Immunohistochemical Characterization of the Nervous System of Culex pipiens (Diptera, Culicidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Samples and Tissue Processing
2.2. Selection of Antibodies and Multiple Sequence Alignment
2.3. Histochemistry and Immunohistochemistry
2.4. Evaluation of Results
3. Results
3.1. Multiple Sequence Alignment
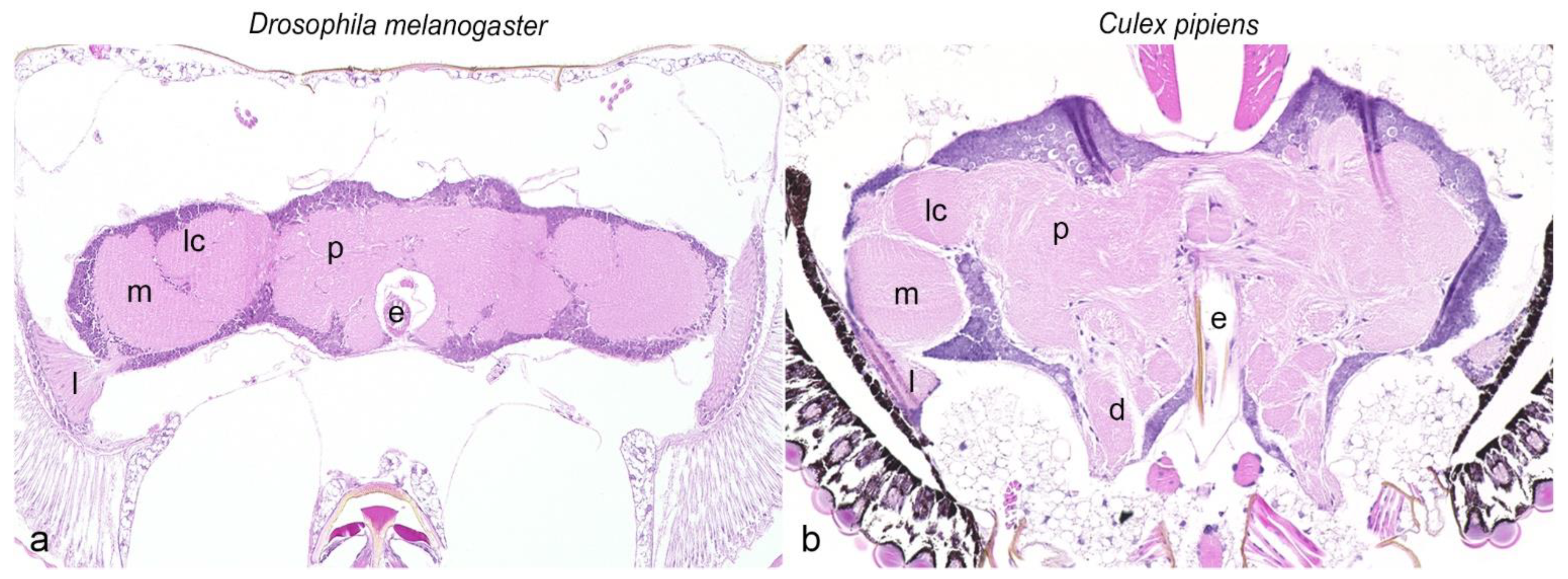
3.2. Visualization of the Nervous System
3.3. Characterization of the Nervous System
3.4. Neurotransmitters and Neurotransmitter-Related Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A global public health threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W. The global distribution of the arbovirus vectors Aedes aegypti and Aedes albopictus. elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; De Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Medlock, J.; Hansford, K.; Versteirt, V.; Cull, B.; Kampen, H.; Fontenille, D.; Hendrickx, G.; Zeller, H.; Van Bortel, W.; Schaffner, F. An entomological review of invasive mosquitoes in Europe. Bull. Entomol. Res. 2015, 105, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Bevins, S.N. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biol. Invasions 2008, 10, 1109–1117. [Google Scholar] [CrossRef]
- Leggewie, M.; Badusche, M.; Rudolf, M.; Jansen, S.; Borstler, J.; Krumkamp, R.; Huber, K.; Kruger, A.; Schmidt-Chanasit, J.; Tannich, E.; et al. Culex pipiens and Culex torrentium populations from central Europe are susceptible to West Nile virus infection. One Health 2016, 2, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, G.A.; Piorkowski, G.; Thirion, L.; Ninove, L.; Giron, S.; Zandotti, C.; Denis, J.; Badaut, C.; Failloux, A.-B.; Grard, G. Vector-borne transmission of the Zika virus asian genotype in Europe. Viruses 2020, 12, 296. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, K.N.; Javed, F.; Ejaz, M.; Ali, M.; Mujaddadi, N.; Khan, A.A.; Khattak, A.A.; Zaib, A.; Ahmad, I.; Saeed, W.K.; et al. The global emergence of Chikungunya infection: An integrated view. Rev. Med. Virol. 2021, e2287. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of Dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Michel, V. Rift Valley fever–epidemiological update and risk of introduction into Europe. EFSA J. 2020, 18, e06041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roesch, F.; Fajardo, A.; Moratorio, G.; Vignuzzi, M. Usutu virus: An arbovirus on the rise. Viruses 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, A.D. West Nile in Europe: An increasing public health problem. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubler, D.J. Human arbovirus infections worldwide. Ann. N. Y. Acad. Sci. 2001, 951, 13–24. [Google Scholar] [CrossRef]
- Martinet, J.P.; Ferté, H.; Failloux, A.B.; Schaffner, F.; Depaquit, J. Mosquitoes of north-western Europe as potential vectors of arboviruses: A review. Viruses 2019, 11, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shocket, M.S.; Verwillow, A.B.; Numazu, M.G.; Slamani, H.; Cohen, J.M.; El Moustaid, F.; Rohr, J.; Johnson, L.R.; Mordecai, E.A. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23 °C and 26 °C. Elife 2020, 9, e58511. [Google Scholar] [CrossRef]
- Becker, N. Die Stechmückenfauna Deutschlands im Wandel der Zeit-Stechmücken als Indikatoren für Klimaveränderung. In Warnsignal Klima: Gefahren für Pflanzen, Tiere und Menschen, 2nd ed.; Lozán, J.L., Grassl, H., Karbe, L., Jendritzky, G., Eds.; Published Electronically; 2014; Chapter 3.2.7; Available online: www.warnsignale.uni-hamburg.de (accessed on 18 March 2020).
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley fever: An emerging mosquito-borne disease. Annu. Rev. Entomol. 2016, 61, 395–415. [Google Scholar] [CrossRef]
