Two Novel Lasiodiplodia Species from Blighted Stems of Acer truncatum and Cotinus coggygria in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates and Specimens
2.2. Morphology and Growth Characterization
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Sequence Alignment and Phylogenetic Analyses
3. Results
3.1. Phylogenetic Analyses
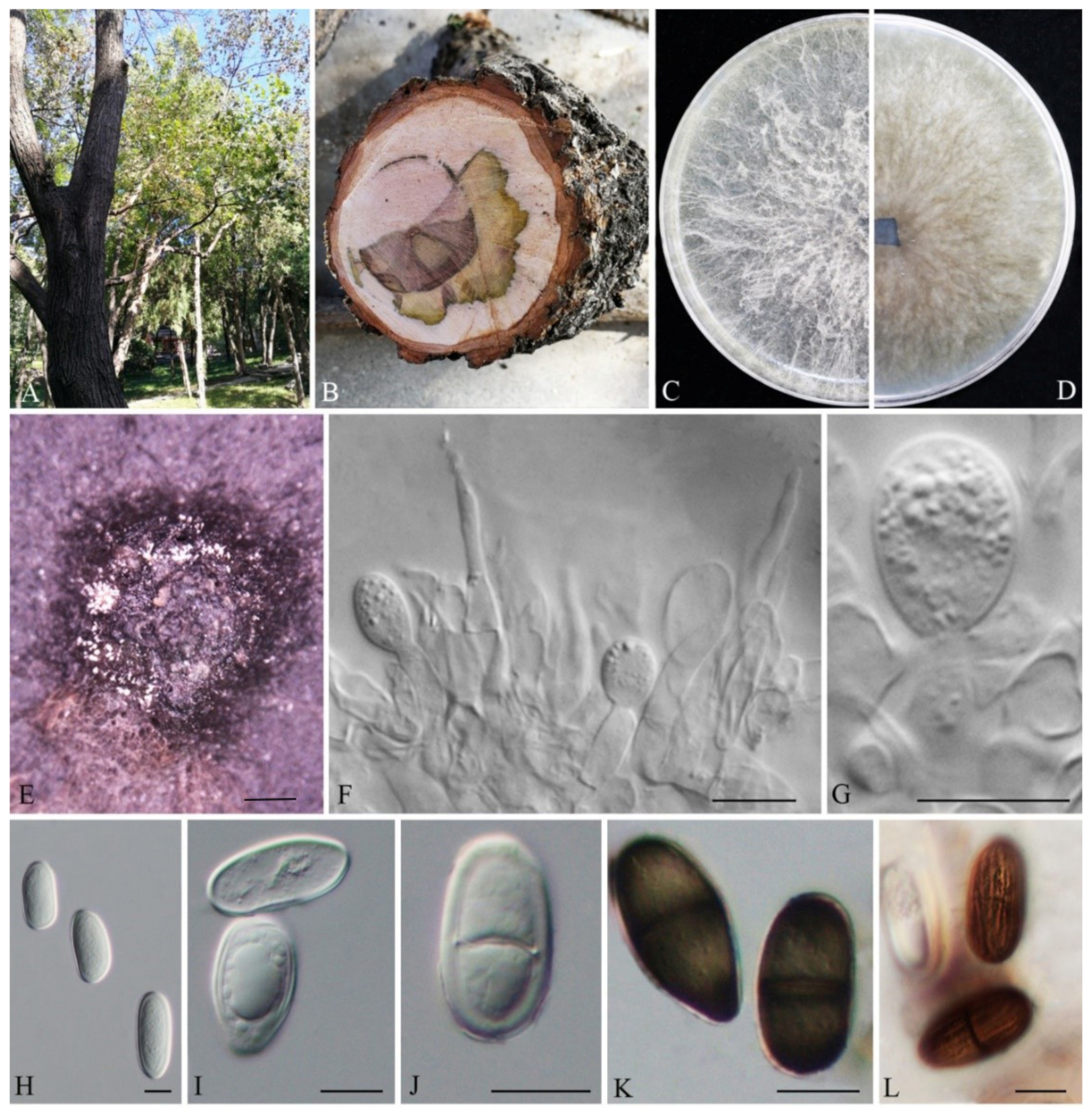
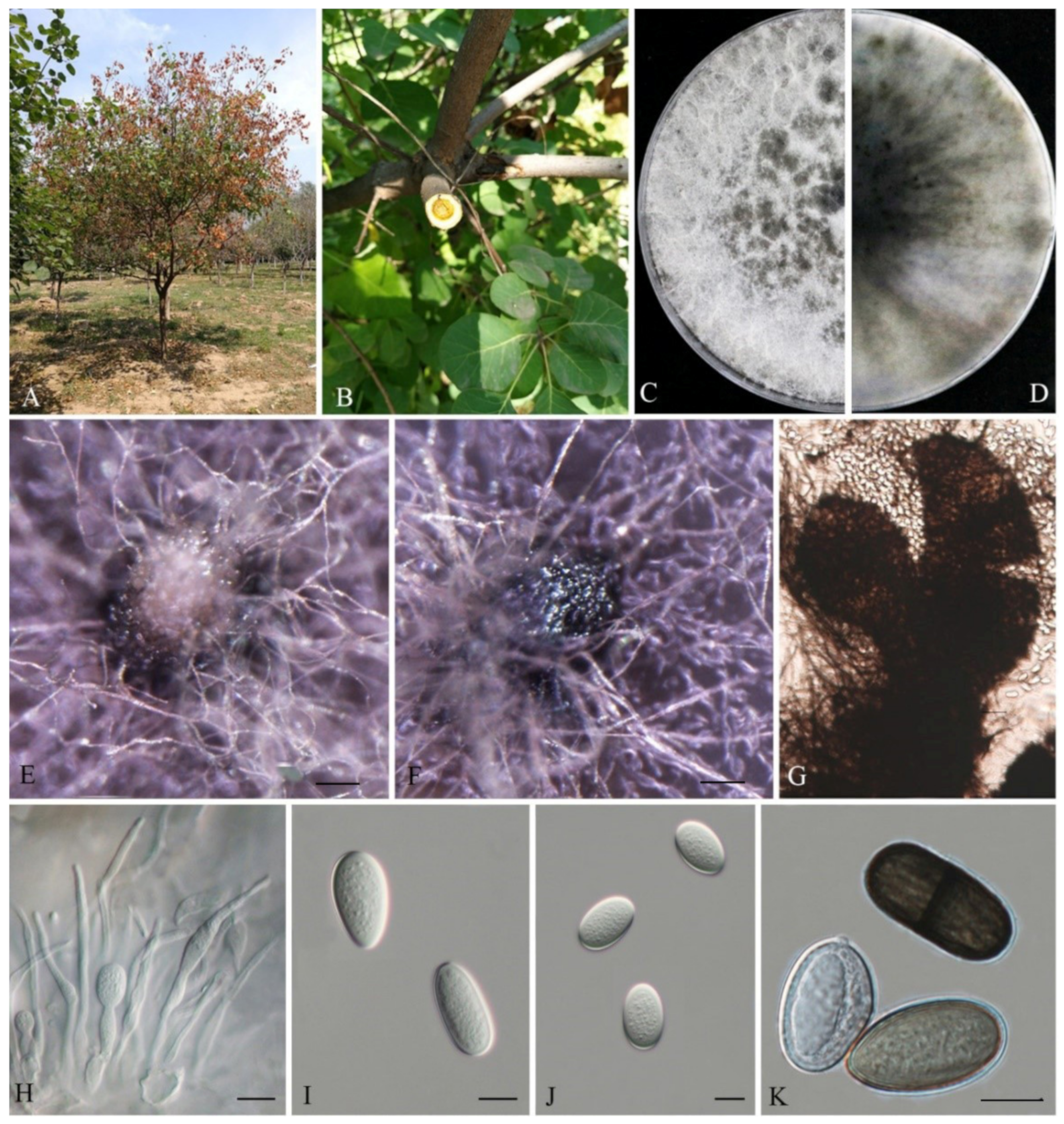
3.2. Taxonomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clendinin, I. Lasiodiplodia Ellis. and Everh. n. gen. Bot. Gaz. 1896, 21, 92–93. [Google Scholar] [CrossRef]
- De Silva, N.I.; Phillips, A.J.L.; Liu, J.-K.; Lumyong, S.; Hyde, K.D. Phylogeny and morphology of Lasiodiplodia species associated with Magnolia forest plants. Sci. Rep. 2019, 9, 14355. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.W.; Lima, N.B.; de Morais, M.A.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.P.; He, W.; Zhang, Y. Does morphology matter in taxonomy of Lasiodiplodia? An answer from Lasiodiplodia hyalina sp. nov. Mycosphere 2017, 8, 1014–1027. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Bhoyroo, V.; Rampadarath, S.; Jeewon, R. Multigene phylogenetics and morphology reveal five novel Lasiodiplodia species associated with blueberries. Life 2021, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.; Phillips, A.; Li, X.; Hyde, K. Botryosphaeriaceae: Current status of genera and species. Mycosphere 2016, 7, 1001–1073. [Google Scholar] [CrossRef]
- Xiao, X.E.; Pu, Z.X.; Wang, W.; Zhu, Z.R.; Li, H.; Crous, P.W. Species of Botryosphaeriaceae associated with citrus branch diseases in China. Persoonia 2021, 47, 106–135. [Google Scholar] [CrossRef]
- Zhang, W.; Groenewald, J.Z.; Lombard, L.; Schumacher, R.K.; Phillips, A.J.L.; Crous, P.W. Evaluating species in Botryosphaeriales. Persoonia 2021, 46, 63–115. [Google Scholar] [CrossRef]
- Dou, Z.P.; He, W.; Zhang, Y. Lasiodiplodia chinensis, a new holomorphic species from China. Mycosphere 2017, 8, 521–532. [Google Scholar] [CrossRef]
- Burgess, T.I.; Barber, P.A.; Mohali, S.; Pegg, G.; de Beer, W.; Wingfield, M.J. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 2006, 98, 423–435. [Google Scholar] [CrossRef]
- Liang, L.; Li, H.; Zhou, L.; Chen, F. Lasiodiplodia pseudotheobromae causes stem canker of Chinese hackberry in China. J. For. Res. 2019, 31, 2571–2580. [Google Scholar] [CrossRef]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridisation in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef]
- Pavlic, D.; Slippers, B.; Coutinho, T.A.; Gryzenhout, M.; Wingfield, M.J. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Stud. Mycol. 2004, 50, 313–322. [Google Scholar]
