The Neonicotinoid Thiacloprid Interferes with the Development, Brain Antioxidants, and Neurochemistry of Chicken Embryos and Alters the Hatchling Behavior: Modulatory Potential of Phytochemicals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Eggs
2.3. Experimental Design
2.3.1. Experiment 1: Dose-Response
Management of Fertilized Egg
Air Cell Injections
Chicken Embryo Incubation
Evaluation of Brain Monoamines and Acetylcholine Esterase Activity
2.3.2. Experiment 2: Antidotal Study
Experiment Design
Antioxidant Status in the Brain Tissue of Embryos
Determination of the Activities of Acetyclcholine Esterase and Na+/K+-ATPase in Brain of Embryos
Determination of Brain Levels of GABA, Monoamines and Amino Acids of Embryos
Transcriptional Analysis of Antioxidant-Related Genes in the Brain Tissue of Embryos
2.4. Behavioral Responses of Hatchlings
2.4.1. Righting Response Test
2.4.2. Level Balance Beam
2.4.3. Startle Response
2.5. Data Analysis
3. Results
3.1. Experiment 1: (Dose-Response Study)
3.1.1. Effect on Mortality and Macroscopic Examination
3.1.2. Effect of Thiacloprid on Acetylcholine Esterase and Monoamine Neurotransmitters
3.2. Experiment 2: Antidotal Therapy Study
3.2.1. Effects on Antioxidant Variables
3.2.2. Effects on AChE and Na+/K+-ATPase Enzymes Activity in the Brain
3.2.3. Effects on the Brain Levels of GABA, Monoamines, and Amino Acids
3.2.4. Transcriptional Profile Changes in Antioxidant-Related Genes
3.2.5. Behavioral Response
Effects on the Vestibular and Motor-Related Righting Response
Effects on Fear-Related Avoidance Behaviors
Effects on Auditory Habituation (Startle Response)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Khalil, S.R.; Zaglool, A.W.; Hendam, B.M.; Moustafa, A.A.; Cocco, R.; Di Cerbo, A.; Alagawany, M. Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids. Biology 2021, 10, 1100. [Google Scholar] [CrossRef]
- Farag, M.R.; Mahmoud, H.K.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Alagawany, M.; Abou-Zeid, S.M. Neurobehavioral, physiological and inflammatory impairments in response to bifenthrin intoxication in Oreochromis niloticus fish: Role of dietary supplementation with Petroselinum crispum essential oil. Aquat. Toxicol. 2021, 231, 105715. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Quistad, G.B. Golden age of insecticide research: Past, present, or future? Annu. Rev. Entomol. 1998, 43, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Ann, J.; Akk, G. Activation and modulation of human alpha4beta2 nicotinic acetylcholine receptors by the neonicotinoids clothianidin and imidacloprid. J. Neurosci. Res. 2011, 89, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Hendawi, M.Y.; Alam, R.T.M.; Abdellatief, S.A. Ameliorative effect of flaxseed oil against thiacloprid-induced toxicity in rats: Hematological, biochemical, and histopathological study. Environ. Sci. Pollut. Res. 2016, 23, 11855–11863. [Google Scholar] [CrossRef]
