Improvement of Bemisia tabaci (Hemiptera: Aleyrodidae) Fitness on Chinese Kale upon Simultaneous Herbivory by Plutella xylostella (Lepidoptera: Plutellidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plant and Insect Cultures
2.2. Bemisia Tabaci Performance
2.3. Plutella Xylostella Performance
2.4. Statistical Analysis
3. Results
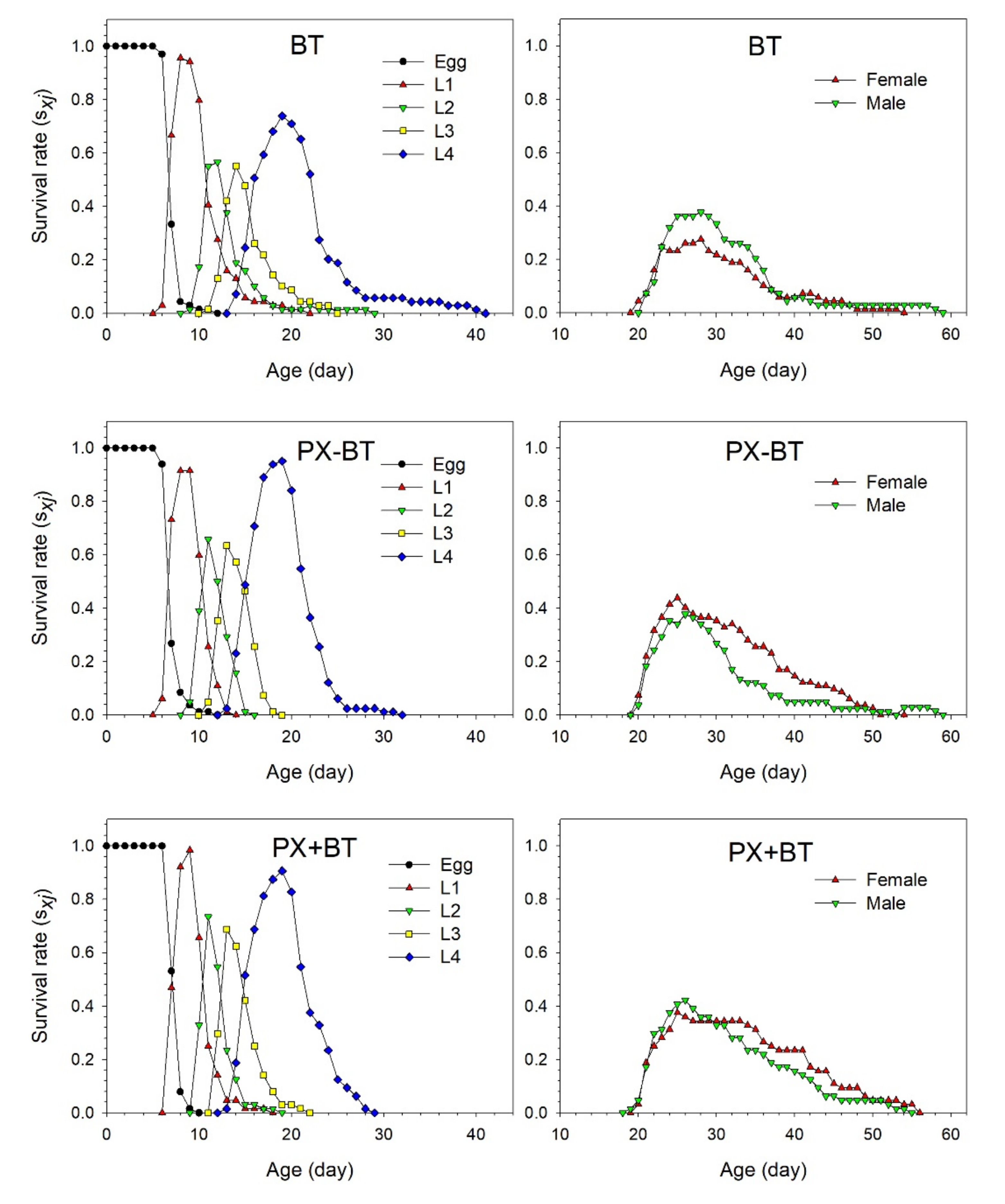
3.1. Performance of B. tabaci
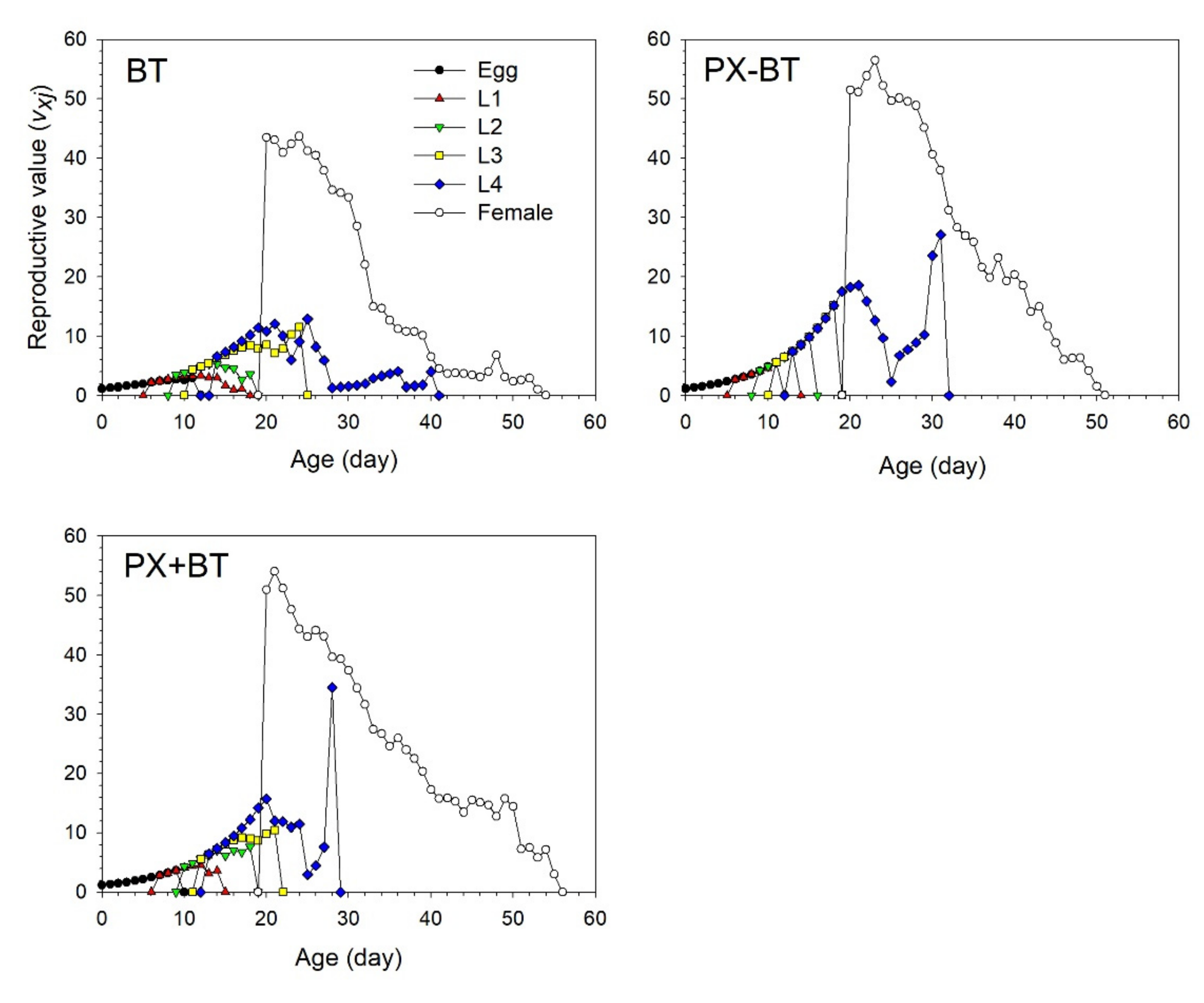
3.2. Life Table Analysis of B. tabaci
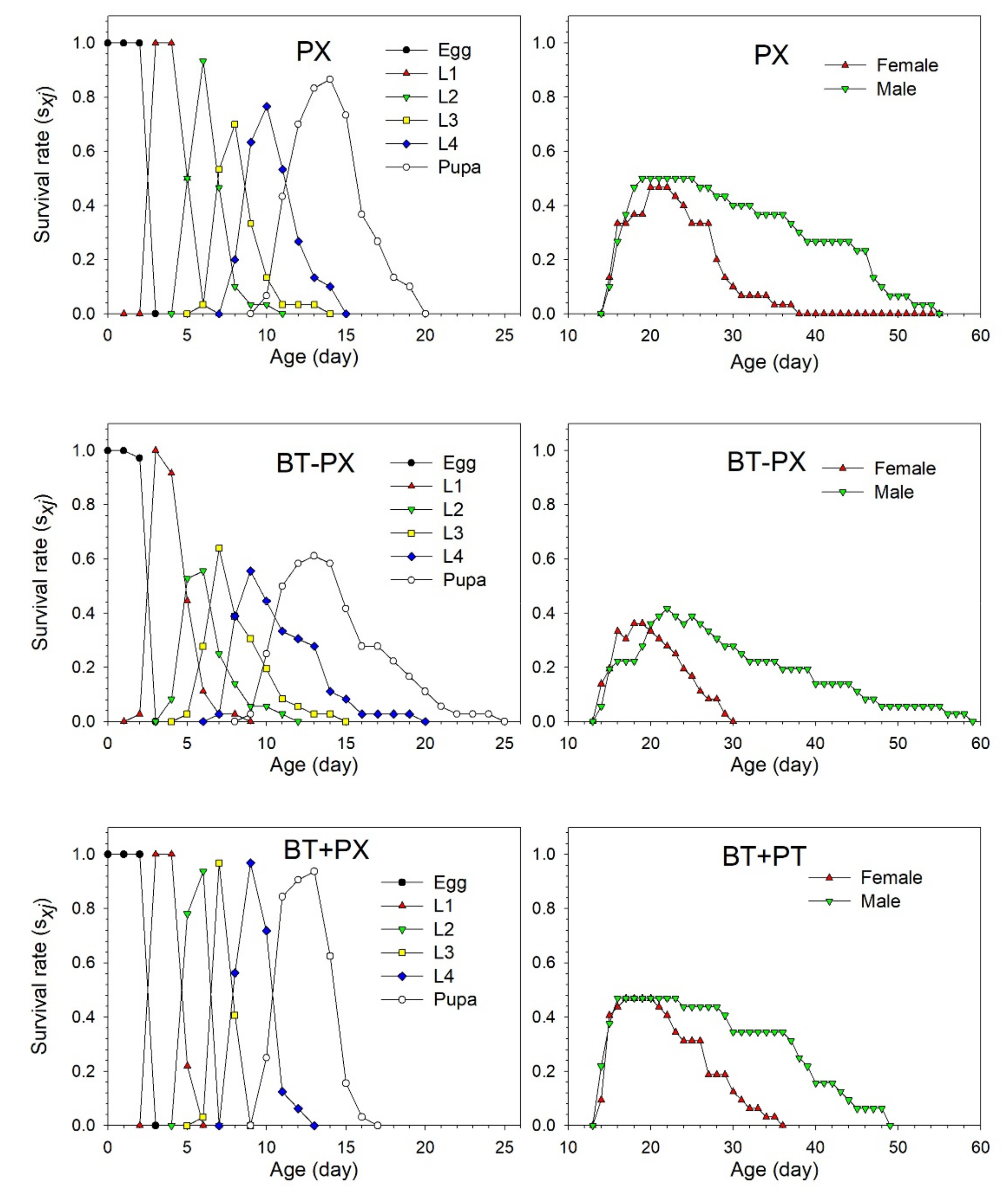
3.3. Performance of DBM
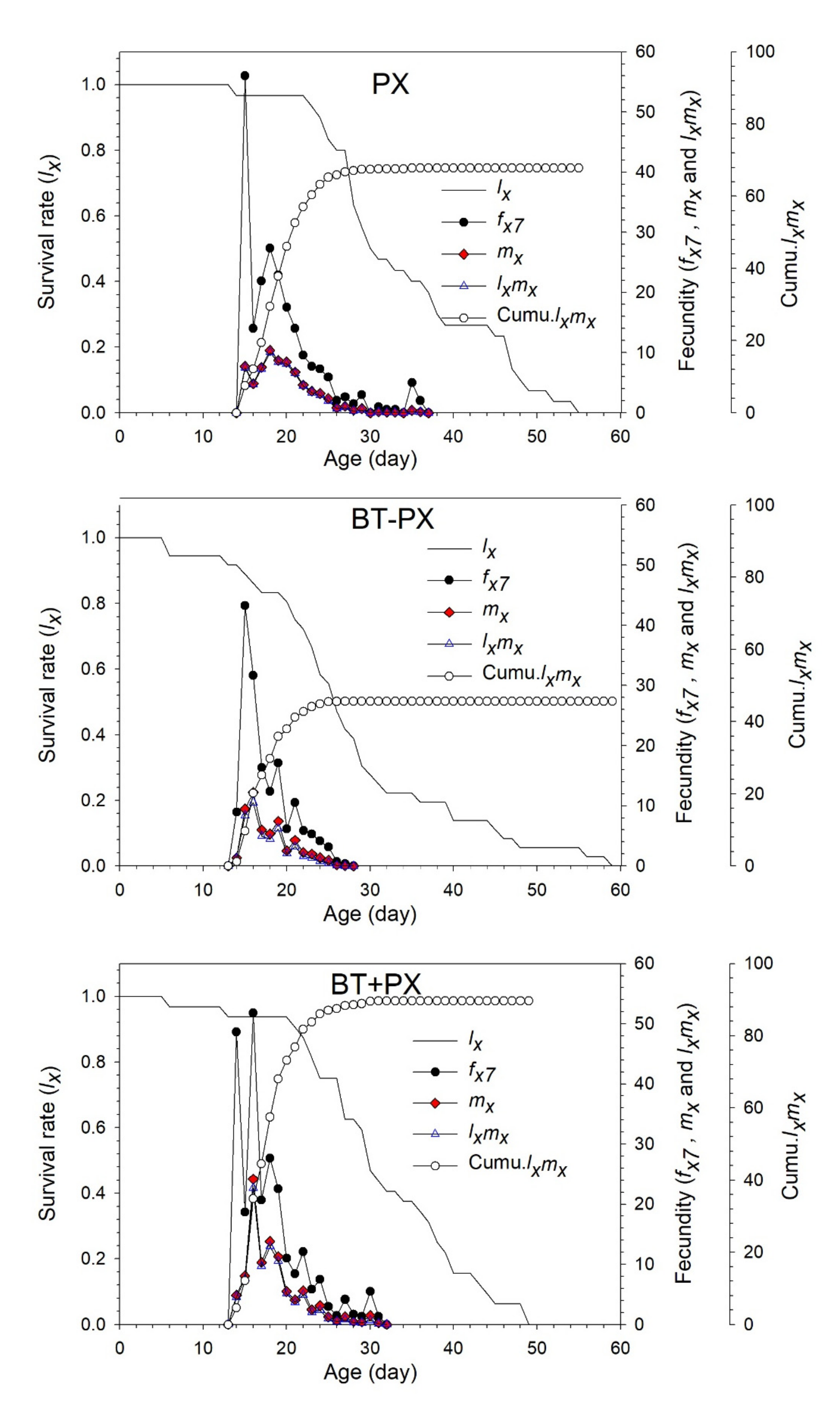
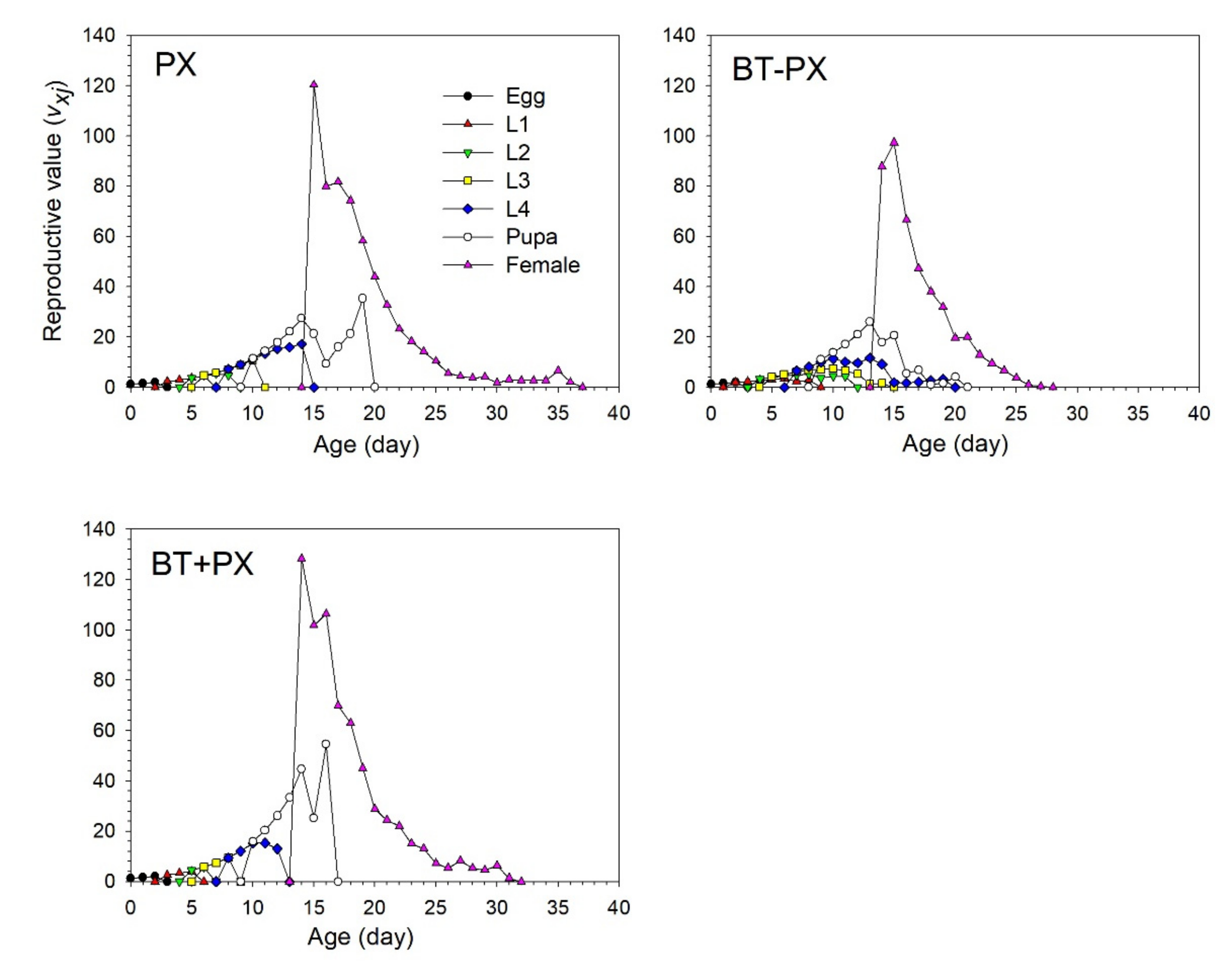
3.4. Life Table Analysis of DBM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dicke, M.; Van Loon, J.J.A.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef]
- Ponzio, C.; Papazian, S.; Albrectsen, B.R.; Dicke, M.; Gols, R. Dual herbivore attack and herbivore density affect metabolic profiles of Brassica nigra leaves. Plant Cell Environ. 2017, 40, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Davidson-Lowe, E.; Szendrei, Z.; Ali, J.G. Asymmetric effects of a leaf-chewing herbivore on aphid population growth. Ecol. Entomol. 2019, 44, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 2010, 127, 617–633. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef]
- Poelman, E.H.; Broekgaarden, C.; Van Loon, J.J.; Dicke, M. Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol. Ecol. 2008, 17, 3352–3365. [Google Scholar] [CrossRef]
- Erb, M.; Robert, C.A.M.; Hibbard, B.E.; Turlings, T.C.J. Sequence of arrival determines plant-mediated interactions between herbivores. J. Ecol. 2011, 99, 7–15. [Google Scholar] [CrossRef]