- Ismail, A.; Cirvilleri, G.; Polizzi, G.; Crous, P.; Groenewald, J.; Lombard, L. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas. Plant Pathol. 2012, 41, 649–660. [Google Scholar] [CrossRef]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; pp. 95–98. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2019, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.P. Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0; Sinauer Associates: Sunderland, UK, 2002. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, X.-W.; Liang, Y.-M.; Tian, C.-M. Lasiodiplodia cinnamomi sp. nov. from Cinnamomum camphora in China. Mycotaxon 2018, 133, 249–259. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Mohammadi Goltapeh, E.; Zare, R.; Phillips, A.J. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010, 25, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Zhao, L.; Sun, X.; He, W.; Zhang, Y.; Dai, Y.-C. Lasiodiplodia spp. associated with Aquilaria crassna in Laos. Mycol. Prog. 2019, 18, 683–701. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Zhou, L.; Chen, F.; Chen, F. Wilt of Acer negundo L. caused by Fusarium nirenbergiae in China. J. For. Res. 2020, 31, 2013–2022. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Y.; Jiang, J.; Hui, O.; Qin, S.; Huang, T. Identification of the pathogen causing brown spot disease of ‘October Glory’. Sci. Silvae Sin. 2015, 51, 142–147. [Google Scholar] [CrossRef]
- Moricca, S.; Uccello, A.; Ginetti, B.; Ragazzi, A. First Report of Neofusicoccum parvum Associated with Bark Canker and Dieback of Acer pseudoplatanus and Quercus robur in Italy. Plant Dis. 2012, 96, 1699. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.X.; Dong, H.X.; Jia, X.Z.; Zhang, X.Y. First report of Botryosphaeria dothidea causing canker of Acer platanoides in China. Plant Dis. 2015, 99, 1857. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, W.; Yang, Q. First report of Alternaria alternata causing leaf spot on Cotinus coggygria Scop. in China. Plant Dis. 2018, 102, 2644. [Google Scholar] [CrossRef]
- Xiong, D.G.; Wang, Y.L.; Ma, J.; Klosterman, S.J.; Xiao, S.; Tian, C. Deep mRNA sequencing reveals stage-specific transcriptome alterations during microsclerotia development in the smoke tree vascular wilt pathogen, Verticillium dahliae. BMC Genom. 2014, 15, 324. [Google Scholar] [CrossRef]
- Fan, S.S.; Huang, Y.J.; Zhang, X.J.; Chen, G.H.; Zhou, J.; Li, X.; Han, M.Z. First report of Botryosphaeria dothidea causing canker on Cotinus coggygria in China. Plant Dis. 2019, 103, 2678. [Google Scholar] [CrossRef]



| Species | Strain | Host | Locality | GenBank Accession Numbers | |||
|---|---|---|---|---|---|---|---|
| ITS | TEF1-α | TUB | RPB2 | ||||