- Kammoun, I.; Sellem, I.; Ben Saad, H.; Boudawara, T.; Nasri, M.; Gharsallah, N.; Mallouli, L.; Amara, I.B. Potential benefits of polysaccharides derived from marine alga Ulva lactuca against hepatotoxicity and nephrotoxicity induced by thiacloprid, an insecticide pollutant. Environ. Toxicol. 2019, 34, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Peer review of the pesticide risk assessment of the active substance thiacloprid. EFSA J. 2019, 17, 5595. [Google Scholar] [CrossRef] [Green Version]
- Salvaggio, A.; Antoci, F.; Messina, A.; Ferrante, M.; Copat, C.; Ruberto, C.; Scalisi, E.M.; Pecoraro, R.; Brundo, M.V. Teratogenic effects of the neonicotinoid thiacloprid on chick embryos (Gallus gallus domesticus). Food Chem. Toxicol. 2018, 118, 812–820. [Google Scholar] [CrossRef]
- Babelova, J.; Sefcikova, Z.; Cikos, S.; Spirkova, A.; Kovarikova, V.; Koppel, J.; Makarevich, A.V.; Chrenek, P.; Fabian, D. Exposure to neonicotinoid insecticides induces embryotoxicity in mice and rabbits. Toxicology 2017, 392, 71–80. [Google Scholar] [CrossRef]
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural Compounds and Neuroprotection: Mechanisms of Action and Novel Delivery Systems. In Vivo 2016, 30, 535–547. [Google Scholar]
- Farag, M.R.; Moselhy, A.A.A.; El-Mleeh, A.; Aljuaydi, S.H.; Ismail, T.A.; Di Cerbo, A.; Crescenzo, G.; Abou-Zeid, S.M. Quercetin alleviates the immunotoxic impact mediated by oxidative stress and inflammation induced by doxorubicin expo-sure in rats. Antioxidants 2021, 10, 1906. [Google Scholar] [CrossRef]
- Guidetti, G.; Di Cerbo, A.; Giovazzino, A.; Rubino, V.; Palatucci, A.T.; Centenaro, S.; Fraccaroli, E.; Cortese, L.; Bonomo, M.G.; Ruggiero, G.; et al. In Vitro Effects of Some Botanicals with Anti-Inflammatory and Antitoxic Activity. J. Immunol. Res. 2016, 2016, 5457010. [Google Scholar] [CrossRef] [Green Version]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Ma, J.; Zhu, W.; Wang, L.; Qu, W.; Su, S.; Zhai, W.; Feng, F.; Liu, W.; Zhang, J. Highly selective separation and purification of chicoric acid from Echinacea purpurea by quality control methods in macroporous adsorption resin column chromatography. J. Sep. Sci. 2019, 42, 1027–1036. [Google Scholar] [CrossRef]
- Rajashekar, C.B.; Oh, M.-M.; Carey, E.E. Organic Crop Management Enhances Chicoric Acid Content in Lettuce. Food Nutr. Sci. 2012, 3, 1296–1302. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic Fractions from Dandelion Leaves and Petals as Modulators of the Antioxidant Status and Lipid Profile in an In Vivo Study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Flanigan, P.M.; Niemeyer, E.D. Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Grignon-Dubois, M.; Rezzonico, B. The economic potential of beach-cast seagrass—Cymodocea nodosa: A promising renewable source of chicoric acid. Bot. Mar. 2013, 56, 303–311. [Google Scholar] [CrossRef]