- Magalhaes, D.M.; Borges, M.; Laumann, R.A.; Moraes, M.C.B. Influence of multiple- and single-species infestations on herbivore-induced cotton volatiles and Anthonomus grandis behaviour. J. Pest Sci. 2019, 91, 1019–1032. [Google Scholar] [CrossRef] [Green Version]
- Heidel, A.J.; Baldwin, I.T. Microarray analysis of salicylic acid- and jasmonic acid-signaling in responses of Nicotiana attenuate to attack by insects from multiple feeding guilds. Plant Cell Environ. 2004, 27, 1362–1373. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Erb, M.; Flors, V.; Karlen, D.; de Lange, E.; Planchamp, C.; D’Alessandro, M.; Turlings, T.C.J.; Ton, J. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J. 2009, 59, 292–302. [Google Scholar] [CrossRef]
- Glauser, G.; Marti, G.; Villard, N.; Doyen, G.A.; Wolfender, J.L.; Turlings, T.C.; Erb, M. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 2011, 68, 901–911. [Google Scholar] [CrossRef]
- Soler, R.; Badenes-Perez, F.R.; Broekgaarden, C.; Zheng, S.J.; David, A.; Boland, W.; Dicke, M. Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: From insect performance to gene transcription. Funct. Ecol. 2012, 26, 156–166. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Xue, M.; Zhang, X. Feeding of whitefly on tobacco decreases aphid performance via increased salicylate signaling. PLoS ONE 2015, 10, e0138584. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct. Ecol. 2014, 28, 1404–1412. [Google Scholar] [CrossRef]
- Eisenring, M.; Glauser, G.; Meissle, M.; Romeis, J. Differential impact of herbivores from three feeding guilds on systemic secondary metabolite induction, phytohormone levels and plant-mediated herbivore interactions. J. Chem. Ecol. 2018, 44, 1178–1189. [Google Scholar] [CrossRef]