| Lasiodiplodia acaciae | CBS 136434T | Acacia sp. | Indonesia | MT587421 | MT592133 | MT592613 | MT592307 |
| L. acerina | JZBHD1902 | Acer truncatum | China | OP117390 | OP141776 | OP141782 | N/A |
| L. acerina | JZBHD1904T | Acer truncatum | China | OP117391 | OP141777 | OP141783 | OP141788 |
| L. acerina | JZBHD1905 | Acer truncatum | China | OP117392 | OP141778 | OP141784 | OP141789 |
| L.americana | CERC1962 | Pistacia vera | USA | KP217060 | KP217068 | KP217076 | N/A |
| L.americana | CERC1961T | Pistacia vera | USA | KP217059 | KP217067 | KP217075 | N/A |
| L.americana | CERC1960 | Pistacia vera | USA | KP217058 | KP217066 | KP217074 | N/A |
| L. aquilariae | CGMCC 3.18471T | Aquilaria crassna | Laos | KY783442 | KY848600 | N/A | KY848562 |
| L. avicenniae | CMW 41467T | Avicennia marina | South Africa | KP860835 | KP860680 | KP860758 | KU587878 |
| L. avicenniae | LAS 199 | Avicennia marina | South Africa | KU587957 | KU587947 | KU587868 | KU587880 |
| L. avicenniarum | MFLUCC 17-2591T | Avicennia marina | Thailand | MK347777 | MK340867 | N/A | N/A |
| L. brasiliense | CMW 35884 | Adansonia sp. | Laos | KU887094 | KU886972 | KU887466 | KU696345 |
| L. brasiliense | CBS 115447 | Psychotria tutcheri | China | MT587422 | MT592134 | MT592614 | MT592308 |
| L. brasiliensis | CMM 4015T | Mangifera indica | Brazil | JX464063 | JX464049 | N/A | N/A |
| L. brasiliensis | CMM 4469 | Anacardium occidentale | Brazil | KT325574 | KT325580 | N/A | N/A |
| L. bruguierae | CMW 41470T | Bruguiera gymnorrhiza | South Africa | KP860833 | KP860678 | KP860756 | KU587875 |
| L. bruguierae | CMW 42480 | Bruguiera gymnorrhiza | South Africa | KP860832 | KP860677 | KP860755 | KU587876 |
| L. caatinguensis | CMM 1325T | Citrus sinensis | Brazil | KT154760 | KT008006 | KT154767 | N/A |
| L. caatinguensis | IBL 381 | Spondias purpurea | Brazil | KT154757 | KT154751 | KT154764 | N/A |
| L. chiangraiensis | MFLUCC 21-0003T | / | Thailand | MW760854 | MW815630 | MW815628 | N/A |
| L. chiangraiensis | GZCC 21-0003 | / | Thailand | MW760853 | MW815629 | MW815627 | N/A |
| L. chinensis | CGMCC 3.18061T | / | China | KX499889 | KX499927 | KX500002 | KX499965 |
| L. chinensis | CGMCC 3.18063 | Canarium parvum | China | KX499891 | KX499929 | KX500004 | KX499967 |
| L. chonburiensis | MFLUCC 16-0376T | Pandanaceae | Thailand | MH275066 | MH412773 | MH412742 | N/A |
| L. cinnamomi | CFCC 51997T | Cinnamomum camphora | China | MG866028 | MH236799 | MH236797 | MH236801 |
| L. cinnamomi | CFCC 51998 | Cinnamomum camphora | China | MG866029 | MH236800 | MH236798 | MH236802 |
| L. citricola | CBS 124707T | Citrus sp. | Iran | GU945354 | GU945340 | KU887505 | KU696351 |