- Zhang, H.L.; Dai, L.H.; Wu, Y.H.; Yu, X.P.; Zhang, Y.Y.; Guan, R.F.; Liu, T.; Zhao, J. Evaluation of hepatocyteprotective and anti-hepatitis B virus properties of Cichoric acid from Cichorium intybus leaves in cell culture. Biol. Pharm. Bull. 2014, 37, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xie, G.; Liu, Q.; Duan, X.; Liu, Z.; Liu, X. Pharmacokinetics, tissue distribution, and plasma protein binding study of chicoric acid by HPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1031, 139–145. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Zhang, L.; Niu, Y.; Liu, Z.; Liu, X. Comparison of chicoric acid, and its metabolites caffeic acid and caftaric acid: In vitro protection of biological macromolecules and inflammatory responses in BV2 microglial cells. Food Sci. Hum. Wellness 2017, 6, 155–166. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Du, Q.; Liu, Z.; Liu, X. Cichoric Acid Reverses Insulin Resistance and Suppresses Inflammatory Responses in the Glucosamine-Induced HepG2 Cells. J. Agric. Food Chem. 2015, 63, 10903–10913. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, Z.; Li, J.; Ren, B.; Zhu, D.; Liu, Q.; Liu, Z.; Liu, X. Chicoric acid supplementation ameliorates cognitive impairment induced by oxidative stress via promotion of antioxidant defense system. RSC Adv. 2017, 7, 36149–36162. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Makino, T.; Ono, T.; Muso, E.; Honda, G. Inhibitory effect of Perilla frutescens and its phenolic constituents on cultured murine mesangial cell proliferation. Planta Med. 1998, 64, 541–545. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Agrahari, S.; Gopal, K. Inhibition of Na+–K+-ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic. Biochem. Physiol. 2008, 92, 57–60. [Google Scholar] [CrossRef]
- Turnell, D.C.; Cooper, J.D. Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin. Chem. 1982, 28, 527–531. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Effects of varying temporal exposure to lead on behavioral development in herring gull (Larus argentatus) chicks. Pharmacol. Biochem. Behav. 1995, 52, 601–608. [Google Scholar] [CrossRef]
- Rogers, L.J.; Drennen, H.D.; Mark, R.F. Inhibition of memory formation in the imprinting period: Irreversible action of cycloheximide in young chickens. Brain Res. 1974, 79, 213–233. [Google Scholar] [CrossRef]