- Mcnutt, D.W.; Underwood, N. Variation in plant-mediated intra- and interspecific interactions among insect herbivores: Effects of host genotype. Ecosphere 2016, 7, e01520. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Robert, C.A.; Herve, M.R.; Hu, L.; Bont, Z.; Erb, M. A mechanism for sequence specificity in plant-mediated interactions between herbivores. New Phytol. 2017, 214, 169–179. [Google Scholar] [CrossRef]
- Stam, J.M.; Dicke, M.; Poelman, E.H. Order of herbivore arrival on wild cabbage populations influences subsequent arthropod community development. Oikos 2018, 127, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.Z.; Huang, H.; Shan, H.W.; Zhang, F.; Wan, F.H.; Liu, T.X. Defense against Pieris rapae in cabbage plants induced by Bemisia tabaci biotype B. Entomol. Exp. Appl. 2013, 147, 293–300. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Xiang, Z.W.; Zhang, S.Z.; Wu, L.N.; Liu, T.X. Adaptations of Plutella xylostella adult females and larvae to waxy host plants. J. Pest Sci. 2021. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef]
- Elbaz, M.; Halon, E.; Malka, O.; Malitsky, S.; Blum, E.; Aharoni, A.; Morin, S. Asymmetric adaptation to indolic and aliphatic glucosinolates in the B and Q sibling species of Bemisia tabaci (Hemiptera: Aleyrodidae). Mol. Ecol. 2012, 21, 4533–4546. [Google Scholar] [CrossRef]
- Malka, O.; Shekhov, A.; Reichelt, M.; Gershenzon, J.; Vassao, D.G.; Morin, S. Glucosinolate desulfation by the phloem-feeding insect Bemisia tabaci. J. Chem. Ecol. 2016, 42, 230–235. [Google Scholar] [CrossRef]
