Species-Specific Flash Patterns Track the Nocturnal Behavior of Sympatric Taiwanese Fireflies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Habitat
2.2. Wild Flash Signal Collection
2.3. Video-Imagery Processing and Analyzing of Flash Signals
2.4. The Measurement and Pattern Matching of FI and FD
2.5. Time-Course Specimen Collection
2.6. Morphological Identification of Species and Sex
2.7. Flash Wavelength Measurement
2.8. Data Statistics
3. Results
3.1. Video-Imagery Distinguished between Flashes from Flying and Perching Individuals
3.2. Discovering a Firefly Species-Specific Luminous Marker for Distinguishing Sympatric A. cerata, L. kagiana, and L. curtithorax
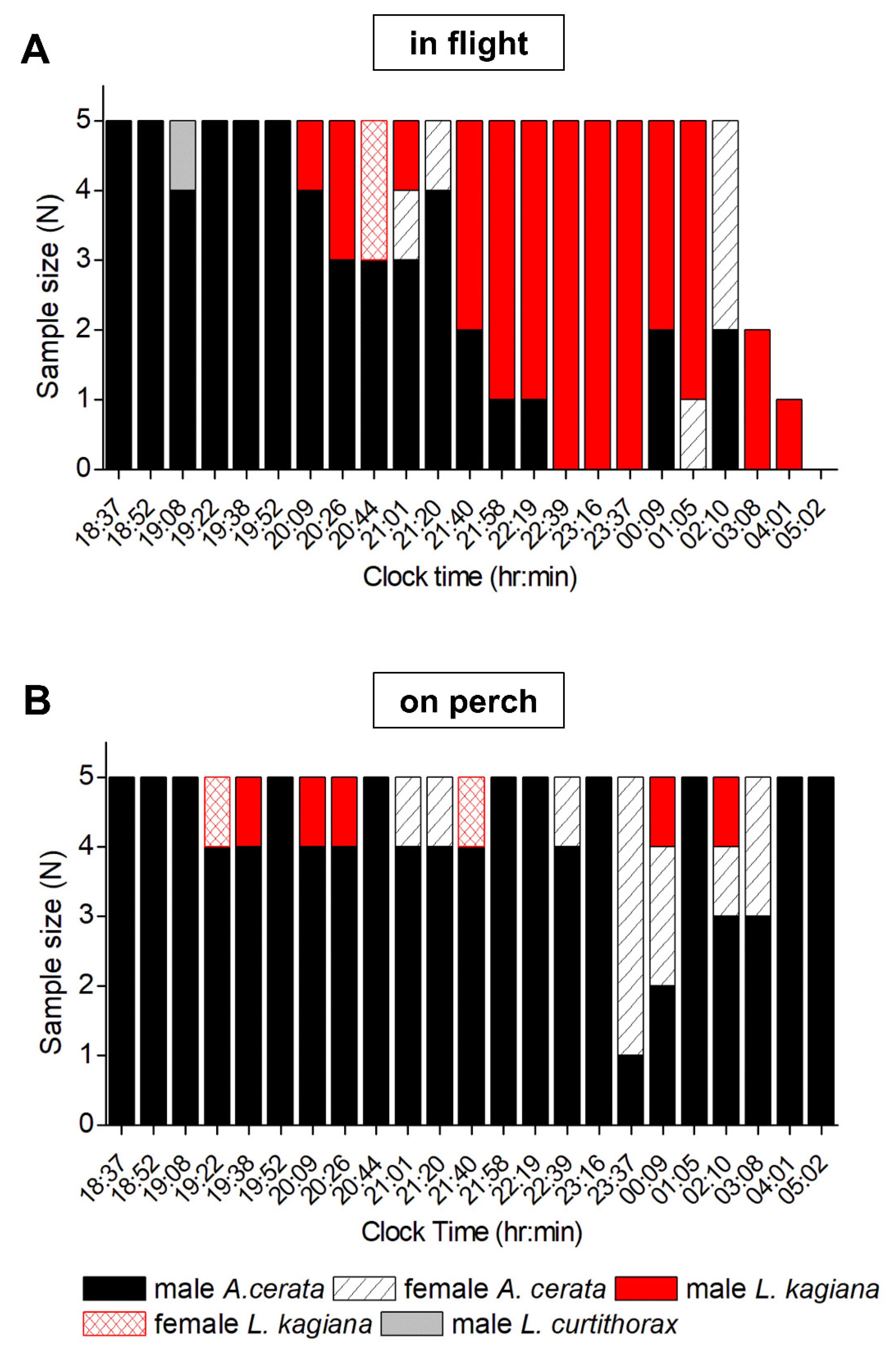
3.3. Evaluating Population Flash Activities during the A. cerata Mating Season
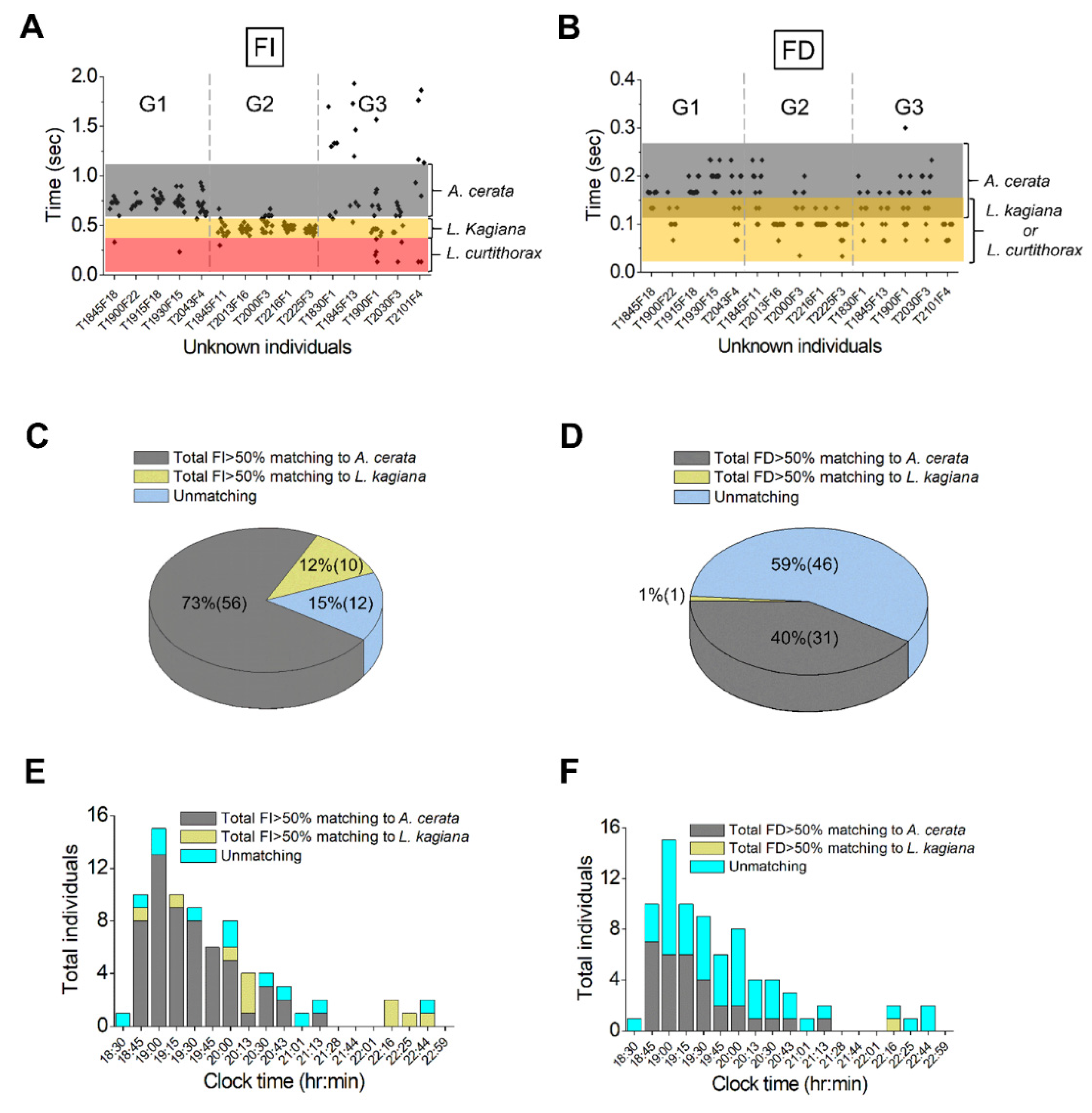
3.4. Species Identification for the Flying Population Using FI and FD Pattern Matching
3.5. Assessing Nocturnal Active-Periods of FI- and FD-Identified Fireflies
4. Discussions
4.1. Discovering a Species-Specificity Luminescent Marker-FI Pattern by Comparing Inter-Species Luminescent Characters
4.2. Application of FI Patterns to Evaluate Species Compositions and Nightly Activities of a Sympatric Firefly Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahlke, T.; Umbers, K.D. Bioluminescence. Curr. Biol. 2016, 26, R313–R314. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.E. Aggressive mimicry in Photuris fireflies: Signal repertoires by femmes fatales. Science 1975, 187, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Cratsley, C.K. Flash signal evolution, mate choice, and predation in fireflies. Annu. Rev. Entomol. 2008, 53, 293–321. [Google Scholar] [CrossRef]
