiates the muscle response to endurance exercise. Exp. Physiol. 2010, 95, 723–735. [Google Scholar] [CrossRef]
- Zoll, J.; Ponsot, E.; Dufour, S.; Doutreleau, S.; Ventura-Clapier, R.; Vogt, M.; Hoppeler, H.; Richard, R.; Flück, M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J. Appl. Physiol. 2006, 100, 1258–1266. [Google Scholar] [CrossRef]
- Puype, J.; Van Proeyen, K.; Raymackers, J.-M.; Deldicque, L.; Hespel, P. Sprint interval training in hypoxia stimulates glycolytic enzyme activity. Med. Sci. Sport. Exerc. 2013, 45, 2166–2174. [Google Scholar] [CrossRef]
- Faiss, R.; Léger, B.; Vesin, J.-M.; Fournier, P.-E.; Eggel, Y.; Dériaz, O.; Millet, G.P. Significant molecular and systemic adaptations after repeated sprint training in hypoxia. PLoS ONE 2013, 8, e56522. [Google Scholar] [CrossRef]
- Warnier, G.; Benoit, N.; Naslain, D.; Lambrecht, S.; Francaux, M.; Deldicque, L. Effects of sprint interval training at different altitudes on cycling performance at sea-level. Sports 2020, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Girard, O.; Beard, A.; Brocherie, F. Repeated sprint training in hypoxia–an innovative method. Dtsch. Z. Für Sportmed. 2019, 5, 115–122. [Google Scholar] [CrossRef]
- Faiss, R.; Girard, O.; Millet, G.P. Advancing hypoxic training in team sports: From intermittent hypoxic training to repeated sprint training in hypoxia. Br. J. Sport Med. 2013, 47, i45–i50. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Faiss, R. Hypoxic conditions and exercise-to-rest ratio are likely paramount. Sports Med. 2012, 42, 1081–1083. [Google Scholar]
- Bowtell, J.L.; Cooke, K.; Turner, R.; Mileva, K.N.; Sumners, D.P. Acute physiological and performance responses to repeated sprints in varying degrees of hypoxia. J. Sci. Med. Sport 2014, 17, 399–403. [Google Scholar] [CrossRef]
- Goods, P.S.; Dawson, B.; Landers, G.J.; Gore, C.J.; Peeling, P. No additional benefit of repeat-sprint training in hypoxia than in normoxia on sea-level repeat-sprint ability. J. Sport. Sci. Med. 2015, 14, 681. [Google Scholar]
- Khaosanit, P.; Hamlin, M.J.; Graham, K.S.; Boonrod, W. Acute effect of different normobaric hypoxic conditions on shuttle repeated sprint performance in futsal players. J. Phys. Educ. Sport 2018, 18, 210–216. [Google Scholar]
- Kon, M.; Nakagaki, K.; Ebi, Y.; Nishiyama, T.; Russell, A.P. Hormonal and metabolic responses to repeated cycling sprints under different hypoxic conditions. Growth Horm. IGF Res. 2015, 25, 121–126. [Google Scholar] [CrossRef]
- Karabiyik, H.; Eser, M.C.; Guler, O.; Yasli, B.C.; Ertetik, G.; Sisman, A.; Koz, M.; Gabrys, T.; Pilis, K.; Karayigit, R. The effects of 15 or 30 s SIT in normobaric hypoxia on aerobic, anaerobic performance and critical power. Int. J. Environ. Res. Public Health 2021, 18, 3976. [Google Scholar] [CrossRef]