- Li, J.X.; Rao, L.L.; Xie, H.; Monika, S.; Chen, L.P.; Liu, Y.Q. Morphology and glucosinolate profiles of chimeric Brassica and the responses of Bemisia tabaci in host selection, oviposition and development. J. Integr. Agr. 2017, 16, 2009–2018. [Google Scholar] [CrossRef]
- Wu, X.X.; Hu, D.X.; Li, Z.X.; Shen, Z.R. Using RAPD-PCR to distinguish biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) in China. Insect Sci. 2002, 9, 1–8. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2019. Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart.zip (accessed on 9 December 2019).
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sinica 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Li, Y.; Meijer, D.; Dicke, M.; Gols, R. Oviposition preference of three lepidopteran species is not affected by previous aphid infestation in wild cabbage. Entomol. Exp. Appl. 2018, 166, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Aphid feeding induces phytohormonal cross-talk without affecting silicon defense against subsequent chewing herbivores. Plants 2020, 9, 1009. [Google Scholar] [CrossRef]
- Kroes, A.; Stam, J.M.; David, A.; Boland, W.; van Loon, J.J.; Dicke, M.; Poelman, E.H. Plant-mediated interactions between two herbivores differentially affect a subsequently arriving third herbivore in populations of wild cabbage. Plant Biol. 2016, 18, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Mayer, R.T.; Inbar, M.; McKenzie, C.L.; Shatters, R.; Borowicz, V.; Albrecht, U.; Powell, C.A.; Doostdar, H. Multitrophic interactions of the silverleaf whitefly, host plants, competing herbivores, and phytopathogens. Arch. Insect Biochem. 2002, 51, 151–169. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zhang, G.Y.; Zhang, Y.J.; Zhang, W.J.; Liu, Z. Interspecific interactions between Bemisia tabaci (Hem., Aleyrodidae) and Liriomyza sativae (Dipt., Agromyzidae). J. Appl. Entomol. 2005, 129, 443–446. [Google Scholar] [CrossRef]