- Vogels, C.B.; Fros, J.J.; Goertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasit. Vectors 2016, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 2015, 1, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Marm Kilpatrick, A. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Gaburro, J.; Bhatti, A.; Harper, J.; Jeanne, I.; Dearnley, M.; Green, D.; Nahavandi, S.; Paradkar, P.N.; Duchemin, J.B. Neurotropism and behavioral changes associated with Zika infection in the vector Aedes aegypti. Emerg. Microbes Infect. 2018, 7, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimstad, P.R.; Ross, Q.E.; Craig, G.B., Jr. Aedes triseriatus (Diptera: Culicidae) and La Crosse Virus: II. Modification of mosquito feeding behavior by virus infection. J. Med. Entomol. 1980, 17, 1–7. [Google Scholar] [CrossRef]
- Girard, Y.A.; Klingler, K.A.; Higgs, S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004, 4, 109–122. [Google Scholar] [CrossRef]
- Gregor, K.M.; Michaely, L.M.; Gutjahr, B.; Rissmann, M.; Keller, M.; Dornbusch, S.; Naccache, F.; Schon, K.; Jansen, S.; Heitmann, A.; et al. Rift Valley fever virus detection in susceptible hosts with special emphasis in insects. Sci. Rep. 2021, 11, 9822. [Google Scholar] [CrossRef]
- Lumley, S.; Hunter, L.; Emery, K.; Hewson, R.; Fooks, A.R.; Horton, D.L.; Johnson, N. Detection of Rift Valley fever virus RNA in formalin-fixed mosquitoes by in situ hybridization (RNAscope(®)). Viruses 2021, 13, 1079. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Higgs, S. How do mosquito vectors live with their viruses? In Microbe-Vector Interactions in Vector-Borne Diseases; Gillespie, S.H., Smith, G.L., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 103–138. [Google Scholar]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenco-de-Oliveira, R.; Sorgine, M.H.; Peixoto, A.A. Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogels, C.B.F.; Fros, J.J.; Pijlman, G.P.; van Loon, J.J.A.; Gort, G.; Koenraadt, C.J.M. Virus interferes with host-seeking behaviour of mosquito. J. Exp. Biol. 2017, 220, 3598–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Chan, K.; Brewster, C.C.; Paulson, S.L. Effects of La Crosse virus infection on the host-seeking behavior and levels of two neurotransmitters in Aedes triseriatus. Parasit. Vectors 2019, 12, 397. [Google Scholar] [CrossRef]
- Platt, K.B.; Linthicum, K.J.; Myint, K.S.A.; Innis, B.L.; Lerdthusnee, K.; Vaughn, D.W. Impact of Dengue virus infection on feeding behavior of Aedes aegypti. Am. J. Trop. Med. Hyg. 1997, 57, 119–125. [Google Scholar] [CrossRef]
- Andrés, M.; Seifert, M.; Spalthoff, C.; Warren, B.; Weiss, L.; Giraldo, D.; Winkler, M.; Pauls, S.; Göpfert, M.C. Auditory efferent system modulates mosquito hearing. Curr. Biol. 2016, 26, 2028–2036. [Google Scholar] [CrossRef] [Green Version]
- Matthews, B.J.; McBride, C.S.; DeGennaro, M.; Despo, O.; Vosshall, L.B. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genom. 2016, 17, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mysore, K.; Flister, S.; Müller, P.; Rodrigues, V.; Reichert, H. Brain development in the yellow fever mosquito Aedes aegypti: A comparative immunocytochemical analysis using cross-reacting antibodies from Drosophila melanogaster. Dev. Genes Evol. 2011, 221, 281–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyhof, J.M.; McIver, S.B. Structural organization of the brain and subesophageal ganglion of male Aedes aegypti (L.) (Diptera: Culicidae). Int. J. Insect Morphol. Embryol. 1989, 18, 13–37. [Google Scholar] [CrossRef]
- Raji, J.I.; Potter, C.J. The number of neurons in Drosophila and mosquito brains. PLoS ONE 2021, 16, e0250381. [Google Scholar] [CrossRef] [PubMed]
- Wheelwright, M.; Whittle, C.R.; Riabinina, O. Olfactory systems across mosquito species. Cell Tissue Res. 2021, 383, 75–90. [Google Scholar] [CrossRef]
- Wolff, G.H.; Lahondère, C.; Vinauger, C.; Riffell, J.A. Neuromodulation and differential learning across mosquito species. bioRxiv 2019, 755017. [Google Scholar] [CrossRef]
- Younger, M.; Didkovsky, N.; Danan, V.; Renier, N. Female Aedes aegypti Brain. Available online: www.mosquitobrains.org (accessed on 10 November 2021).