- Gobeli, A.; Crossley, D.; Johnson, J.; Reyna, K. The effects of neonicotinoid exposure on embryonic development and organ mass in northern bobwhite quail (Colinus virginianus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 9–15. [Google Scholar] [CrossRef]
- Hussein, M.; Singh, V. Effect on chick embryos development after exposure to neonicotinoid insecticide imidacloprid. J. Anat. Soc. India 2016, 65, 83–89. [Google Scholar] [CrossRef]
- Seifert, J.; Stollberg, J. Antagonism of a neonicotinoid insecticide imidacloprid at neuromuscular receptors. Environ. Toxicol. Pharmacol. 2005, 20, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Li, Y.; Huang, X.F.; Zheng, J.F.; Yang, J.; Diao, H.; Yuan, Y.; Xu, Y.; Liu, M.; Shi, H.J.; et al. Reproductive effects of two neonicotinoid insecticides on mouse sperm function and early embryonic development in vitro. PLoS ONE 2013, 8, e70112. [Google Scholar] [CrossRef]
- Fabian, D.; Bukovská, A.; Juhás, Š.; Koppel, J. Apoptotic processes and DNA cytosine methylation in mouse embryos arrested at the 2-cell stage. Zygote 2009, 17, 269–279. [Google Scholar] [CrossRef]
- Aydin, B. Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pestic. Biochem. Physiol. 2011, 100, 165–171. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Zhang, Y.; Zhang, P.; Jiang, H. Inhibition and recovery of biomarkers of earthworm Eisenia fetida after exposure to thiacloprid. Environ. Sci. Pollut. Res. Int. 2015, 22, 9475–9482. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Bhardwaj, S.; Srivastava, L.P. Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its No Observed Effect Level (NOEL). J. Toxicol. Sci. 2010, 35, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wang, J. Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J. Agric. Food Chem. 2015, 63, 1856–1862. [Google Scholar] [CrossRef]
- Shukla, S.; Jhamtani, R.C.; Dahiya, M.S.; Agarwal, R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017, 4, 240–244. [Google Scholar] [CrossRef]
- Yan, S.H.; Wang, J.H.; Zhu, L.S.; Chen, A.M.; Wang, J. Thiamethoxam induces oxidative stress and antioxidant response in zebrafish (Danio Rerio) livers. Environ. Toxicol. 2016, 31, 2006–2015. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.O.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Salah, A.S.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquac. 2020, 12, 2481–2492. [Google Scholar] [CrossRef]
- Alagawany, M.; Nasr, M.; Abdulaziz, A.-A.; Alhotan, R.A.; Azzam, M.M.; Reda, F.M. Impact of dietary cold-pressed chia oil on growth, blood chemistry, haematology, immunity and antioxidant status of growing Japanese quail. Ital. J. Anim. Sci. 2020, 19, 896–904. [Google Scholar] [CrossRef]