- Xue, M.; Wang, C.X.; Bi, M.J.; Li, Q.L.; Liu, T.X. Induced defense by Bemisia tabaci Biotype B (Hemiptera: Aleyrodidae) in tobacco against Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 2010, 39, 883–891. [Google Scholar] [CrossRef]
- Baumgartner, J.; Delucchi, V.; Von Arx, R.; Rubli, D. Whitefly (Bemisia tabaci Genn., Stern.: Aleyrodidae) infestation patterns as influenced by cotton, weather and Heliothis: Hypotheses testing by using simulation models. Agr. Ecosyst. Environ. 1986, 17, 49–59. [Google Scholar] [CrossRef]
- Inbar, M.; Doostdar, H.; Leibee, G.L.; Mayer, R.T. The role of plant rapidly induced responses in asymmetric interspecific interactions among insect herbivores. J. Chem. Ecol. 1999, 25, 1961–1979. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Karban, R.; Colfer, R.G. How leaf domatia and induced plant resistance affect herbivores, natural enemies and plant performance. Oikos 2000, 89, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xu, Y.H.; Wu, H.M.; Liu, X.Y.; Zhang, L.; Wang, J.R.; Rao, Q. Plant defense responses induced by two herbivores and consequences for whitefly Bemisia tabaci. Front. Physiol. 2019, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.J.; Broekgaarden, C.; Zheng, S.J.; Snoeren, T.A.; van Loon, J.J.; Gols, R.; Dicke, M. Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol. 2013, 197, 1291–1299. [Google Scholar] [CrossRef]
- Chretien, L.T.; David, A.; Daikou, E.; Boland, W.; Gershenzon, J.; Giron, D.; Dicke, M.; Lucas-Barbosa, D. Caterpillars induce jasmonates in flowers and alter plant responses to a second attacker. New Phytol. 2018, 217, 1279–1291. [Google Scholar] [CrossRef]