- Katz, P.S.; Harris-Warrick, R.M. The evolution of neuronal circuits underlying species-specific behavior. Curr. Opin. Neurobiol. 1999, 9, 628–633. [Google Scholar] [CrossRef]
- Roberts, D.B. Drosophila melanogaster: The model organism. Entomol. Exp. Appl. 2006, 121, 93–103. [Google Scholar] [CrossRef]
- Górska-Andrzejak, J.; Makuch, R.; Stefan, J.; Görlich, A.; Semik, D.; Pyza, E. Circadian expression of the presynaptic active zone protein Bruchpilot in the lamina of Drosophila melanogaster. Dev. Neurobiol. 2013, 73, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wagh, D.A.; Rasse, T.M.; Asan, E.; Hofbauer, A.; Schwenkert, I.; Dürrbeck, H.; Buchner, S.; Dabauvalle, M.C.; Schmidt, M.; Qin, G.; et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 2006, 49, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Jung, A.G.; Labarrera, C.; Jansen, A.M.; Qvortrup, K.; Wild, K.; Kjaerulff, O. A mutational analysis of the endophilin-A N-BAR domain performed in living flies. PLoS ONE 2010, 5, e9492. [Google Scholar] [CrossRef]
- Rogulja-Ortmann, A.; Picao-Osorio, J.; Villava, C.; Patraquim, P.; Lafuente, E.; Aspden, J.; Thomsen, S.; Technau, G.M.; Alonso, C.R. The RNA-binding protein ELAV regulates Hox RNA processing, expression and function within the Drosophila nervous system. Development 2014, 141, 2046–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, T.; Krukkert, K.; Roos, J.; Davis, G.; Klämbt, C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron 2000, 26, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Halter, D.A.; Urban, J.; Rickert, C.; Ner, S.S.; Ito, K.; Travers, A.A.; Technau, G.M. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development 1995, 121, 317–332. [Google Scholar] [CrossRef]
- Xiong, W.-C.; Okano, H.; Patel, N.H.; Blendy, J.A.; Montell, C. Repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev. 1994, 8, 981–994. [Google Scholar] [CrossRef] [Green Version]
- Takagawa, K.; Salvaterra, P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci. Res. 1996, 24, 237–243. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koushika, S.P.; Lisbin, M.J.; White, K. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr. Biol. 1996, 6, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Wittle, A.E.; Kamdar, K.P.; Finnerty, V. The Drosophila cinnamon gene is functionally homologous to Arabidopsis cnx1 and has a similar expression pattern to the mammalian gephyrin gene. Mol. Gen. Genet. MGG 1999, 261, 672–680. [Google Scholar] [CrossRef]
- Kurz, S.; King, J.G.; Dinglasan, R.R.; Paschinger, K.; Wilson, I.B.H. The fucomic potential of mosquitoes: Fucosylated N-glycan epitopes and their cognate fucosyltransferases. Insect Biochem. Mol. Biol. 2016, 68, 52–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.; Knipp, S.; Bicker, G. Embryonic differentiation of serotonin-containing neurons in the enteric nervous system of the locust (Locusta migratoria). J. Comp. Neurol. 2007, 501, 38–51. [Google Scholar] [CrossRef]