- Ghiradella, H.; Schmidt, J.T. Fireflies at one hundred plus: A new look at flash control. Integr. Comp. Biol. 2004, 44, 203–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trimmer, B.A.; Aprille, J.R.; Dudzinski, D.M.; Lagace, C.J.; Lewis, S.M.; Michel, T.; Qazi, S.; Zayas, R.M. Nitric oxide and the control of firefly flashing. Science 2001, 292, 2486–2488. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, Y.; Kim, H.G.; Choi, K.J.; Kweon, H.S.; Park, S.; Jeong, K.H. Biologically inspired LED lens from cuticular nanostructures of firefly lantern. Proc. Natl. Acad. Sci. USA 2012, 109, 18674–18678. [Google Scholar] [CrossRef]
- Yeh, H.W.; Ai, H.W. Development and applications of bioluminescent and chemiluminescent reporters and biosensors. Annu. Rev. Anal. Chem. 2019, 12, 129–150. [Google Scholar] [CrossRef]
- Fallon, T.R.; Lower, S.E.; Chang, C.H.; Bessho-Uehara, M.; Martin, G.J.; Bewick, A.J.; Behringer, M.; Debat, H.J.; Wong, I.; Day, J.C.; et al. Firefly genomes illuminate parallel origins of bioluminescence in beetles. eLife 2018, 7, e36495. [Google Scholar] [CrossRef]
- Carlson, A.D.; Copeland, J. Communication in insects. 1. flash communication in fireflies. Q. Rev. Biol. 1985, 60, 415–436. [Google Scholar] [CrossRef]
- Lewis, S.M.; Wong, C.H.; Owens, A.C.S.; Fallon, C.; Jepsen, S.; Thancharoen, A.; Wu, C.; de Cock, R.; Novak, M.; Lopez-Palafox, T.; et al. A global perspective on firefly extinction threats. Bioscience 2020, 70, 157–167. [Google Scholar] [CrossRef]
- Buck, J.B. The anatomy and physiology of the light organ in fireflies. Ann. Ny. Acad. Sci. 1948, 49, 397–485. [Google Scholar] [CrossRef]