- Kroes, A.; van Loon, J.J.A.; Dicke, M. Density-dependent interference of aphids with caterpillar-induced defenses in Arabidopsis: Involvement of phytohormones and transcription factors. Plant Cell Physiol. 2015, 56, 98–106. [Google Scholar] [CrossRef]
- Hunter, M.D. Multiple approaches to estimating the relative importance of top-down and bottom-up forces on insect populations: Experiments, life tables, and time-series analysis. Basic Appl. Ecol. 2001, 2, 295–309. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Atlihan, R.; Kasap, I.; Ozgokce, M.S.; Polat-Akkopru, E.; Chi, H. Population growth of Dysaphis pyri (Hemiptera: Aphididae) on different pear cultivars with discussion on curve fitting in life table studies. J. Econ. Entomol. 2017, 110, 1890–1898. [Google Scholar] [CrossRef]
- Qayyum, A.Q.; Aziz, M.A.; Iftikhar, A.; Hafeez, F.; Atlihan, R. Demographic parameters of Lipaphis erysimi (Hemiptera: Aphididae) on different cultivars of Brassica vegetables. J. Econ. Entomol. 2018, 111, 1885–1894. [Google Scholar] [CrossRef]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Saeed, R.; Sayyed, A.H.; Shad, S.A.; Zaka, S.M. Effect of different host plants on the fitness of diamond-back moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2010, 29, 178–182. [Google Scholar] [CrossRef]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]


| Stage | BT | PX − BT | PX + BT |
|---|---|---|---|
| Egg | 7.4 ± 0.1 a | 7.4 ± 0.1 a | 7.6 ± 0.1 a |
| First instar | 4.5 ± 0.2 a | 3.6 ± 0.1 b | 3.5 ± 0.1 b |
| Second instar | 2.4 ± 0.1 a | 2.1 ± 0.0 b | 2.1 ± 0.1 ab |
| Third instar | 2.9 ± 0.2 a | 2.5 ± 0.1 b | 2.6 ± 0.1 ab |