- Herrera-Molina, R.; Sarto-Jackson, I.; Montenegro-Venegas, C.; Heine, M.; Smalla, K.H.; Seidenbecher, C.I.; Beesley, P.W.; Gundelfinger, E.D.; Montag, D. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J. Biol. Chem. 2014, 289, 8973–8988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geffard, M.; Vieillemaringe, J.; Heinrich-Rock, A.-M.; Duris, P. Anti-acetylcholine antibodies and first immunocytochemical application in insect brain. Neurosci. Lett. 1985, 57, 1–6. [Google Scholar] [CrossRef]
- Eichler, K.; Li, F.; Litwin-Kumar, A.; Park, Y.; Andrade, I.; Schneider-Mizell, C.M.; Saumweber, T.; Huser, A.; Eschbach, C.; Gerber, B.; et al. The complete connectome of a learning and memory centre in an insect brain. Nature 2017, 548, 175–182. [Google Scholar] [CrossRef]
- Salvaterra, P.M.; McCAMAN, R.E. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: A study using two temperature-sensitive mutants. J. Neurosci. 1985, 5, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Paschinger, K.; Rendic, D.; Wilson, I.B. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj. J. 2009, 26, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Humeau, Y.; Candiani, S.; Ghirardi, M.; Poulain, B.; Montarolo, P. Functional roles of synapsin: Lessons from invertebrates. Semin. Cell Dev. Biol. 2011, 22, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Lardi-Studler, B.; Smolinsky, B.; Petitjean, C.M.; Koenig, F.; Sidler, C.; Meier, J.C.; Fritschy, J.-M.; Schwarz, G. Vertebrate-specific sequences in the gephyrin E-domain regulate cytosolic aggregation and postsynaptic clustering. J. Cell Sci. 2007, 120, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- Smolinsky, B.; Eichler, S.A.; Buchmeier, S.; Meier, J.C.; Schwarz, G. Splice-specific functions of gephyrin in molybdenum cofactor biosynthesis. J. Biol. Chem. 2008, 283, 17370–17379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeneweg, F.L.; Trattnig, C.; Kuhse, J.; Nawrotzki, R.A.; Kirsch, J. Gephyrin: A key regulatory protein of inhibitory synapses and beyond. Histochem. Cell Biol. 2018, 150, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Miller, M.A. When tissue antigens and antibodies get along: Revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet. Pathol. 2014, 51, 42–87. [Google Scholar] [CrossRef] [Green Version]
- Harzsch, S.; Hansson, B.S. Brain architecture in the terrestrial hermit crab Coenobita clypeatus (Anomura, Coenobitidae), a crustacean with a good aerial sense of smell. BMC Neurosci. 2008, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anlauf, E.; Derouiche, A. Glutamine synthetase as an astrocytic marker: Its cell type and vesicle localization. Front. Endocrinol. 2013, 4, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, I.; Bodega, G.; Fernández, B. Glutamine synthetase in brain: Effect of ammonia. Neurochem. Int. 2002, 41, 123–142. [Google Scholar] [CrossRef]
- Weihrauch, D.; Donini, A.; O’Donnell, M.J. Ammonia transport by terrestrial and aquatic insects. J. Insect Physiol. 2012, 58, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Jan, L.Y.; Jan, Y.N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J. Physiol. 1976, 262, 215–236. [Google Scholar] [CrossRef]