- Riley, W.B.; Rosa, S.P.; Lima da Silveira, L.F. A comprehensive review and call for studies on firefly larvae. PeerJ 2021, 9, e12121. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.E. Studies on the Flash Communication System in Photinus Fireflies; Museum of Zoology, University of Michigan: Ann Arbor, MI, USA, 1966; p. 95. [Google Scholar]
- Seliger, H.H.; Buck, J.B.; Fastie, W.G.; Mcelroy, W.D. Flash patterns in Jamaican fireflies. Biol. Bull. 1964, 127, 159–172. [Google Scholar] [CrossRef]
- Buck, J. Synchronous rhythmic flashing of fireflies. II. Q. Rev. Biol. 1988, 63, 265–289. [Google Scholar] [CrossRef]
- Moiseff, A.; Copeland, J. Firefly synchrony: A behavioral strategy to minimize visual clutter. Science 2010, 329, 181. [Google Scholar] [CrossRef]
- Takeda, M.; Amano, T.; Katoh, K.; Higuchi, H. The habitat requirement of the Genji-firefly Luciola cruciata (Coleoptera: Lampyridae), a representative endemic species of Japanese rural landscapes. Biodivers. Conserv. 2006, 15, 191–203. [Google Scholar] [CrossRef]
- Kazama, S.; Matsumoto, S.; Ranjan, S.P.; Hamamoto, H.; Sawamoto, M. Characterization of firefly habitat using a geographical information system with hydrological simulation. Ecol. Model. 2007, 209, 392–400. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Lewis, S.M. The impact of artificial light at night on nocturnal insects: A review and synthesis. Ecol. Evol. 2018, 8, 11337–11358. [Google Scholar] [CrossRef]
- Picchi, M.S.; Avolio, L.; Azzani, L.; Brombin, O.; Camerini, G. Fireflies and land use in an urban landscape: The case of Luciola italica L. (Coleoptera: Lampyridae) in the city of Turin. J. Insectig. Conserv. 2013, 17, 797–805. [Google Scholar] [CrossRef]