- Zupet, P.; Princi, T.; Finderle, Z. Effect of hypobaric hypoxia on heart rate variability during exercise: A pilot field study. Eur. J. Appl. Physiol. 2009, 107, 345–350. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hoshikawa, Y.; Miyashita, M. Effects of acute exposure to simulated altitude on heart rate variability during exercise. J. Appl. Physiol. 1996, 81, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Simon, C.; Piquard, F.; Ehrhart, J.; Brandenberger, G. Effects of increased training load on vagal-related indexes of heart rate variability: A novel sleep approach. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2813–H2818. [Google Scholar] [CrossRef] [PubMed]
- Petrassi, F.A.; Hodkinson, P.D.; Walters, P.L.; Gaydos, S.J. Hypoxic hypoxia at moderate altitudes: Review of the state of the science. Aviat. Space Environ. Med. 2012, 83, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef] [PubMed]
- Galvin, H.M.; Cooke, K.; Sumners, D.P.; Mileva, K.N.; Bowtell, J.L. Repeated sprint training in normobaric hypoxia. Br. J. Sport. Med. 2013, 47, i74–i79. [Google Scholar] [CrossRef]
- Tian, Z.; Kim, B.-Y.; Bae, M.-J. A study on the effect of wearing masks on stress response. Memory 2020, 8, 12. [Google Scholar] [CrossRef]
- Sandoval, D.A.; Matt, K.S. Effects of the oral contraceptive pill cycle on physiological responses to hypoxic exercise. High Alt. Med. Biol. 2003, 4, 61–72. [Google Scholar] [CrossRef]
- Laurent, C.M.; Green, J.M.; Bishop, P.A.; Sjökvist, J.; Schumacker, R.E.; Richardson, M.T.; Curtner-Smith, M. Effect of gender on fatigue and recovery following maximal intensity repeated sprint performance. J. Sport. Med. Phys. Fit. 2010, 50, 243–253. [Google Scholar]
- Laurent, C.M.; Vervaecke, L.S.; Kutz, M.R.; Green, J.M. Sex-specific responses to self-paced, high-intensity interval training with variable recovery periods. J. Strength Cond. Res. 2014, 28, 920–927. [Google Scholar] [CrossRef]
- Sandoval, D.A.; Matt, K.S. Gender differences in the endocrine and metabolic responses to hypoxic exercise. J. Appl. Physiol. 2002, 92, 504–512. [Google Scholar] [CrossRef][Green Version]
- Esbjornsson-Liljedahl, M.; Bodin, K.; Jansson, E. Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. J. Appl. Physiol. 2002, 93, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Storer, T.W.; Davis, J.A.; Caiozzo, V.J. Accurate prediction of VO2max in cycle ergometry. Med. Sci. Sport. Exerc. 1990, 22, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Ware, L.; Capodilupo, E.R. Patterns of endogenous and exogeneous ovarian hormone modulation on recovery metrics across the menstrual cycle. BMJ Open Sport Exerc. Med. 2021, 7, e001047. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Heather, A.K. Myths and methodologies: Reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Kelly, E.E.; Collier, S.R.; Fernhall, B. Cardiac autonomic modulation during recovery from acute endurance versus resistance exercise. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Bartels, R.; Brito, L.C.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Karayigit, R.; Naderi, A.; Akca, F.; Cruz, C.J.G.D.; Sarshin, A.; Yasli, B.C.; Ersoz, G.; Kaviani, M. Effects of Different Doses of Caffeinated Coffee on Muscular Endurance, Cognitive Performance, and Cardiac Autonomic Modulation in Caffeine Naive Female Athletes. Nutrients 2020, 13, 2. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psych. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Brocherie, F.; Girard, O.; Faiss, R.; Millet, G.P. Effects of repeated-sprint training in hypoxia on sea-level performance: A meta-analysis. Sport. Med. 2017, 47, 1651–1660. [Google Scholar] [CrossRef]
- Girard, O.; Brocherie, F.; Millet, G.P. Effects of altitude/hypoxia on single-and multiple- sprint performance: A comprehensive review. Sport. Med. 2017, 47, 1931–1949. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ohiwa, N.; Honda, A.; Matsubayashi, T.; Ikeda, T.; Akimoto, T.; Suzuki, T.; Hirano, Y.; Rusell, A.P. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiol. Rep. 2015, 3, e12267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogawa, T.; Hayashi, K.; Ichinose, M.; Wada, H.; Nishiyasu, T. Metabolic response during intermittent graded sprint running in moderate hypobaric hypoxia in competitive middle- distance runners. Eur. J. Appl. Physiol. 2007, 99, 39–46. [Google Scholar] [CrossRef]
- Freese, E.C.; Gist, N.H.; Cureton, K.J. Physiological responses to an acute bout of sprint interval cycling. J. Strength Cond. Res. 2013, 27, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Patton, J.F.; Frederick, F.A. Comparative anaerobic power of men and women. Aviat. Space Environ. Med. 1986, 57, 636–641. [Google Scholar]
- Magal, M.; Liette, N.C.; Crowley, S.K.; Hoffman, J.R.; Thomas, K.S. Sex-based performance responses to an acute sprint interbal cycling training session in collegiate athletes. Res. Q. Exerc. Sport 2021, 92, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Billaut, F. Tissue oxygenation in men and women during repeated-sprint exercise. Int. J. Sport. Physiol. Perform. 2012, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.J.; Alvarez, L.; Millet, G.P.; Borrani, F. Changes in muscle and cerebral deoxygenation and perfusion during repeated sprints in hypoxia to exhaustion. Front. Physiol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Ochi, G.; Yamada, Y.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Byun, K.; Dan, I.; Soya, H. Neural basis for reduced executive performance with hypoxic exercise. Neuroimage 2018, 171, 75–83. [Google Scholar] [CrossRef]
- Povea, C.; Schmitt, L.; Brugniaux, J.; Nicolet, G.; Richalet, J.-P.; Fouillot, J.-P. Effects of intermittent hypoxia on heart rate variability during rest and exercise. High Alt. Med. Biol. 2005, 6, 215–225. [Google Scholar] [CrossRef]