- Nässel, D.R. Neurotransmitters and neuromodulators in the insect visual system. Prog. Neurobiol. 1991, 37, 179–254. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Estevez-Lao, T.Y.; Mirzai, H.E. The neurotransmitters serotonin and glutamate accelerate the heart rate of the mosquito Anopheles gambiae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 188, 49–57. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.; Sun, X.; Meinertzhagen, I.A.; Nassel, D.R. Glutamate, GABA and acetylcholine signaling components in the lamina of the Drosophila visual system. PLoS ONE 2008, 3, e2110. [Google Scholar] [CrossRef] [Green Version]
- Cayre, M.; Buckingham, S.; Yagodin, S.; Sattelle, D. Cultured insect mushroom body neurons express functional receptors for acetylcholine, GABA, glutamate, octopamine, and dopamine. J. Neurophysiol. 1999, 81, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Devaud, J.-M.; Clouet-Redt, C.; Bockaert, J.; Grau, Y.; Parmentier, M.-L. Widespread brain distribution of the Drosophila metabotropic glutamate receptor. Neuroreport 2008, 19, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, F.W. Acetylcholine, GABA, glutamate and NO as putative transmitters indicated by immunocytochemistry in the olfactory mushroom body system of the insect brain. Acta Biol. Hung. 2000, 51, 355–362. [Google Scholar] [CrossRef]
- Wafford, K.A.; Sattelle, D.B. L-glutamate receptors on the cell body membrane of an identified insect motor neurone. J. Exp. Biol. 1989, 144, 449–462. [Google Scholar] [CrossRef]
- Liu, W.W.; Wilson, R.I. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc. Natl. Acad. Sci. USA 2013, 110, 10294–10299. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.B.; Waagepetersen, H.S.; Bak, L.K.; Schousboe, A.; Sonnewald, U. The glutamine–glutamate/GABA cycle: Function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem. Res. 2015, 40, 402–409. [Google Scholar] [CrossRef]
- Mustard, J.A.; Jones, L.; Wright, G.A. GABA signaling affects motor function in the honey bee. J. Insect Physiol. 2020, 120, 103989. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, F.; Frambach, I.; Elekes, K. GABAergic synaptic connections in mushroom bodies of insect brains. Acta Biol. Hung. 2008, 59, 173–181. [Google Scholar] [CrossRef]
- Wilson, R.I.; Laurent, G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 2005, 25, 9069–9079. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Awasaki, T.; Ito, K. Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J. Comp. Neurol. 2009, 514, 74–91. [Google Scholar] [CrossRef]
- Bicker, G.; Schäfer, S.; Kingan, T.G. Mushroom body feedback interneurones in the honeybee show GABA-like immunoreactivity. Brain Res. 1985, 360, 394–397. [Google Scholar] [CrossRef]
- Distler, P. Histochemical demonstration of GABA-like immunoreactivity in cobalt labeled neuron individuals in the insect olfactory pathway. Histochemistry 1989, 91, 245–249. [Google Scholar] [CrossRef]
- Jacobsen, B. GABA Immunostaining of the Olfactory Pathway in the Heliothine Moth Brain. Master’s Thesis, Department of Biology, Norwegian University of Science and Technology, Trondheim, Norway, 2012. [Google Scholar]
- Homberg, U.; Kingan, T.G.; Hildebrand, J.G. Immunocytochemistry of GABA in the brain and suboesophageal ganglion of Manduca sexta. Cell Tissue Res. 1987, 248, 1–24. [Google Scholar] [CrossRef]