- Iguchi, Y. Temperature-dependent geographic variation in the flashes of the firefly Luciola cruciata (Coleoptera: Lampyridae). J. Nat. Hist. 2010, 44, 861–867. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Meyer-Rochow, V.B.; Yang, E.C. Short- and mid-wavelength artificial light influences the flash signals of Aquatica ficta fireflies (Coleoptera: Lampyridae). PLoS ONE 2018, 13, e0191576. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Lewis, S.M. Narrow-spectrum artificial light silences female fireflies (Coleoptera: Lampyridae). Insect. Conserv. Diver. 2021, 14, 199–210. [Google Scholar] [CrossRef]
- Hagen, O.; Santos, R.; Schlindwein, M.; Viviani, V. Artificial night lighting reduces firefly (Coleoptera: Lampyridae) occurrence in Sorocaba, Brazil. Adv. Entomol. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Case, J.F.; Buck, J. Control of flashing in fireflies. 2. role of central nervous system. Biol. Bull. 1963, 125, 234–250. [Google Scholar] [CrossRef]
- Case, J.F. Flight studies on photic communication by the firefly Photinus pyralis. Integr. Comp. Biol. 2004, 44, 250–258. [Google Scholar] [CrossRef][Green Version]
- Tathawee, T.; Wattanachaiyingcharoen, W.; Suwannakom, A.; Prasarnpun, S. Flash communication pattern analysis of fireflies based on computer vision. Int. J. Adv. Intell. Inform. 2020, 6, 60–71. [Google Scholar] [CrossRef]
- Konno, J.; Hatta-Ohashi, Y.; Akiyoshi, R.; Thancharoen, A.; Silalom, S.; Sakchoowong, W.; Yiu, V.; Ohba, N.; Suzuki, H. TiLIA: A software package for image analysis of firefly flash patterns. Ecol. Evol. 2016, 6, 3026–3031. [Google Scholar] [CrossRef]