- Al Haddad, H.; Mendez-Villanueva, A.; Bourdon, P.C.; Buchheit, M. Effect of acute hypoxia on post-exercise parasympathetic reactivation in healthy men. Front. Physiol. 2012, 3, 289. [Google Scholar] [CrossRef] [PubMed]
- Aras, D.; Coskun, B. The changes on the HRV after a Wingate anaerobic test in different simulated altitudes in healthy, physically-active adults. Acta Med. Mediterr. 2016, 32, 1683. [Google Scholar]
- Botek, M.; Krejčí, J.; De Smet, S.; Gába, A.; McKune, A.J. Heart rate variability and arterial oxygen saturation response during extreme normobaric hypoxia. Auton. Neurosci. 2015, 190, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Boss, C.J.; Mellor, A.; O’Hara, J.P.; Tsakirides, C.; Woods, D.R. The effects of sex on cardiopulmonary responses to acute normobaric hypoxia. High Alt. Med. Biol. 2016, 17, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Philadelphy, M.; Gatterer, H.; Burtscher, J.; Faulbaher, M.; Nachbauer, W.; Likar, R. Physiological responses in humans acutely exposed to high altitude (3480 m): Minute ventilation and oxygenation are predictive for the development of acute mountain sickness. High Alt. Med. Biol. 2019, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; McLellan, C.; Minahan, C. A clustered repeated-sprint running protocol for team-sport athletes performed in normobaric hypoxia. J. Sport. Sci. Med. 2015, 14, 857. [Google Scholar]
- Calbet, J.; Boushel, R.; Rådegran, G.; Søndergaard, H.; Wagner, P.D.; Saltin, B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R291–R303. [Google Scholar] [CrossRef]
- Goodall, S.; Ross, E.Z.; Romer, L.M. Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee-extensor contractions. J. Appl. Physiol. 2010, 109, 1842–1851. [Google Scholar] [CrossRef]
- Faiss, R.; Rapillard, A. Repeated Sprint Training in Hypoxia: Case Report of Performance Benefits in a Professional Cyclist. Front. Sport. Act. Living 2020, 2, 35. [Google Scholar] [CrossRef]
| Pre SIT | Post SIT | Pre SIT | Post SIT | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Females | Males | |||
| SDNN | ||||
| CON | 80.4 (43.3) | 29.6 (16.9) | 83.7 (24.5) | 20.8 (11.5) |
| NOR | 74.7 (42.4) | 26.3 (8.9) | 90.2 (28.3) | 23.7 (13.9) |
| MHYP | 83.7 (45.8) | 26.3 (20.4) | 99.8 (69.5) | 26.8 (24.2) |
| HHYP | 89.2 (53.9) | 28.5 (26.1) | 92.7 (68.3) | 29.1 (23.9) |
| SDSD | ||||
| CON | 116.0 (71.9) | 21.9 (12.7) | 93.0 (21.0) | 16.1 (14.1) |
| NOR | 126.8 (97.1) | 17.2 (5.7) | 108.2 (39.3) | 14.5 (8.7) |
| MHYP | 137.5 (97.5) | 23.2 (13.1) | 116.8 (51.5) | 18.0 (11.7) |
| HHYP | 135.2 (90.8) | 24.4 (15.7) | 111.7 (46.2) | 19.0 (10.0) |
| RMSSD | ||||
| CON | 95.8 (65.6) | 23.5 (14.5) | 74.2 (16.5) | 14.6 (12.8) |
| NOR | 98.1 (76.3) | 15.9 (8.5) | 79.9 (22.8) | 15.6 (10.2) |
| MHYP | 114.9 (82.1) | 24.9 (16.3) | 97.2 (49.3) | 14.3 (12.9) |
| HHYP | 128.2 (100.7) | 23.6 (12.2) | 105.0 (49.9) | 19.0 (16.2) |
| TP | ||||