- Hoskins, S.G.; Homberg, U.; Kingan, T.G.; Christensen, T.A.; Hildebrand, J.G. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res. 1986, 244, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Matute, C.; Streit, P.; Nässel, D. Insect optic lobe neurons identifiable with monoclonal antibodies to GABA. Histochemistry 1986, 84, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Bicker, G. Distribution of GABA-like immunoreactivity in the brain of the honeybee. J. Comp. Neurol. 1986, 246, 287–300. [Google Scholar] [CrossRef]
- Hamasaka, Y.; Wegener, C.; Nässel, D.R. GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J. Neurobiol. 2005, 65, 225–240. [Google Scholar] [CrossRef]
- Leitch, B.; Laurent, G. GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system. J. Comp. Neurol. 1996, 372, 487–514. [Google Scholar] [CrossRef]
- Liu, X.; Buchanan, M.E.; Han, K.-A.; Davis, R.L. The GABA-A receptor RDL suppresses the conditioned stimulus pathway for olfactory learning. J. Neurosci. 2009, 29, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.; Roorda, R.D.; Lima, S.Q.; Zemelman, B.V.; Morcillo, P.; Miesenböck, G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 2002, 36, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.R.; Wilson, R.I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 2008, 452, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Kashin, P. The role of gamma-aminobutyric acid in mosquito-host interactions: A hypothesis. Ann. Entomol. Soc. Am. 1969, 62, 695–702. [Google Scholar] [CrossRef]
- Wolff, G.H.; Riffell, J.A. Olfaction, experience and neural mechanisms underlying mosquito host preference. J. Exp. Biol. 2018, 221, jeb157131. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, R.; Zhang, B.; Zhao, T.; Wang, P.; Liang, G.; Cheng, G. Blood meal acquisition enhances arbovirus replication in mosquitoes through activation of the GABAergic system. Nat. Commun. 2017, 8, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neckameyer, W.S.; Leal, S.M. Biogenic amines as circulating hormones in insects. In Hormones, Brain and Behavior; Pfaff, D.W., Arnold, A.P., Fahrbach, S.E., Etgen, A.M., Rubin, R.T., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 3, pp. 141–165. [Google Scholar] [CrossRef]
- Alekseyenko, O.V.; Chan, Y.B.; Li, R.; Kravitz, E.A. Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. USA 2013, 110, 6151–6156. [Google Scholar] [CrossRef] [Green Version]
- Drobysheva, D.; Ameel, K.; Welch, B.; Ellison, E.; Chaichana, K.; Hoang, B.; Sharma, S.; Neckameyer, W.; Srinakevitch, I.; Murphy, K.J.; et al. An optimized method for histological detection of dopaminergic neurons in Drosophila melanogaster. J. Histochem. Cytochem. 2008, 56, 1049–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinauger, C.; Lahondère, C.; Cohuet, A.; Lazzari, C.R.; Riffell, J.A. Learning and memory in disease vector insects. Trends Parasitol. 2016, 32, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.P.; Schwartz, A.; Gramsbergen, J.B.; Loeschcke, V. Dopamine levels in the mosquito Aedes aegypti during adult development, following blood feeding and in response to heat stress. J. Insect Physiol. 2006, 52, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, Y.; Irie, K.; Satho, T.; Aonuma, H.; Dieng, H.; Ahmad, A.H.; Nakashima, Y.; Mishima, K.; Kashige, N.; Miake, F. Elevation of dopamine level reduces host-seeking activity in the adult female mosquito Aedes albopictus. Parasites Vectors 2012, 5, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, R.H. Insect neurotransmission: Neurotransmitters and their receptors. Pharmacol. Ther. 1996, 69, 117–142. [Google Scholar] [CrossRef]