- Wang, Z.; Scott, W.C.; Williams, E.S.; Ciarlo, M.; DeLeo, P.C.; Brooks, B.W. Identification of novel uncertainty factors and thresholds of toxicological concern for health hazard and risk assessment: Application to cleaning product ingredients. Environ. Int. 2018, 113, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Xie, G.; Wang, J.; Hou, X.; Wang, X.; Wu, W.; Liu, X. Chicoric acid prevents obesity by attenuating hepatic steatosis, inflammation and oxidative stress in high-fat diet-fed mice. Food Res. Int. 2013, 54, 345–353. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.S.; Park, E.; Kim, S.; Lee, S.Y.; Kim, C.S.; Kim, D.K.; Kim, S.J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef]
- Coelho, V.R.; Vieira, C.G.; de Souza, L.P.; Moyses, F.; Basso, C.; Papke, D.K.; Pires, T.R.; Siqueira, I.R.; Picada, J.N.; Pereira, P. Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci. 2015, 122, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, A.; Milanovic, I.; Pavlovic, N.; Cebovic, T.; Vukmirovic, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [Green Version]
- Schreck, E.; Geret, F.; Gontier, L.; Treilhou, M. Neurotoxic effect and metabolic responses induced by a mixture of six pesticides on the earthworm Aporrectodea caliginosa nocturna. Chemosphere 2008, 71, 1832–1839. [Google Scholar] [CrossRef]
- Goyal, S.; Sandhu, H.S.; Brar, R.S. Histopathological alterations induced after oral sub-acute thiacloprid toxicity in Gallus domesticus. Vet. Arh. 2010, 80, 673–682. [Google Scholar]
- Liu, M.; Wang, G.; Zhang, S.Y.; Zhong, S.; Qi, G.L.; Wang, C.J.; Chuai, M.; Lee, K.K.; Lu, D.X.; Yang, X. From the Cover: Exposing Imidacloprid Interferes With Neurogenesis Through Impacting on Chick Neural Tube Cell Survival. Toxicol. Sci. 2016, 153, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Kour, K.; Bani, S. Chicoric acid regulates behavioral and biochemical alterations induced by chronic stress in experimental Swiss albino mice. Pharmacol. Biochem. Behav. 2011, 99, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Han, J.; Shimozono, H.; Villareal, M.O.; Isoda, H. Caffeoylquinic Acid-Rich Purple Sweet Potato Extract, with or without Anthocyanin, Imparts Neuroprotection and Contributes to the Improvement of Spatial Learning and Memory of SAMP8 Mouse. J. Agric. Food Chem. 2013, 61, 5037–5045. [Google Scholar] [CrossRef]
- Kondo, S.; El Omri, A.; Han, J.; Isoda, H. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funct. Foods 2015, 14, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Alkam, T.; Nitta, A.; Mizoguchi, H.; Itoh, A.; Nabeshima, T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25–35). Behav. Brain Res. 2007, 180, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Srivastava, M.K.; Kapoor, U.; Srivastava, L.P. A 90 days oral toxicity of imidacloprid in female rats: Morphological, biochemical and histopathological evaluations. Food Chem. Toxicol. 2010, 48, 1185–1190. [Google Scholar] [CrossRef]