- Carlson, A.D.; Copeland, J.; Raderman, R.; Bulloch, A.G.M. Role of interflash intervals in a firefly courtship (Photinus macdermotti). Anim. Behav. 1976, 24, 786–792. [Google Scholar] [CrossRef]
- Sharma, U.; Goswami, A.; Phukan, M.; Chandra Rajbongshi, S.; Gohain Barua, A. Temperature dependence of the flash duration of the firefly Luciola praeusta. Photochem. Photobiol. Sci. 2014, 13, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Yokoyama, J.; Ohba, N.; Kawata, M. Geographic differences in flash intervals and pre-mating isolation between populations of the Genji-firefly, Luciola cruciata. Ecol. Entomol. 2005, 30, 241–245. [Google Scholar] [CrossRef]
- Jeng, M.L.; Lai, J.; Yang, P.S. Lampyridae: A synopsis of aquatic fireflies with description of a new species (Coleoptera). Water Beetles China 2003, 3, 539–562. [Google Scholar]
- Jeng, M.L.; Yang, P.S.; Engel, M.S. The firefly genus Vesta in Taiwan (Coleoptera: Lampyridae). J. Kansas Entomol. Soc. 2007, 80, 265–280. [Google Scholar] [CrossRef]
- Jeng, M.L.; Yang, P.S.; Sato, M.; Lai, J.; Chang, J.C. The genus Curtos (Coleoptera, Lampyridae, Luciolinae) of Taiwan and Japan. Jpn. J. Syst. Entomol. 1998, 4, 331–347. [Google Scholar]
- Ballantyne, L.; Fu, X.H.; Lambkin, C.; Jeng, M.L.; Faust, L.; Wijekoon, W.M.C.D.; Li, D.Q.; Zhu, T.F. Studies on south-east Asian fireflies: Abscondita, a new genus with details of life history, flashing patterns and behaviour of Abs. chinensis (L.) and Abs. terminalis (Olivier) (Coleoptera: Lampyridae: Luciolinae). Zootaxa 2013, 3721, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Jeng, M.L.; South, A.; Ho, J.Z.; Yang, P.S. Evidence