| L. citricola | CBS 124706 | Citrus sp. | Iran | GU945353 | GU945339 | KU887504 | KU696350 |
| L. clavispora | CGMCC 3.19594T | Vaccinium uliginosum | China | MK802166 | N/A | MK816339 | MK809507 |
| L. clavispora | CGMCC 3.19595 | Vaccinium uliginosum | China | MK802165 | N/A | MK816338 | MK809506 |
| L. cotini | JZBPG1901 | Cotinus coggygria | China | OP117387 | OP141773 | OP141779 | OP141785 |
| L. cotini | JZBPG1903 | Cotinus coggygria | China | OP117388 | OP141774 | OP141780 | OP141786 |
| L. cotini | JZBPG1905T | Cotinus coggygria | China | OP117389 | OP141775 | OP141781 | OP141787 |
| L. crassispora | CBS 118741T | Santalum album | Australia | DQ103550 | DQ103557 | KU887506 | KU696353 |
| L. crassispora | CMW 13488 | Eucalyptus urophylla | Venezuela | DQ103552 | DQ103559 | KU887507 | KU696352 |
| L. crassispora | WAC 12533 | Santalum album | Australia | DQ103550 | DQ103557 | KU887506 | KU696353 |
| L. curvata | CGMCC 3.18456T | Aquilaria crassna | Laos | KY783437 | KY848596 | KY848529 | KY848557 |
| L. curvata | CGMCC 3.18476 | Aquilaria crassna | Laos | KY783443 | KY848601 | KY848532 | KY848563 |
| L. endophytica | MFLUCC 18-1121T | Magnolia acuminata | China | MK501838 | MK584572 | MK550606 | N/A |
| L. euphorbicola | CMW 3609T | Jatropha curcas | Brazil | KF234543 | KF226689 | KF254926 | N/A |
| L. euphorbiicola | CMW 33350T | Adansonia digitata | Botswana | KU887149 | KU887026 | KU887455 | KU696346 |
| L. euphorbiicola | CMW 36231 | Adansonia digitata | Zimbabwe | KU887187 | KU887063 | KU887494 | KU696347 |
| L. euphorbiaceicola | CMW 33268T | Adansonia sp. | Senegal | KU887131 | KU887008 | KU887430 | KU887367 |
| L. exigua | BL184T | Retama raetam | Tunisia | KJ638318 | KJ638337 | N/A | N/A |
| L. exigua | CBS 137785 | Retama raetam | Tunisia | KJ638317 | KJ638336 | KU887509 | KU696355 |
| L. fujianensis | CGMCC 3.19593T | Vaccinium uliginosum | China | MK802164 | MK887178 | MK816337 | MK809505 |
| L. gilanensis | CBS 124704T | Citrus sp. | Iran | GU945351 | GU945342 | KU887511 | KU696357 |
| L. gilanensis | CBS 124705 | Citrus sp. | Iran | GU945352 | GU945341 | KU887510 | KU696356 |
| L. gonubiensis | CMW 14077T | Syzygium cordatum | South Africa | AY639595 | DQ103566 | DQ458860 | KU696359 |
| L. gonubiensis | CMW 14078 | Syzygium cordatum | South Africa | AY639594 | DQ103567 | EU673126 | KU696358 |
| L. gravistriata | CMM 4564T | Anacardium humile | Brazil | KT250949 | KT250950 | N/A | N/A |
| L. gravistriata | CMM 4565 | Anacardium humile | Brazil | KT250947 | KT266812 | N/A | N/A |
| L. guilinensis | CGMCC3.20378T | Citrus sinensis | China | MW880672 | MW884175 | MW884204 | MW884149 |
| L. guilinensis | CGMCC3.20379 | Citrus unshiu | China | MW880673 | MW884176 | MW884205 | MW884150 |