- Deglise, P.; Grunewald, B.; Gauthier, M. The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 2002, 321, 13–16. [Google Scholar] [CrossRef]
- Tison, L.; Holtz, S.; Adeoye, A.; Kalkan, O.; Irmisch, N.S.; Lehmann, N.; Menzel, R. Effects of sublethal doses of thiacloprid and its formulation Calypso® on the learning and memory performance of honey bees. J. Exp. Biol. 2017, 220, 3695–3705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velisek, J.; Stara, A. Effect of thiacloprid on early life stages of common carp (Cyprinus carpio). Chemosphere 2018, 194, 481–487. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Goldstein, L.B.; Bullman, S.; Tu, T.; Khan, W.A.; Dechkovskaia, A.M.; Abdel-Rahman, A.A. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J. Toxicol. Environ. Health A 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Sano, K.; Isobe, T.; Yang, J.; Win-Shwe, T.T.; Yoshikane, M.; Nakayama, S.F.; Kawashima, T.; Suzuki, G.; Hashimoto, S.; Nohara, K.; et al. In utero and Lactational Exposure to Acetamiprid Induces Abnormalities in Socio-Sexual and Anxiety-Related Behaviors of Male Mice. Front. Neurosci. 2016, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T. Effects of maternal clothianidin exposure on behavioral development in F(1) generation mice. Toxicol. Ind. Health 2012, 28, 697–707. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Miyamoto, J.; Matsumiya, T. Rosmarinic acid and caffeic acid reduce the defensive freezing behavior of mice exposed to conditioned fear stress. Psychopharmacology 2002, 164, 233–235. [Google Scholar] [CrossRef]
- Pereira, P.; Tysca, D.; Oliveira, P.; da Silva Brum, L.F.; Picada, J.N.; Ardenghi, P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol. Res. 2005, 52, 199–203. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Li, S.; Yang, J. Rosmarinic acid protects rat hippocampal neurons from cerebral ischemia/reperfusion injury via the Akt/JNK3/caspase-3 signaling pathway. Brain Res. 2017, 1657, 9–15. [Google Scholar] [CrossRef]
- Fonteles, A.A.; de Souza, C.M.; de Sousa Neves, J.C.; Menezes, A.P.; Santos do Carmo, M.R.; Fernandes, F.D.; de Araujo, P.R.; de Andrade, G.M. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav. Brain Res. 2016, 297, 91–103. [Google Scholar] [CrossRef]
- Hasanein, P.; Mahtaj, A.K. Ameliorative effect of rosmarinic acid on scopolamine-induced memory impairment in rats. Neurosci. Lett. 2015, 585, 23–27. [Google Scholar] [CrossRef]
- Park, D.H.; Park, S.J.; Kim, J.M.; Jung, W.Y.; Ryu, J.H. Subchronic administration of rosmarinic acid, a natural prolyl oligopeptidase inhibitor, enhances cognitive performances. Fitoterapia 2010, 81, 644–648. [Google Scholar] [CrossRef]