| Fourth instar | 7.4 ± 0.3 a | 6.7 ± 0.1 b | 6.8 ± 0.1 ab |
| Egg-adult | 24.1 ± 0.6 a | 22.2 ± 0.2 b | 22.6 ± 0.3 b |
| Female adult longevity | 10.8 ± 1.2 c | 14.7 ± 1.2 b | 19.2 ± 1.6 a |
| Male adult longevity | 11.8 ± 1.1 a | 10.8 ± 1.2 a | 12.8 ± 1.4 a |
| Oviposition days | 9.9 ± 1.1 c | 14.2 ± 1.2 b | 18.1 ± 1.6 a |
| Fecundity (egg/female) | 66.2 ± 9.6 b | 116.8 ± 11.3 a | 115.9 ± 10.9 a |
| TPOP | 24.2 ± 1.0 a | 22.3 ± 0.3 a | 22.6 ± 0.4 a |
| Nf/N ratio | 0.362 ± 0.06 a | 0.476 ± 0.06 a | 0.391 ± 0.06 a |
| Population Parameters | BT | PX − BT | PX + BT |
|---|---|---|---|
| Intrinsic rate of increase (r) (d−1) | 0.11 ± 0.01 b | 0.14 ± 0.01 a | 0.13 ± 0.01 ab |
| Net reproductive rate (R0) (egg) | 24.00 ± 5.15 b | 55.54 ± 8.35 a | 45.27 ± 8.20 a |
| Finite rate of increase (λ) (d−1) | 1.12 ± 0.01 b | 1.15 ± 0.01 a | 1.14 ± 0.01 ab |
| Mean generation time (T) (d) | 28.59 ± 0.42 a | 28.59 ± 0.39 a | 29.62 ± 0.54 a |
| Stage | PX | BT − PX | BT + PX |
|---|---|---|---|
| Egg | 3.0 ± 0.0 a | 3.0 ± 0.0 a | 3.0 ± 0.0 a |
| First instar | 2.5 ± 0.1 a | 2.5 ± 0.2 ab | 2.2 ± 0.1 b |
| Second instar | 2.1 ± 0.1 a | 1.8 ± 0.1 a | 1.8 ± 0.1 a |
| Third instar | 1.8 ± 0.1 b | 2.1 ± 0.1 a | 1.5 ± 0.1 c |
| Fourth instar | 2.7 ± 0.1 a | 2.8 ± 0.1 a | 2.5 ± 0.1 a |
| Pupa | 4.7 ± 0.1 a | 4.7 ± 0.1 a | 4.0 ± 0.1 b |
| Egg-adult | 16.7 ± 0.3 a | 16.8 ± 0.5 a | 14.9 ± 0.1 b |
| Female adult longevity | 11.9 ± 1.0 a | 8.5 ± 0.7 b | 12.6 ± 1.2 a |
| Male adult longevity | 24.8 ± 2.1 a | 19.1 ± 2.8 a | 23.6 ± 1.7 a |
| Oviposition days | 8.8 ± 0.8 a | 6.4 ± 0.5 b | 7.7 ± 1.1 ab |
| Fecundity(egg/female) | 145.4 ± 16.9 ab | 109.4 ± 20.9 b | 191.3 ± 29.9 a |
| APOP | 0.6 ± 0.2 a | 0.4 ± 0.2 a | 1.5 ± 0.6 a |