| L. henanica | CGMCC3.19176T | Vaccinium uliginosum | China | MH729351 | MH729357 | MH729360 | MH729354 |
| L. hormozganensis | CBS 124709T | Olea sp. | Iran | GU945355 | GU945343 | KU887515 | KU696361 |
| L. hormozganensis | CBS 124708 | Mangifera indica | Iran | GU945356 | GU945344 | KU887514 | KU696360 |
| L. huangyanensis | CGMCC 3.20380T | Citrus lata | China | MW880674 | MW884177 | MW884206 | MW884151 |
| L. huangyanensis | CGMCC 3.20381 | Citrus unshiu | China | MW880675 | MW884178 | MW884207 | MW884152 |
| L.hyalina | CGMCC 3.17975T | Acacia confusa | China | KX499879 | KX499917 | KX499992 | KX499955 |
| L. hyalina | CGMCC 3.18383 | / | China | KY767661 | KY751302 | KY751299 | KY751296 |
| L. indica | IBP 01T | angiospermic wood | India | KM376151 | N/A | N/A | N/A |
| L. iranensis | CBS 124710T | Salvadora persica | Iran | GU945348 | GU945336 | KU887516 | KU696363 |
| L. iranensis | CBS 124711 | Juglans sp. | Iran | GU945347 | GU945335 | KU887517 | KU696362 |
| L. irregularis | CGMCC3.18468T | Aquilaria crassna | Laos | KY783472 | KY848610 | KY848553 | KY848592 |
| L. jatrophicola | CMM 3610T | Jatropha curcas | Brazil | KF234544 | KF226690 | KF254927 | N/A |
| L.jatrophicola | CMW36237 | Adansonia sp. | Brazil | KU887121 | KU886998 | KU887499 | KU696348 |
| L.jatrophicola | CMW36239 | Adansonia sp. | Brazil | KU887123 | KU887000 | KU887501 | KU696349 |
| L. krabiensis | MFLUCC 17-2617T | Bruguiera sp. | Thailand | MN047093 | MN077070 | N/A | N/A |
| L. laeliocattleyae | CBS 130992T | Mangifera indica | Egypt | KU507487 | KU507454 | KU887508 | KU696354 |
| L. laeliocattleyae | BOT 29 | Mangifera indica | Egypt | JN814401 | JN814428 | N/A | N/A |
| L. laeliocattleyae | CBS 167.28 | Laeliocattleya sp. | Italy | KU507487 | KU507454 | MT592618 | MT592313 |
| L. laosensis | CGMCC 3.18464T | Aquilaria crassna | Laos | KY783471 | KY848609 | KY848552 | KY848591 |
| L. laosensis | CGMCC 3.18473 | Aquilaria crassna | Laos | KY783450 | KY848603 | KY848536 | KY848570 |
| L. lignicola | CBS 134112T | / | Thailand | JX646797 | KU887003 | JX646845 | KU696364 |
| L. lignicola | MFLUCC 11-0435 | / | Thailand | JX646797 | JX646862 | JX646845 | KP872470 |
| L. lignicola | MFLUCC 11-0656 | / | Thailand | JX646798 | JX646863 | JX646846 | N/A |
| L. linhaiensis | CGMCC 3.20386T | Citrus unshiu | China | MW880677 | MW884180 | MW884209 | MW884154 |
| L. linhaiensis | CGMCC 3.20383 | Citrus sinensis | China | MW880678 | MW884181 | MW884210 | MW884155 |
| L. loidaceae | DSM 112340T | Lodoicea maldivica | Mexico | MW274148 | MW604230 | MW604240 | MW604219 |
| L. loidaceae | DSM 112341 | Lodoicea maldivica | Mexico | MW274146 | MW604229 | MW604239 | MW604218 |