- Kantar-Gok, D.; Hidisoglu, E.; Er, H.; Acun, A.D.; Olgar, Y.; Yargicoglu, P. Changes of auditory event-related potentials in ovariectomized rats injected with d-galactose: Protective role of rosmarinic acid. Neurotoxicology 2017, 62, 64–74. [Google Scholar] [CrossRef]
- Kantar Gok, D.; Hidisoglu, E.; Ocak, G.A.; Er, H.; Acun, A.D.; Yargicoglu, P. Protective role of rosmarinic acid on amyloid beta 42-induced echoic memory decline: Implication of oxidative stress and cholinergic impairment. Neurochem. Int. 2018, 118, 1–13. [Google Scholar] [CrossRef]
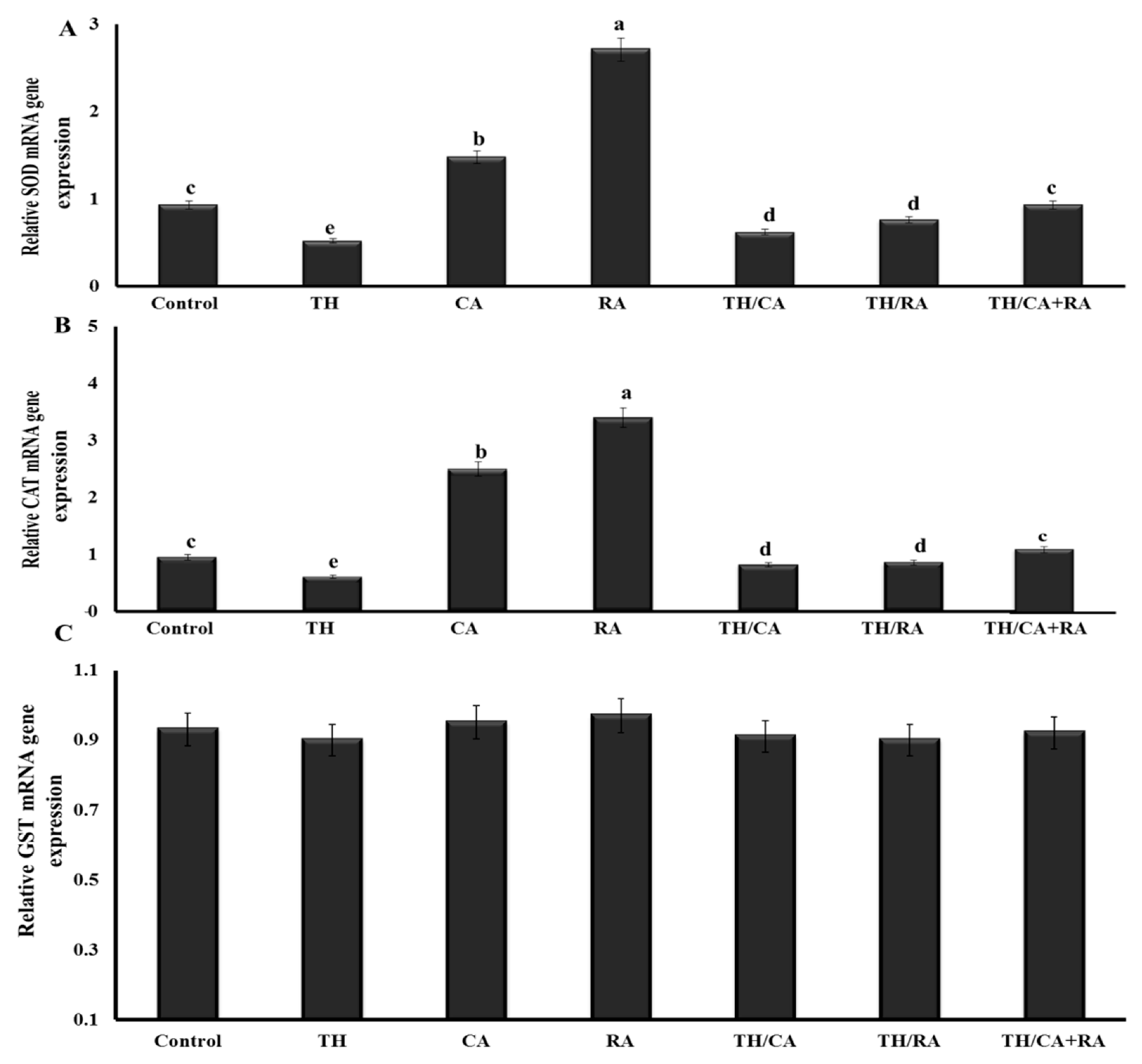
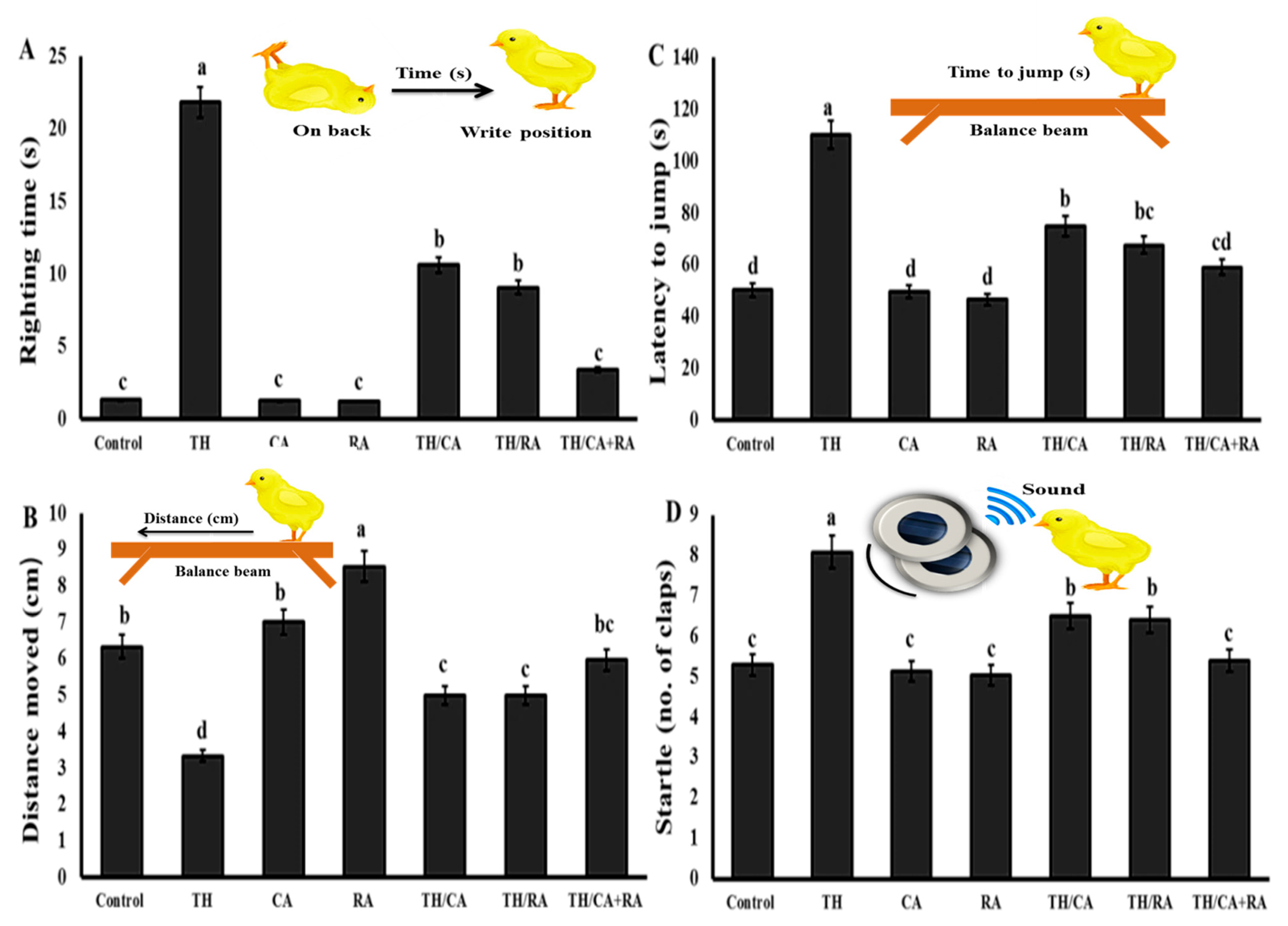
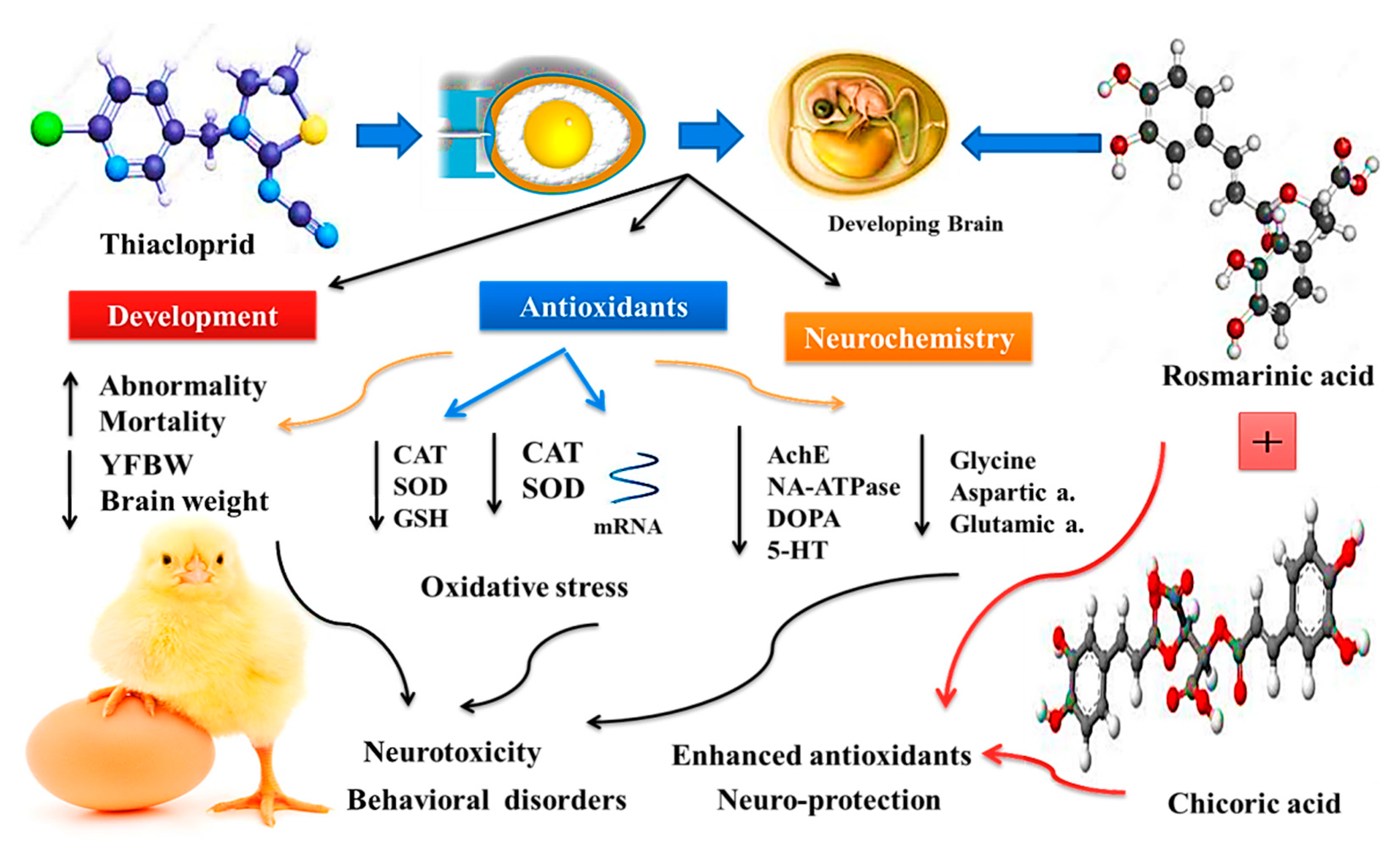
| Gene | Sequences (5′->3′) | Accession Number |
|---|---|---|
| SOD1 | F: AAAATTACCGGCTTGTCTGATG R: CGCTGGTACACCCATTTG | NM_205064 |
| CAT | F: TGCAAGGCGAAAGTGTTTGA R: CAGATTCTCCAGCAACAGTGGA | NM_001031215 |
| GST-α | F: GGAGAGAGCCTGGATTGATATG R: GGTTGTAGCTCGTTCAGTGAT | NM_001001776 |
| β-actin | F: CCCAAAGCCAACAGAGAGAA R: CCATCACCAGAGTCCATCAC | NM_205518 |
| Parameters | Experimental Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | Vehicle | TH0.1 | TH1 | TH10 | TH100 | ||
| Mortality rate (%) | 15.00 ± 0.28 d | 15.00 ± 0.028 d | 15.18 ± 0.39 d | 20.00 ± 0.28 c | 23.33 ± 0.01 b | 39.99 ± 0.96 a | <0.001 |
| Abnormality rate (%) | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 10.50 ± 0.57 c | 21.34 ± 0.09 b | 30.50 ± 1.21 a | <0.001 |
| YFBW 1 | 39.12 ± 0.05 a | 38.36 ± 0.02 a | 38.30 ± 0.01 a | 27.56 ± 0.01 b | 25.23 ± 0.01 c | 22.45 ± 0.09 d | <0.001 |
| Relative brain weight (g) | 0.55 ± 0.01 a | 0.55 ± 0.00 a | 0.54 ± 0.00 a | 0.42 ± 0.00 b | 0.34 ± 0.0.02 c | 0.30 ± 0.00 c | <0.001 |
| AchE (ng/mg protein) | 2.64 ± 0.04 a | 2.58 ± 0.02 ab | 2.47 ± 0.03 b | 1.53 ± 0.02 c | 1.40 ± 0.07 c | 0.99 ± 0.01 d | <0.001 |
| Dopamine (ng/g) | 13.35 ± 0.07 a | 13.31 ± 0.14 a | 13.21 ± 0.06 a | 9.11 ± 0.00 b | 8.5 ± 0.12 c | 6.18 ± 0.03 d | <0.001 |
| 5-HT (ng/g) 1 | 50.57 ± 0.06 a | 50.57 ± 0.09 a | 50.35 ± 0.02 a | 42.67 ± 0.84 b | 34.43 ± 3.33 c | 19.91 ± 2.22 d | <0.001 |
| Parameters | Experimental Groups | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | TH | CA | RA | TH/CA | TH/RA | TH/CA+RA | ||
| Antioxidants markers | ||||||||
| SOD (U/g tissue) | 7.85 ± 0.20 a | 3.59 ± 0.30 c | 7.97 ± 0.22 a | 7.99 ± 0.12 a | 5.81 ± 0.06 b | 6.47 ± 0.13 b | 7.40 ± 0.29 a | <0.001 |
| CAT (U/g tissue) | 5.59 ± 0.22 ab | 3.13 ± 0.07 d | 5.56 ± 0.19 ab | 6.09 ± 0.03 a | 4.80 ± 0.21 c | 5.04 ± 0.20 bc | 5.17 ± 0.03 bc | <0.001 |
| GSH (nmol/g tissue) | 17.10 ± 0.05 a | 13.84 ± 0.91 b | 17.18 ± 0.04 a | 17.24 ± 0.00 a | 16.56 ± 0.32 a | 17.07 ± 0.28 a | 17.0 ± 0.00 a | <0.001 |
| AchE and monoamines neurotransmitters | ||||||||
| AchE (ng/mg protein) | 2.61 ± 0.00 b | 1.70 ± 0.05 f | 2.70 ± 0.00 b | 2.81 ± 0.00 a | 1.90 ± 0.00 e | 2.10 ± 0.00 d | 2.45 ± 0.01 c | <0.001 |