for two male morphs of Luciola cerata Olivier (Coleoptera: Lampyridae) exhibiting distinct mating behavior, with implications for sexual selection. Coleopt. Bull. 2010, 64, 235–242. [Google Scholar] [CrossRef]
- Jeng, M.L.; Lai, J.; Yang, P.S. A synopsis of the firefly fauna at six national parks in Taiwan (Coleoptera: Lampyridae). Formos. Entomol. 1999, 19, 65–91. [Google Scholar]
- Ohba, N.; Yang, P.S. Flash patterns and communication system of the Taiwan firefly, Luciola cerata Olivier. Sci. Rep. Yokosuka City Mus. 2003, 50, 1–12. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Stanger-Hall, K.F.; Lloyd, J.E. Flash signal evolution in Photinus fireflies: Character displacement and signal exploitation in a visual communication system. Evolution 2015, 69, 666–682. [Google Scholar] [CrossRef]
- Woods, W.A., Jr.; Hendrickson, H.; Mason, J.; Lewis, S.M. Energy and predation costs of firefly courtship signals. Am. Nat. 2007, 170, 702–708. [Google Scholar] [CrossRef]
- Hermann, S.L.; Xue, S.S.; Rowe, L.; Davidson-Lowe, E.; Myers, A.; Eshchanov, B.; Bahlai, C.A. Thermally moderated firefly activity is delayed by precipitation extremes. R. Soc. Open Sci. 2016, 3, 160712. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y. The influence of temperature on flash interval in the genji-firefly Luciola cruciata (Coleoptera: Lampyridae). Entomol. Rev. Jpn. 2002, 57, 119–122. [Google Scholar]
- Lloyd, J.E. Flashes of Photuris fireflies: Their value and use in recognizing species. Fla. Entomol. 1969, 52, 29–35. [Google Scholar] [CrossRef]