| CON | 2963.9 (1647.3) | 248.5 (140.9) | 2180.9 (1259.1) | 170.3 (239.4) |
| NOR | 2882.2 (1751.1) | 230.4 (80.9) | 2446.2 (1018.8) | 163.3 (234.1) |
| MHYP | 3326.8 (1532.9) | 244.3 (140.7) | 2401.8 (874.4) | 176.4 (250.5) |
| HHYP | 3286.2 (1461.4) | 271.6 (164.7) | 2542.3 (692.9) | 145.2 (121.1) |
| LF | ||||
| CON | 859.8 (363.3) | 143.8 (99.3) | 1105.3 (993.0) | 80.8 (161.3) |
| NOR | 865.0 (456.6) | 122.8 (79.2) | 1230.3 (868.9) | 102.6 (164.7) |
| MHYP | 1168.2 (691.5) | 140.9 (106.5) | 1242.0 (748.4) | 79.6 (101.4) |
| HHYP | 1161.5 (728.8) | 163.0 (128.7) | 1213.0 (661.0) | 84.2 (62.5) |
| HF | ||||
| CON | 1740.8 (1447.0) | 87.6 (58.1) | 849.7 (452.3) | 62.8 (109.7) |
| NOR | 1636.0 (1374.2) | 113.8 (58.2) | 1033.3 (517.1) | 106.1 (159.6) |
| MHYP | 1701.0 (1000.0) | 86.6 (75.4) | 1239.0 (554.0) | 151.1 (197.0) |
| HHYP | 1784.3 (1136.0) | 98.2 (95.7) | 1398.4 (688.9) | 131.3 (140.7) |
| LF/HF | ||||
| CON | 0.8 (0.4) | 5.5 (1.8) | 1.4 (1.7) | 5.5 (5.0) |
| NOR | 1.3 (0.7) | 5.3 (1.8) | 1.8 (1.8) | 5.1 (5.2) |
| MHYP | 0.9 (0.4) | 5.2 (2.6) | 1.4 (1.0) | 4.0 (4.2) |
| HHYP | 0.9 (0.4) | 5.3 (2.7) | 1.4 (1.1) | 3.2 (3.3) |
| Pre SIT | Post SIT | Pre SIT | Post SIT | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Females | Males | |||
| Response Accuracy (%) | ||||
| CON | 92.2 (2.6) | 92.0 (2.7) | 93.1 (2.7) | 93.4 (2.0) |
| NOR | 92.6 (2.6) | 92.6 (2.5) | 92.9 (1.8) | 92.9 (2.2) |
| MHYP | 92.8 (2.8) | 92.9 (2.6) | 93.2 (3.1) | 92.2 (2.5) |
| HHYP | 93.1 (2.6) | 93.8 (2.0) | 93.3 (1.7) | 91.6 (1.8) |
| Reaction Time (ms) | ||||
| CON | 525.2 (24.2) | 522.4 (23.7) | 516.1 (24.3) | 520.3 (17.5) |
| NOR | 545.8 (41.3) | 527.3 (32.5) | 529.2 (25.4) | 522.3 (32.9) |
| MHYP | 531.5 (44.3) | 532.4 (20.4) | 529.2 (26.7) | 536.2 (35.1) |
| HHYP | 520.4 (20.4) | 535.4 (20.1) | 528.3 (19.5) | 537.7 (30.6) |
| Lactate (mmol) | ||||
| CON | 1.1 (0.1) | 10.2 (1.1) | 1.0 (0.2) | 11.8 (2.1) |
| NOR | 1.1 (0.2) | 10.2 (1.4) | 1.0 (0.1) | 11.9 (2.1) |
| MHYP | 1.0 (0.2) | 11.1 (1.9) | 1.1 (0.2) | 12.3 (1.7) |
| HHYP | 1.0 (0.1) | 11.6 (1.9) * | 1.0 (0.2) | 13.3 (1.6) * |
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Heart Rate | ||||||||
| CON | NOR | MHYP | HHYP | CON | NOR | MHYP | HHYP | |
| Sprint 1 | 162.1 (9.0) | 163.6 (8.3) | 164.3 (4.8) | 164.3 (6.0) | 165.3 (6.8) | 166.0 (5.5) | 165.3 (4.6) | 166.4 (4.5) |
| Sprint 2 | 168.0 (5.7) | 165.6 (8.2) | 166.9 (8.5) | 166.3 (7.7) | 167.6 (8.0) | 168.5 (7.0) | 167.4 (5.1) | 167.2 (4.7) |
| Sprint 3 | 171.1 (7.8) | 170.3 (6.3) | 168.9 (8.0) | 168.2 (3.8) | 173.3 (7.3) | 172.3 (8.7) | 170.6 (6.0) | 169.3 (5.7) |
| Sprint 4 | 170.2 (7.2) | 172.4 (6.8) | 171.2 (7.8) | 169.3 (8.7) | 176.0 (7.0) | 176.5 (6.6) | 174.4 (5.1) | 172.5 (6.1) |
| Sprint 5 | 171.4 (7.3) | 172.7 (7.9) | 170.2 (7.9) | 171.1 (7.0) | 179.2 (7.7) | 178.6 (6.7) | 179.3 (6.9) | 177.4 (8.5) |