| NA-ATPase (ng/mg protein) | 25.39 ± 0.29 a | 16.19 ± 0.00 c | 26.21 ± 0.00 a | 26.53 ± 0.56 a | 20.49 ± 1.14 b | 21.24 ± 0.57 b | 24.02 ± 0.57 a | <0.001 |
| Serotonin (ng/g) | 51.49 ± 0.01 b | 42.70 ± 0.40 d | 55.15 ± 0.00 a | 55.87 ± 0.01 a | 46.02 ± 0.58 c | 48.54 ± 0.59 bc | 51.36 ± 1.76 b | <0.001 |
| Dopamine (ng/g) | 13.50 ± 0.03 a | 10.22 ± 0.00 e | 13.66 ± 0.00 a | 13.96 ± 019 a | 11.00 ± 0.15 d | 12.22 ± 0.15 c | 12.98 ± 0.49 b | <0.001 |
| GABA (ng/g) | 120.03 ± 0.00 b | 85.68 ± 0.00 d | 122.34 ± 0.02 b | 135.82 ± 7.19 a | 99.44 ± 0.26 c | 100.36 ± 0.34 c | 100.93 ± 0.85 c | <0.001 |
| Amino acids neurotransmitters | ||||||||
| Glycine (nmol/mg protein) | 2.60 ± 0.00 a | 1.26 ± 0.00 b | 2.70 ± 0.60 a | 2.82 ± 0.22 a | 1.90 ± 0.34 ab | 2.20 ± 0.17 ab | 2.36 ± 0.01 ab | <0.05 |
| Aspartic acid (nmol/mg protein) | 3.10 ± 0.00 a | 1.18 ± 0.00 b | 3.15 ± 0.57 a | 3.56 ± 0.00 a | 2.43 ± 0.01 a | 2.49 ± 0.01 a | 2.91 ± 0.28 a | <0.001 |
| Glutamic acid (nmol/mg protein) | 0.62 ± 0.01 a | 0.37 ± 0.00 b | 0.62 ± 0.06 a | 0.63 ± 0.04 a | 0.60 ± 0.03 a | 0.61 ± 0.00 a | 0.62 ± 0.01 a | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.R.; Alagawany, M.; Moselhy, A.A.A.; Said, E.N.; Ismail, T.A.; Di Cerbo, A.; Pugliese, N.; Ahmed, M.M. The Neonicotinoid Thiacloprid Interferes with the Development, Brain Antioxidants, and Neurochemistry of Chicken Embryos and Alters the Hatchling Behavior: Modulatory Potential of Phytochemicals. Biology 2022, 11, 73. https://doi.org/10.3390/biology11010073
Farag MR, Alagawany M, Moselhy AAA, Said EN, Ismail TA, Di Cerbo A, Pugliese N, Ahmed MM. The Neonicotinoid Thiacloprid Interferes with the Development, Brain Antioxidants, and Neurochemistry of Chicken Embryos and Alters the Hatchling Behavior: Modulatory Potential of Phytochemicals. Biology. 2022; 11(1):73. https://doi.org/10.3390/biology11010073
Chicago/Turabian StyleFarag, Mayada R., Mahmoud Alagawany, Attia A. A. Moselhy, Enas N. Said, Tamer A. Ismail, Alessandro Di Cerbo, Nicola Pugliese, and Mona M. Ahmed. 2022. "The Neonicotinoid Thiacloprid Interferes with the Development, Brain Antioxidants, and Neurochemistry of Chicken Embryos and Alters the Hatchling Behavior: Modulatory Potential of Phytochemicals" Biology 11, no. 1: 73. https://doi.org/10.3390/biology11010073
APA StyleFarag, M. R., Alagawany, M., Moselhy, A. A. A., Said, E. N., Ismail, T. A., Di Cerbo, A., Pugliese, N., & Ahmed, M. M. (2022). The Neonicotinoid Thiacloprid Interferes with the Development, Brain Antioxidants, and Neurochemistry of Chicken Embryos and Alters the Hatchling Behavior: Modulatory Potential of Phytochemicals. Biology, 11(1), 73. https://doi.org/10.3390/biology11010073