- Wicker-Thomas, C.; Hamann, M. Interaction of dopamine, female pheromones, locomotion and sex behavior in Drosophila melanogaster. J. Insect Physiol. 2008, 54, 1423–1431. [Google Scholar] [CrossRef]
- Siju, K.P.; Hansson, B.S.; Ignell, R. Immunocytochemical localization of serotonin in the central and peripheral chemosensory system of mosquitoes. Arthropod Struct. Dev. 2008, 37, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Huser, A.; Eschment, M.; Gullu, N.; Collins, K.A.N.; Bopple, K.; Pankevych, L.; Rolsing, E.; Thum, A.S. Anatomy and behavioral function of serotonin receptors in Drosophila melanogaster larvae. PLoS ONE 2017, 12, e0181865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vleugels, R.; Verlinden, H.; Broeck, J.V. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2015, 2, e314. [Google Scholar] [CrossRef]
- Barnstedt, O.; Owald, D.; Felsenberg, J.; Brain, R.; Moszynski, J.P.; Talbot, C.B.; Perrat, P.N.; Waddell, S. Memory-relevant mushroom body output synapses are cholinergic. Neuron 2016, 89, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Buchner, E.; Buchner, S.; Crawford, G.; Mason, W.T.; Salvaterra, P.M.; Sattelle, D.B. Choline acetyltransferase-like immunoreactivity in the brain of Drosophila melanogaster. Cell Tissue Res. 1986, 246, 57–62. [Google Scholar] [CrossRef]
- Kahsai, L.; Winther, Å.M.E. Chemical neuroanatomy of the Drosophila central complex: Distribution of multiple neuropeptides in relation to neurotransmitters. J. Comp. Neurol. 2011, 519, 290–315. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Breer, H. Cholinergic nerve terminals in the central nervous system of insects. J. Neuroendocrinol. 1990, 2, 241–256. [Google Scholar] [CrossRef]
- Clark, J.; Meisner, S.; Torkkeli, P.H. Immunocytochemical localization of choline acetyltransferase and muscarinic ACh receptors in the antenna during development of the sphinx moth Manduca sexta. Cell Tissue Res. 2005, 320, 163–173. [Google Scholar] [CrossRef]
- Leitinger, G.; Simmons, P.J. Cytochemical evidence that acetylcholine is a neurotransmitter of neurons that make excitatory and inhibitory outputs in the locust ocellar visual system. J. Comp. Neurol. 2000, 416, 345–355. [Google Scholar] [CrossRef]
- Lutz, E.M.; Tyrer, N.M. Immunohistochemical localization of choline acetyltransferase in the central nervous system of the locust. Brain Res. 1987, 407, 173–179. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Ho, Y.W.; Crawford, G.D.; Salvaterra, P.M.; Mason, W.T. Immunocytochemical staining of central neurones in Periplaneta americana using monoclonal antibodies to choline acetyltransferase. Tissue Cell 1986, 18, 51–61. [Google Scholar] [CrossRef]
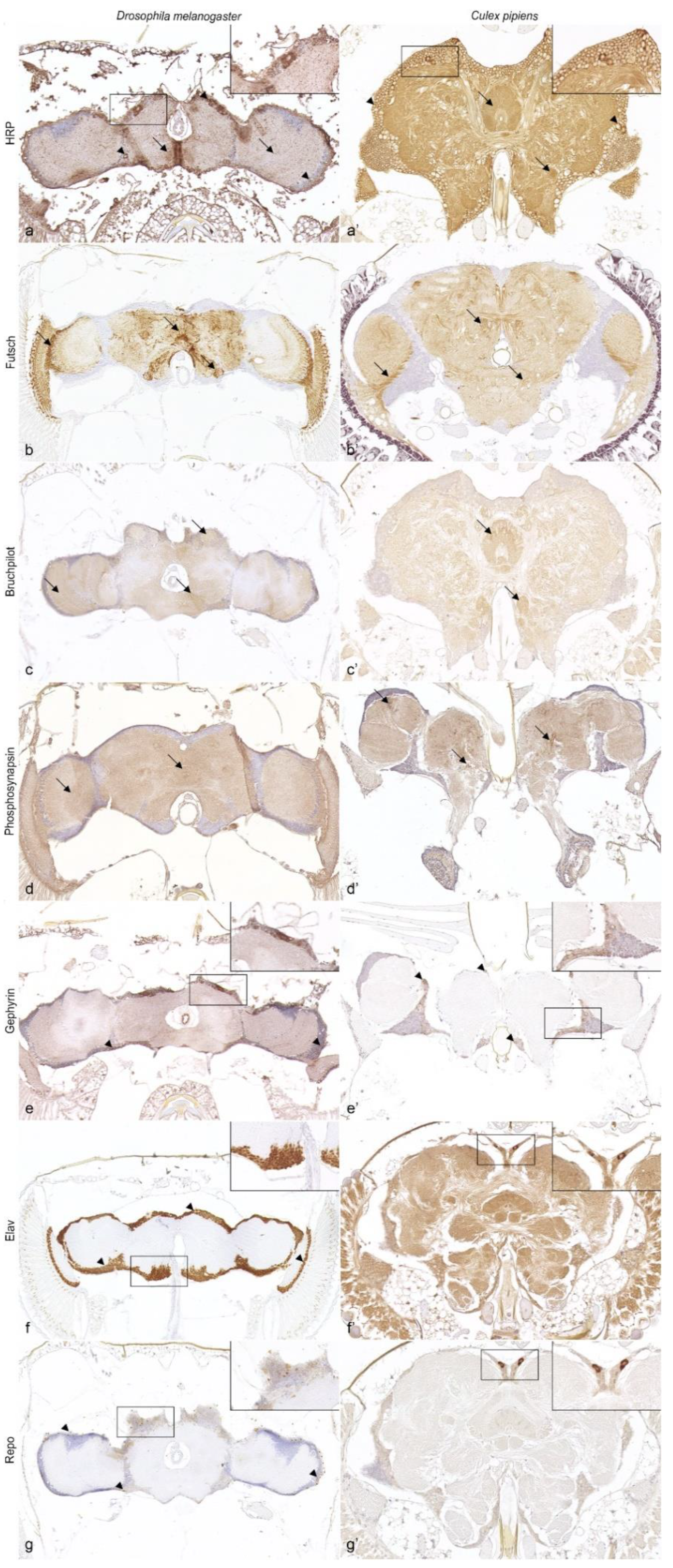
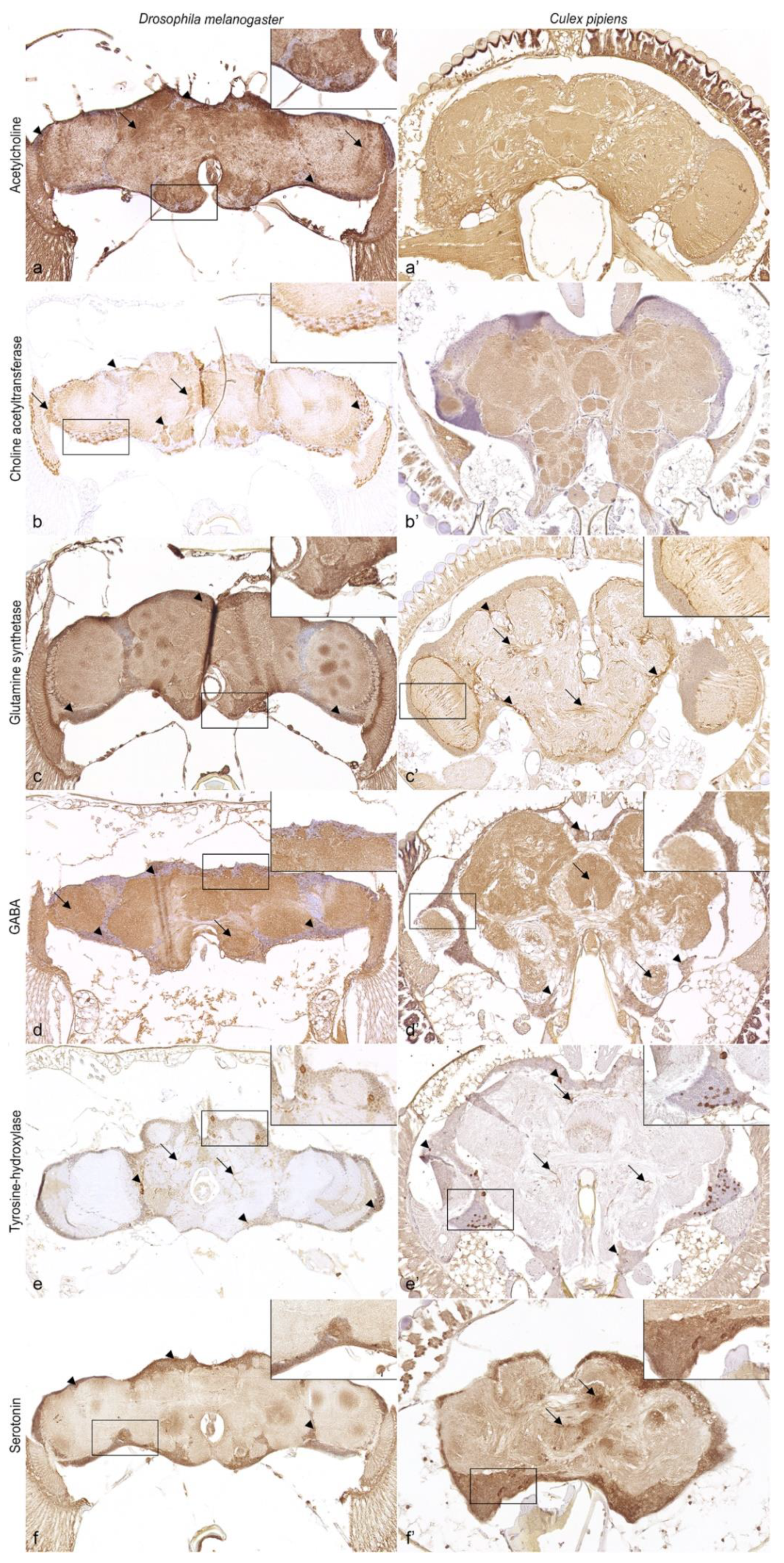
| Primary Antibody | Epitope | Clonality/Host Species | Dilution | Epitope Retrieval | Secondary Antibody | Source | Reference | |