- Goh, K.S.; Li, C.W. A photocytes-associated fatty acid-binding protein from the light organ of adult Taiwanese firefly, Luciola cerata. PLoS ONE 2011, 6, e29576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarfati, R.; Hayes, J.C.; Sarfati, E.; Peleg, O. Spatio-temporal reconstruction of emergent flash synchronization in firefly swarms via stereoscopic 360-degree cameras. J. R. Soc. Interface 2020, 17, 20200179. [Google Scholar] [CrossRef] [PubMed]
- Jaya, S.; Latha, M. An analysis of pattern recognition and machine learning approaches on medical images. In Applications of Artificial Intelligence for Smart Technology; Swarnalatha, P., Prabu, S., Eds.; IGI Global: Vellore, India, 2021; pp. 35–54. [Google Scholar]
- Patel, A.R.; Ramaiya, K.K.; Bhatia, C.V.; Shah, H.N.; Bhavsar, S.N. Artificial intelligence: Prospect in mechanical engineering field—a review. In Data Science and Intelligent Applications: Proceedings of ICDSIA 2020; Kotecha, K., Piuri, V., Shah, H.N., Patel, R., Xhafa, F., Eds.; Lecture Notes on Data Engineering and Communications Technologies; Springer: Singapore, 2021; pp. 267–282. [Google Scholar]
- Lewis, S.M.; Thancharoen, A.; Wong, C.H.; Lopez-Palafox, T.; Santos, P.V.; Wu, C.S.; Faust, L.; De Cock, R.; Owens, A.C.S.; Lemelin, R.H.; et al. Firefly tourism: Advancing a global phenomenon toward a brighter future. Conserv. Sci. Pract. 2021, 3, e391. [Google Scholar] [CrossRef]
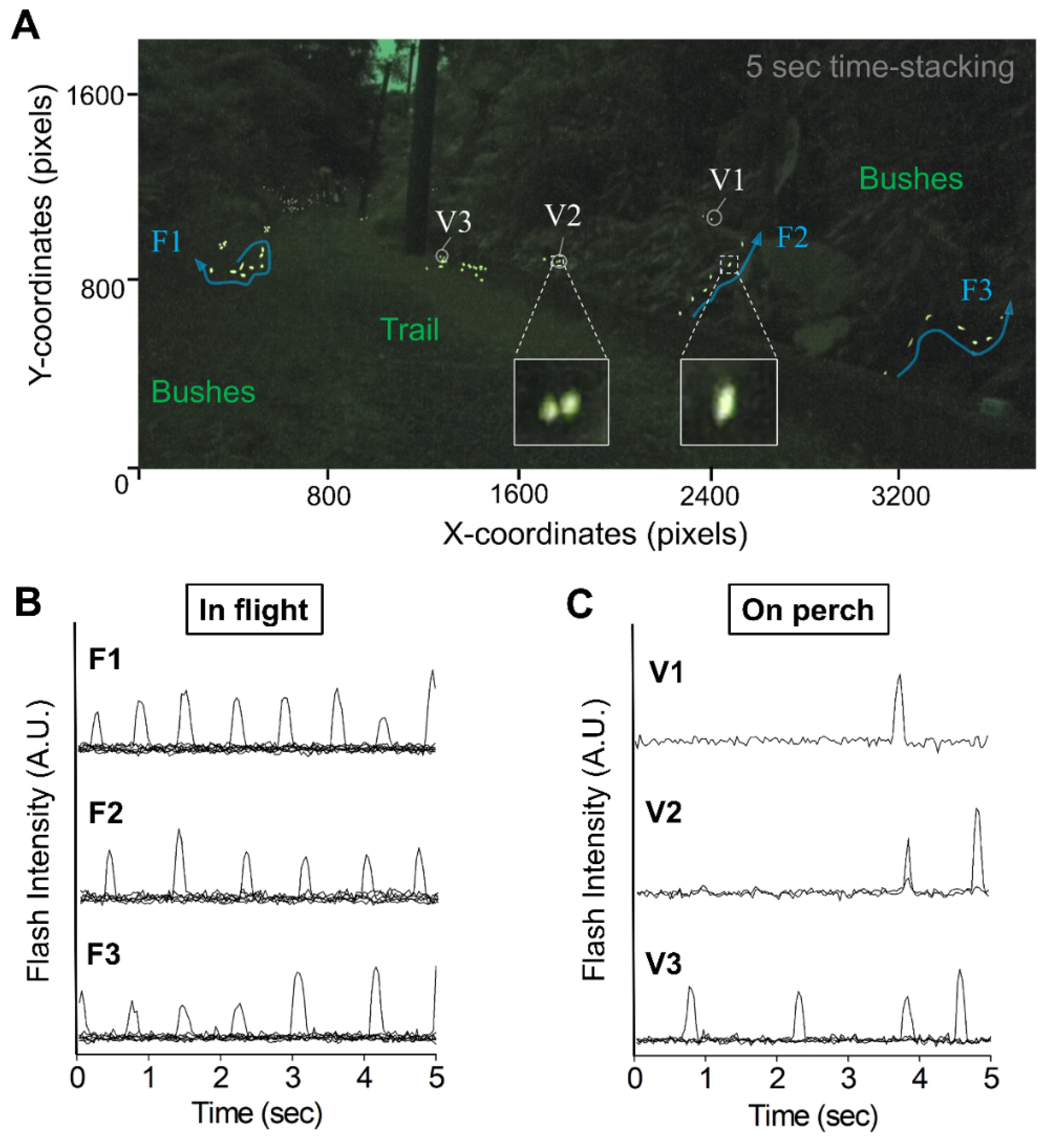
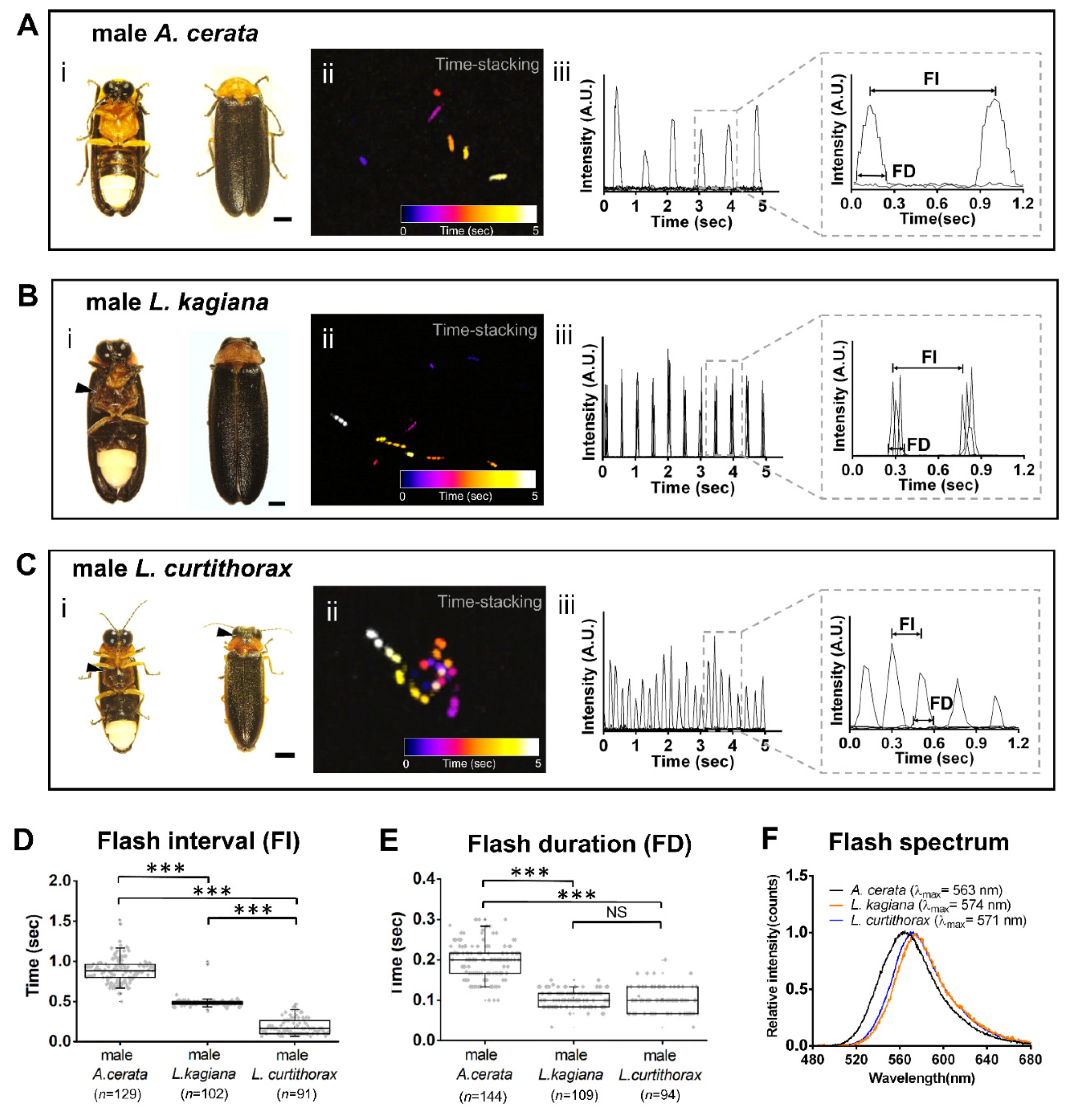
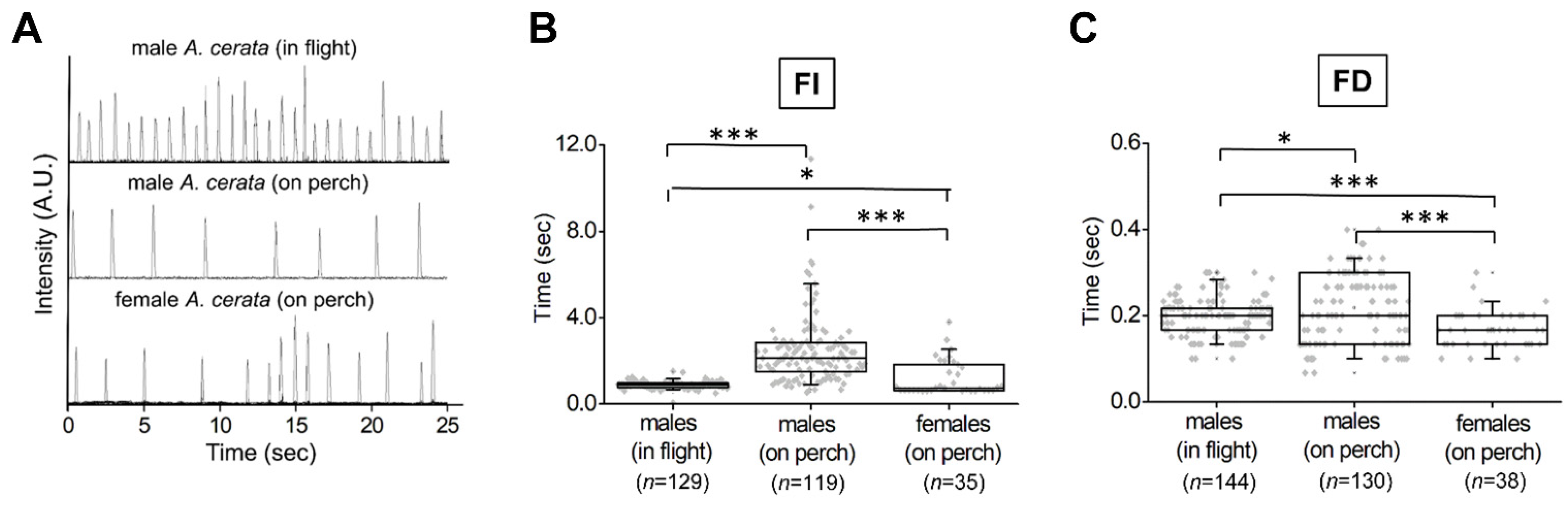
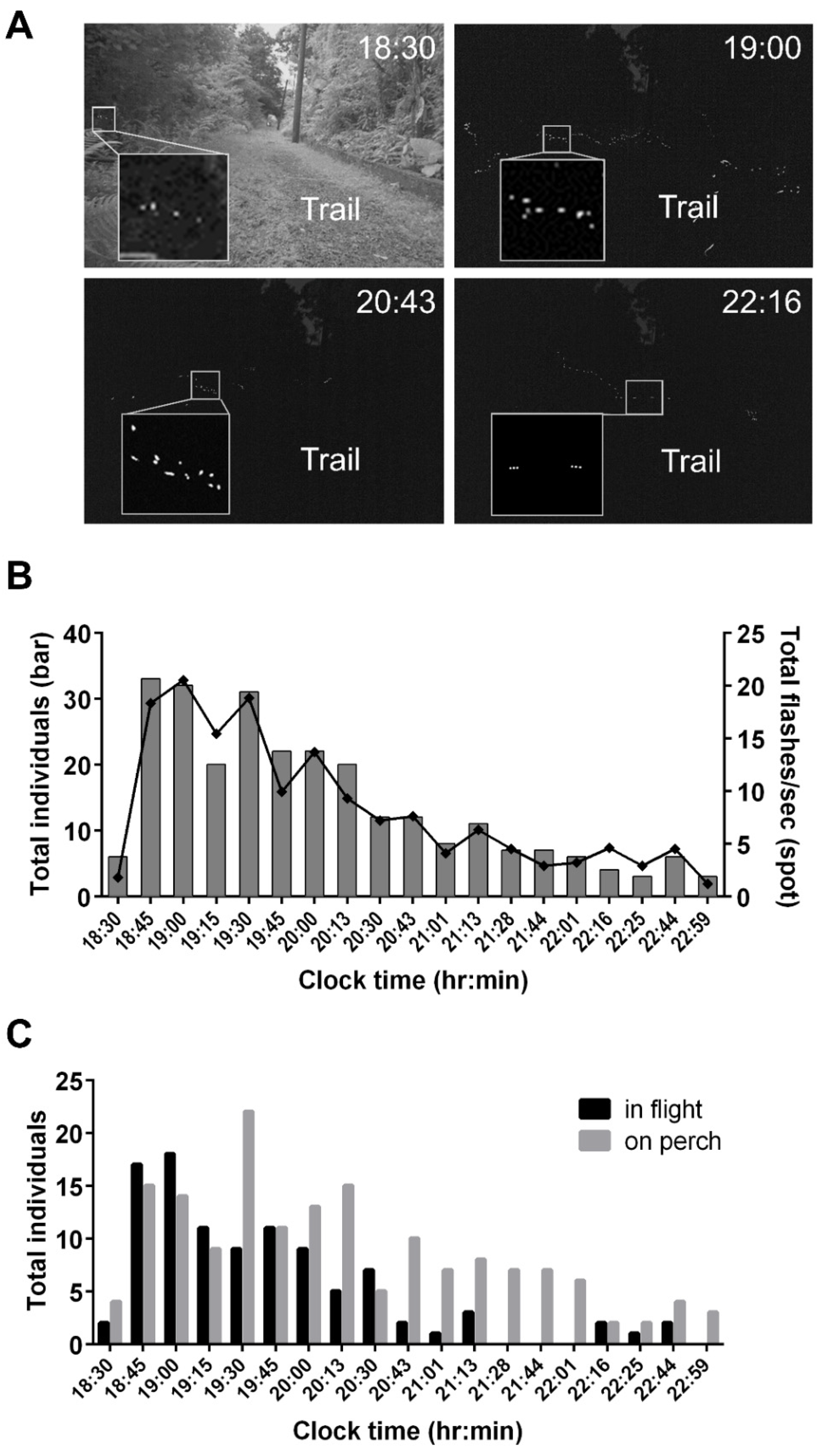
| Species | N | n | Ave Time * | Median * | Q1–Q3* | Min–Max* |
|---|---|---|---|---|---|---|
| (a) FI | ||||||