| L. macroconidia | CGMCC 3.18479T | Aquilaria crassna | Laos | KY783438 | KY848597 | KY848530 | KY848558 |
| L. macrospora | CMM 3833T | Jatropha curcas | Brazil | KF234557 | KF226718 | KF254941 | N/A |
| L. magnoliae | MFLUCC 18-0948T | Magnolia acuminata | China | MK499387 | MK568537 | MK521587 | N/A |
| L. mahajangana | CMW 27801T | Terminalia catappa | Madagascar | FJ900595 | FJ900641 | FJ900630 | KU696365 |
| L. mahajangana | CMW 27818 | Terminalia catappa | Madagascar | FJ900596 | FJ900642 | FJ900631 | KU696366 |
| L. mahajangana | CBS:125267 | Terminalia sambesiaca | Tanzania | MT587428 | MT592140 | MT592622 | MT592318 |
| L. margaritacea | CBS 122519T | Adansonia gibbosa | Australia | EU144050 | EU144065 | KU887520 | KU696367 |
| L. margaritacea | CBS 138291 | Combretum obovatum | Zambia | KP872322 | KP872351 | KP872381 | KP872431 |
| L. marypalme | CMM 2275T | Carica papaya | Brazil | KC484843 | KC481567 | N/A | N/A |
| L. marypalme | CMM 2272 | Carica papaya | Brazil | KC484842 | KC481566 | N/A | N/A |
| L. mediterranea | CBS 137783T | Quercus ilex | Italy | KJ638312 | KJ638331 | KU887521 | KU696368 |
| L. mediterranea | CBS 137784 | Vitis vinifera | Italy | KJ638311 | KJ638330 | KU887522 | KU696369 |
| L. mexicanense | DSM 112342T | Chamaedorea seifrizii | Mexico | MW274151 | MW604234 | MW604243 | MW604222 |
| L. mexicanense | AGQMy 0015 | Chamaedorea seifrizii | Mexico | MW274150 | MW604233 | MW604242 | MW604221 |
| L. microcondia | CGMCC 3.18485T | Aquilaria crassna | Laos | KY783441 | KY848614 | N/A | KY848561 |
| L. missouriana | UCD 2193MOT | Vitis sp. | USA | HQ288225 | HQ288267 | HQ288304 | KU696370 |
| L. missouriana | UCD 2199MO | Vitis sp. | USA | HQ288226 | HQ288268 | HQ288305 | KU696371 |
| L. mitidjana | ALG111T | Citrus sp. | Algeria | MN104115 | MN159114 | N/A | N/A |
| L. mitidjana | ALG112 | Citrus sp. | Algeria | MN104116 | MN159115 | N/A | N/A |
| L. nanpingensis | CGMCC3.19596T | Vaccinium uliginosum | China | MK802167 | N/A | MK816340 | MK809508 |
| L. nanpingensis | CGMCC3.19597 | Vaccinium uliginosum | China | MK802168 | N/A | MK816341 | MK809509 |
| L. pandanicola | MFLUCC 16-0265T | Pandanaceae | Thailand | MH275068 | MH412774 | MH412744 | N/A |
| L. pandanicola | GBLZ 16BO-008T | Litchi chinensis | China | MN540679 | N/A | MN539183 | N/A |
| L.paraphysoide | CGMCC 3.19174T | Vaccinium uliginosum | China | MH729349 | MH729355 | MH729358 | MH729352 |
| L.paraphysoides | CGMCC 3.19175 | Vaccinium uliginosum | China | MH729350 | MH729356 | MH729359 | MH729353 |
| L. parva | CBS 456.78T | / | USA | EF622083 | EF622063 | KU887523 | KU696372 |
| L. parva | CBS 494.78 | Cassava-field soil | Colombia | EF622084 | EF622064 | EU673114 | KU696373 |