| TPOP | 17.4 ± 0.6 a | 16.3 ± 0.5 a | 16.3 ± 0.6 a |
| Nf/N ratio | 0.5 ± 0.1 a | 0.4 ± 0.1 a | 0.5 ± 0.1 a |
| Population Parameters | PX | BT − PX | BT + PX |
|---|---|---|---|
| Intrinsic rate of increase (r) (d−1) | 0.22 ± 0.01 a | 0.21 ± 0.01 a | 0.25 ± 0.02 a |
| Net reproductive rate (R0) (egg) | 67.83 ± 9.29 b | 45.58 ± 8.35 b | 89.69 ± 11.74 a |
| Finite rate of increase (λ) (d−1) | 1.24 ± 0.01 a | 1.23 ± 0.02 a | 1.28 ± 0.02 a |
| Mean generation time (T) (d) | 19.52 ± 0.58 a | 18.10 ± 0.61 a | 18.28 ± 0.50 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Xu, L.-L.; Yu, W.-Y.; Zhang, S.-Z.; Liu, T.-X. Improvement of Bemisia tabaci (Hemiptera: Aleyrodidae) Fitness on Chinese Kale upon Simultaneous Herbivory by Plutella xylostella (Lepidoptera: Plutellidae). Biology 2022, 11, 72. https://doi.org/10.3390/biology11010072
Jiang J, Xu L-L, Yu W-Y, Zhang S-Z, Liu T-X. Improvement of Bemisia tabaci (Hemiptera: Aleyrodidae) Fitness on Chinese Kale upon Simultaneous Herbivory by Plutella xylostella (Lepidoptera: Plutellidae). Biology. 2022; 11(1):72. https://doi.org/10.3390/biology11010072
Chicago/Turabian StyleJiang, Jun, Li-Li Xu, Wen-Yuan Yu, Shi-Ze Zhang, and Tong-Xian Liu. 2022. "Improvement of Bemisia tabaci (Hemiptera: Aleyrodidae) Fitness on Chinese Kale upon Simultaneous Herbivory by Plutella xylostella (Lepidoptera: Plutellidae)" Biology 11, no. 1: 72. https://doi.org/10.3390/biology11010072
APA StyleJiang, J., Xu, L.-L., Yu, W.-Y., Zhang, S.-Z., & Liu, T.-X. (2022). Improvement of Bemisia tabaci (Hemiptera: Aleyrodidae) Fitness on Chinese Kale upon Simultaneous Herbivory by Plutella xylostella (Lepidoptera: Plutellidae). Biology, 11(1), 72. https://doi.org/10.3390/biology11010072