|---|---|---|---|---|---|---|---|---|
| Culex | Drosophila | |||||||
| Nervous Tissue | ||||||||
| Brp | Presynaptic active zone assembly protein | mc, mouse | 1:50 | 1:50 | heated citrate buffer * | GAM | # nc82, DSHB | [45] |
| Elav | Neuronal protein | mc, mouse | - | 1:50,000 | heated citrate buffer * | GAM | # 9F8A9, DSHB | [53] |
| Futsch | Microtubule-associated protein | mc, mouse | 1:800 | 1:1600 | none | GAM | # 22C10, DSHB | [48] |
| Gephyrin | Postsynaptic neuronal assembly protein, glial cells | pc, rabbit | 1:16,000 | 1:4000 | heated citrate buffer * | GAR | # PA5-29036, Thermo Fisher | [54] |
| HRP | Fucosylated N-glycans | pc, rabbit | 1:25,000 | 1:20,000 | heated citrate buffer * | GAR | # 323-005-021, Jackson Immunoresearch | [55,56] |
| Phosphosynapsin | Synapsin 1 | pc, rabbit | 1:50 | 1:50 | PK # | GAR | # PA5-38528, Thermo Fisher | [57] |
| Repo | Glial homeoprotein | mc, mouse | - | 1:1600 | heated citrate buffer * | GAM | # 8D12, DSHB | [50] |
| Neurotransmitters | ||||||||
| ACh | Acetylcholine | pc, rabbit | - | 1:100 | PK # | GAR | # AB5522, Merck Millipore | [58] |
| GABA | γ-aminobutyric acid | pc, rabbit | 1:6000 | 1:3000 | none | GAR | # A2052, Sigma-Aldrich | [59] |
| 5HT | Serotonin | pc, rabbit | 1:60,000 | 1:6000 | heated citrate buffer * | GAR | # S5545, Sigma-Aldrich | [59] |
| Neurotransmitter-Related Enzymes | ||||||||
| ChAT | Choline acetyltransferase | mc, mouse | - | 1:1600 | heated citrate buffer * | GAM | # ChAT4B1, DSHB | [60] |
| GS | Glutamine synthetase | pc, rabbit | 1:8000 | 1:2000 | heated citrate buffer * | GAR | # PA5-28940, Thermo Fisher | [36] |
| TH | Tyrosine-hydroxylase | mc, mouse | 1:80 | 1:80 | heated citrate buffer * | GAM | # 22941, Immunostar | [40] |
| Primary Antibody Specificity | Drosophila | Culex |
|---|---|---|
| Neural Tissue | ||
| Brp | + | + |
| Elav | + | − * |
| Futsch | + | + |
| Gephyrin | + | + |
| HRP | + | + |
| Phosphosynapsin | + | + |
| Repo | + | − * |
| Neurotransmitters | ||
| 5HT | + | + |
| ACh | + | − * |
| GABA | + | + |
| Neurotransmitter-Related Enzymes | ||
| ChAT | + | − * |
| GS | + | + |
| TH | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregor, K.M.; Becker, S.C.; Hellhammer, F.; Baumgärtner, W.; Puff, C. Immunohistochemical Characterization of the Nervous System of Culex pipiens (Diptera, Culicidae). Biology 2022, 11, 57. https://doi.org/10.3390/biology11010057
Gregor KM, Becker SC, Hellhammer F, Baumgärtner W, Puff C. Immunohistochemical Characterization of the Nervous System of Culex pipiens (Diptera, Culicidae). Biology. 2022; 11(1):57. https://doi.org/10.3390/biology11010057
Chicago/Turabian StyleGregor, Katharina M., Stefanie C. Becker, Fanny Hellhammer, Wolfgang Baumgärtner, and Christina Puff. 2022. "Immunohistochemical Characterization of the Nervous System of Culex pipiens (Diptera, Culicidae)" Biology 11, no. 1: 57. https://doi.org/10.3390/biology11010057
APA StyleGregor, K. M., Becker, S. C., Hellhammer, F., Baumgärtner, W., & Puff, C. (2022). Immunohistochemical Characterization of the Nervous System of Culex pipiens (Diptera, Culicidae). Biology, 11(1), 57. https://doi.org/10.3390/biology11010057