| L. plurivora | STE-U 5803T | Prunus salicina | South Africa | EF445362 | EF445395 | KP872421 | KP872479 |
| L. plurivora | STE-U 4583 | Vitis vinifera | South Africa | AY343482 | EF445396 | KP872422 | KP872480 |
| L. ponkanicola | CGMCC3.20388T | Citrus reticulata | China | MW880685 | MW884188 | MW884214 | MW884159 |
| L. pontae | CMM 1277T | Spondias purpurea | Brazil | KT151794 | KT151791 | KT151797 | N/A |
| L. pontae | CBS 117454 | Eucalyptus urophylla | Venezuela | MT587432 | MT592144 | MT592626 | N/A |
| L. pseudotheobromae | CBS 116459T | Gmelina arborea | Costa Rica | EF622077 | EF622057 | EU673111 | KU696376 |
| L.pseudotheobromae | CGMCC 3.18047 | Pteridium aquilinum | China | KX499876 | KX499914 | KX499989| | KX499952 |
| L. pseudotheobromae | CBS 121772 | Acacia mellifera | Namibia | EU101310 | EU101355 | MT592627 | MT592323 |
| L. pyriformis | CBS 121770T | Acacia mellifera | Namibia | EU101307 | EU101352 | KU887527 | KU696378 |
| L. pyriformis | CBS 121771 | Acacia mellifera | Namibia | EU101308 | EU101353 | KU887528 | KU696379 |
| L. rubropurpurea | WAC 12535T | Eucalyptus grandis | Australia | DQ103553 | DQ103571 | EU673136 | KU696380 |
| L. rubropurpurea | WAC 12536 | Eucalyptus grandis | Australia | DQ103554 | DQ103572 | KU887530 | KU696381 |
| L. sterculiae | CBS342.78T | Sterculia oblonga | Germany | KX464140 | KX464634 | KX464908 | KX463989 |
| L. subglobosa | CMM 3872T | Jatropha curcas | Brazil | KF234558 | KF226721 | KF254942 | N/A |
| L. subglobosa | CMM 4046 | Jatropha curcas | Brazil | KF234560 | KF226723 | KF254944 | N/A |
| L. swieteniae | MFLUCC 18-0244T | Swietenia mahagoni | Thailand | MK347789 | MK340870 | MK412877 | N/A |
| L. syzygii | MFLUCC 19-0257T | Syzygium samarangense | Thailand | MT990531 | MW016943 | MW014331 | N/A |
| L. syzygii | CBS:120512 | Syzygium samarangense | Thailand | MT587434 | MT592147 | MT592632 | N/A |
| L. syzygii | GUCC 9719.2 | Syzygium samarangense | Thailand | MW081991 | MW087101 | MW087104 | N/A |
| L. tenuiconidia | CGMCC 3.18449T | Aquilaria crassna | Laos | KY783466 | KY848619 | N/A | KY848586 |
| L. thailandica | CBS 138760T | Mangifera indica | Thailand | KJ193637 | KJ193681 | N/A | N/A |
| L. thailandica | CGMCC 3.18384 | Albizia chinensis | China | KY767663 | KY751304 | KY751301 | KY751298 |
| L. thailandica | MUCC<JPN>:2738 | Bryophyllum pinnatum | Japan | LC567321 | LC567750 | LC567780 | LC567810 |
| L. theobromae | CBS 164.96T | / | Papua New Guinea | AY640255 | AY640258 | KU887532 | KU696383 |
| L. theobromae | CBS 111530 | Leucospermum sp. | USA | EF622074 | EF622054 | KU887531 | KU696382 |
| L. tropica | CGMCC 3.18477T | Aquilaria crassna | Laos | KY783454 | KY848616 | KY848540 | KY848574 |