| Sprint 6 | 170.9 (6.3) | 173.7 (6.5) | 171.0 (7.6) | 173.6 (4.8) | 182.9 (6.5) | 182.3 (7.6) | 180.9 (8.0) | 180.3 (8.2) |
| Ratings of perceived exertion | ||||||||
| Sprint 1 | 14.4 (1.3) | 14.6 (0.7) | 14.6 (1.1) | 14.9 (1.3) | 13.3 (1.8) | 14.0 (1.8) | 13.4 (1.8) | 13.6 (1.8) |
| Sprint 2 | 15.7 (1.4) | 15.4 (1.5) | 15.5 (1.4) | 15.6 (1.5) | 14.7 (1.2) | 15.3 (2.0) | 14.3 (2.7) | 15.0 (19) |
| Sprint 3 | 16.3 (1.6) | 16.3 (1.3) | 16.8 (1.2) | 16.4 (1.1) | 15.5 (1.9) | 16.3 (2.3) | 15.6 (2.1) | 16.8 (2.2) |
| Sprint 4 | 17.6 (1.7) | 17.0 (1.5) | 17.5 (1.6) | 17.3 (1.7) | 17.3 (1.7) | 17.7 (1.7) | 16.7 (2.1) | 17.7 (2.0) |
| Sprint 5 | 18.3 (1.5) | 18.1 (1.2) | 18.4 (1.5) | 18.3 (1.5) | 18.1 (1.5) | 18.3 (1.2) | 17.6 (1.9) | 18.3 (1.6) |
| Sprint 6 | 19.0 (1.0) | 18.5 (1.4) | 19.0 (1.1) | 18.6 (1.3) | 18.6 (1.8) | 18.8 (1.4) | 18.1 (1.6) | 18.9 (1.3) |
| SpO2 | ||||||||
| Sprint 1 | 90.9 (1.7) | 90.4 (1.1) | 87.3 (2.1) | 85.6 (2.6) | 90.3 (1.7) | 90.3 (1.5) | 87.6 (2.2) | 87.5 (2.0) |
| Sprint 2 | 90.4 (1.8) | 90.2 (1.3) | 87.6 (1.3) | 85.1 (1.8) | 90.3 (1.9) | 90.1 (2.7) | 86.4 (1.5) | 85.6 (1.7) |
| Sprint 3 | 90.0 (2.1) | 89.6 (1.8) | 87.2 (1.0) | 85.0 (1.6) | 89.8 (2.3) | 90.1 (2.6) | 86.0 (1.8) | 84.3 (1,9) |
| Sprint 4 | 89.8 (2.1) | 89.1 (1.9) | 86.8 (1.2) | 84.8 (1.7) | 89.6 (2.3) | 89.3 (2.9) | 85.5 (2.2) | 83.7 (1.6) |
| Sprint 5 | 89.6 (2.1) | 89.2 (2.2) | 86.3 (1.6) | 84.4 (2.0) | 89.4 (2.6) | 89.0 (2.7) | 84.7 (1.8) | 83.3 (1.4) |
| Sprint 6 | 89.6 (2.1) | 89.3 (1.3) | 86.1 (1.3) | 84.0 (2.1) | 89.4 (2.5) | 88.7 (2.6) | 84.3 (2.3) | 82.8 (1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karayigit, R.; Ramirez-Campillo, R.; Yasli, B.C.; Gabrys, T.; Benesova, D.; Esen, O. High Dose of Acute Normobaric Hypoxia Does Not Adversely Affect Sprint Interval Training, Cognitive Performance and Heart Rate Variability in Males and Females. Biology 2022, 11, 1463. https://doi.org/10.3390/biology11101463
Karayigit R, Ramirez-Campillo R, Yasli BC, Gabrys T, Benesova D, Esen O. High Dose of Acute Normobaric Hypoxia Does Not Adversely Affect Sprint Interval Training, Cognitive Performance and Heart Rate Variability in Males and Females. Biology. 2022; 11(10):1463. https://doi.org/10.3390/biology11101463
Chicago/Turabian StyleKarayigit, Raci, Rodrigo Ramirez-Campillo, Burak Caglar Yasli, Tomasz Gabrys, Daniela Benesova, and Ozcan Esen. 2022. "High Dose of Acute Normobaric Hypoxia Does Not Adversely Affect Sprint Interval Training, Cognitive Performance and Heart Rate Variability in Males and Females" Biology 11, no. 10: 1463. https://doi.org/10.3390/biology11101463
APA StyleKarayigit, R., Ramirez-Campillo, R., Yasli, B. C., Gabrys, T., Benesova, D., & Esen, O. (2022). High Dose of Acute Normobaric Hypoxia Does Not Adversely Affect Sprint Interval Training, Cognitive Performance and Heart Rate Variability in Males and Females. Biology, 11(10), 1463. https://doi.org/10.3390/biology11101463