| A. cerata | 10 | 129 | 0.89 ± 0.16 | 0.88 | 0.8–0.97 | 0.6–1.2 |
| L. kagiana | 7 | 102 | 0.49 ± 0.09 | 0.48 | 0.47–0.5 | 0.42–0.53 |
| L. curtithorax | 5 | 91 | 0.2 ± 0.14 | 0.17 | 0.1–0.27 | 0.07–0.43 |
| (b) FD | ||||||
| A. cerata | 10 | 144 | 0.2 ± 0.04 | 0.2 | 0.17–0.22 | 0.1–0.29 |
| L. kagiana | 7 | 109 | 0.11 ± 0.02 | 0.1 | 0.08–0.12 | 0.07–0.15 |
| L. curtithorax | 5 | 94 | 0.1 ± 0.03 | 0.1 | 0.07–0.13 | 0.03–0.2 |
| Sex | N | n | Ave Time * | Median * | Q1–Q3 * | Min–Max * |
|---|---|---|---|---|---|---|
| (a) FI | ||||||
| Male | 11 | 119 | 2.48 ± 1.63 | 2.13 | 1.4–2.83 | 0.53–4.83 |
| Female | 3 | 35 | 1.2 ± 0.81 | 0.73 | 0.63–1.83 | 0.6–2.97 |
| (b) FD | ||||||
| Male | 11 | 130 | 0.22 ± 0.08 | 0.2 | 0.13–0.3 | 0.07–0.4 |
| Female | 3 | 38 | 0.17 ± 0.04 | 0.17 | 0.13–0.2 | 0.1–0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, K.-S.; Lee, C.-M.; Wang, T.-Y. Species-Specific Flash Patterns Track the Nocturnal Behavior of Sympatric Taiwanese Fireflies. Biology 2022, 11, 58. https://doi.org/10.3390/biology11010058
Goh K-S, Lee C-M, Wang T-Y. Species-Specific Flash Patterns Track the Nocturnal Behavior of Sympatric Taiwanese Fireflies. Biology. 2022; 11(1):58. https://doi.org/10.3390/biology11010058
Chicago/Turabian StyleGoh, King-Siang, Chia-Ming Lee, and Tzi-Yuan Wang. 2022. "Species-Specific Flash Patterns Track the Nocturnal Behavior of Sympatric Taiwanese Fireflies" Biology 11, no. 1: 58. https://doi.org/10.3390/biology11010058
APA StyleGoh, K.-S., Lee, C.-M., & Wang, T.-Y. (2022). Species-Specific Flash Patterns Track the Nocturnal Behavior of Sympatric Taiwanese Fireflies. Biology, 11(1), 58. https://doi.org/10.3390/biology11010058