| L. vaccinii | CGMCC 3.19022T | Vaccinium uliginosum | China | MH330318 | MH330327 | MH330324 | MH330321 |
| L. vaccinii | CGMCC 3.19023 | Vaccinium uliginosum | China | MH330319 | MH330329 | MH330326 | MH330322 |
| L. venezuelensis | WAC 12539T | Acacia mangium | Venezuela | DQ103547 | DQ103568 | KU887533 | KP872490 |
| L. venezuelensis | WAC 12540 | Acacia mangium | Venezuela | DQ103548 | DQ103569 | KU887534 | KP872491 |
| L. viticola | CBS 128313T | Vitis vinifera | USA | HQ288227 | HQ288269 | HQ288306 | KU696385 |
| L. viticola | UCD 2604MO | Vitis vinifera | USA | HQ288228 | HQ288270 | HQ288307 | KU696386 |
| L. vitis | CBS 124060T | Vitis vinifera | Italy | KX464148 | KX464642 | KX464917 | KX463994 |
| Diplodia mutila | CMW 7060T | Fraxinus excelsior | Netherlands | AY236955 | AY236904 | AY236933 | EU339574 |
| D. seriata | CBS 112555T | Vitis vinifera | Portugal | AY259094 | AY573220 | DQ458856 | N/A |
| Species | Length of Conidia (μm) | Width of Conidia (μm) | Average L/W of Conidia | L/W Range of Conidia | Length of Paraphyses (μm) | Width of Paraphyses (μm) | Size of Conidiomata (μm) | Reference |
|---|---|---|---|---|---|---|---|---|
| L. acerina | (21.64-)21.97–30.83 (-30.96) | (10.61-)11.48–15.87(-16.72) | 2.00 | 1.58–2.61 | 39.4 | 3 | 2525 | This study |
| L. henanica | (14-)19–26(-27) | 10–13 (-15) | 1.86 | 1.17–2.60 | 105 | 4 | 520 | [6] |
| L. huangyanensis | (21-)28–32.5(-34) | (13-)14–16(-17) | 2.00 | - | 82 | 3–4 | - | [9] |
| L. cinnamomi | (17.5-)18.7–21.1(-22.4) | (11.5-)12.7–14.1(-15.5) | 1.50 | - | 106 | 3–4 | - | [29] |
| L. citricola | (20-)22–27(-31) | (10.9-)12–17(-19) | 1.60 | - | 125 | 3–4 | - | [30] |
| L. cotini | (19.38-)20–27(-28.81) | (12.51-)13.61–16.55(-16.62) | 1.58 | 1.40–1.69 | 41.9 | 2.6 | 415 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, G.; Zhao, J.; Liu, J.; Tan, X.; Qin, W. Two Novel Lasiodiplodia Species from Blighted Stems of Acer truncatum and Cotinus coggygria in China. Biology 2022, 11, 1459. https://doi.org/10.3390/biology11101459
Qiao G, Zhao J, Liu J, Tan X, Qin W. Two Novel Lasiodiplodia Species from Blighted Stems of Acer truncatum and Cotinus coggygria in China. Biology. 2022; 11(10):1459. https://doi.org/10.3390/biology11101459
Chicago/Turabian StyleQiao, Guanghang, Juan Zhao, Juanjuan Liu, Xiaoqian Tan, and Wentao Qin. 2022. "Two Novel Lasiodiplodia Species from Blighted Stems of Acer truncatum and Cotinus coggygria in China" Biology 11, no. 10: 1459. https://doi.org/10.3390/biology11101459
APA StyleQiao, G., Zhao, J., Liu, J., Tan, X., & Qin, W. (2022). Two Novel Lasiodiplodia Species from Blighted Stems of Acer truncatum and Cotinus coggygria in China. Biology, 11(10), 1459. https://doi.org/10.3390/biology11101459






