Tetrapeptides Modelled to the Androgen Binding Site of ZIP9 Stimulate Expression of Tight Junction Proteins and Tight Junction Formation in Sertoli Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture of Rat-Derived Sertoli Cells
2.2. Peptides Used in the Study
2.3. Preparation of Cell Lysates from 93RS2 Cells
2.4. Western Blotting
2.5. Immunofluorescence
2.6. Cell-Surface Labeling with Testosterone-BSA-FITC
2.7. Silencing ZIP9 Expression by siRNA
2.8. Reverse Transcription PCR (RT-PCR)
2.9. Transepithelial Electrical Resistance (TER)
2.10. Statistical Analysis
3. Results
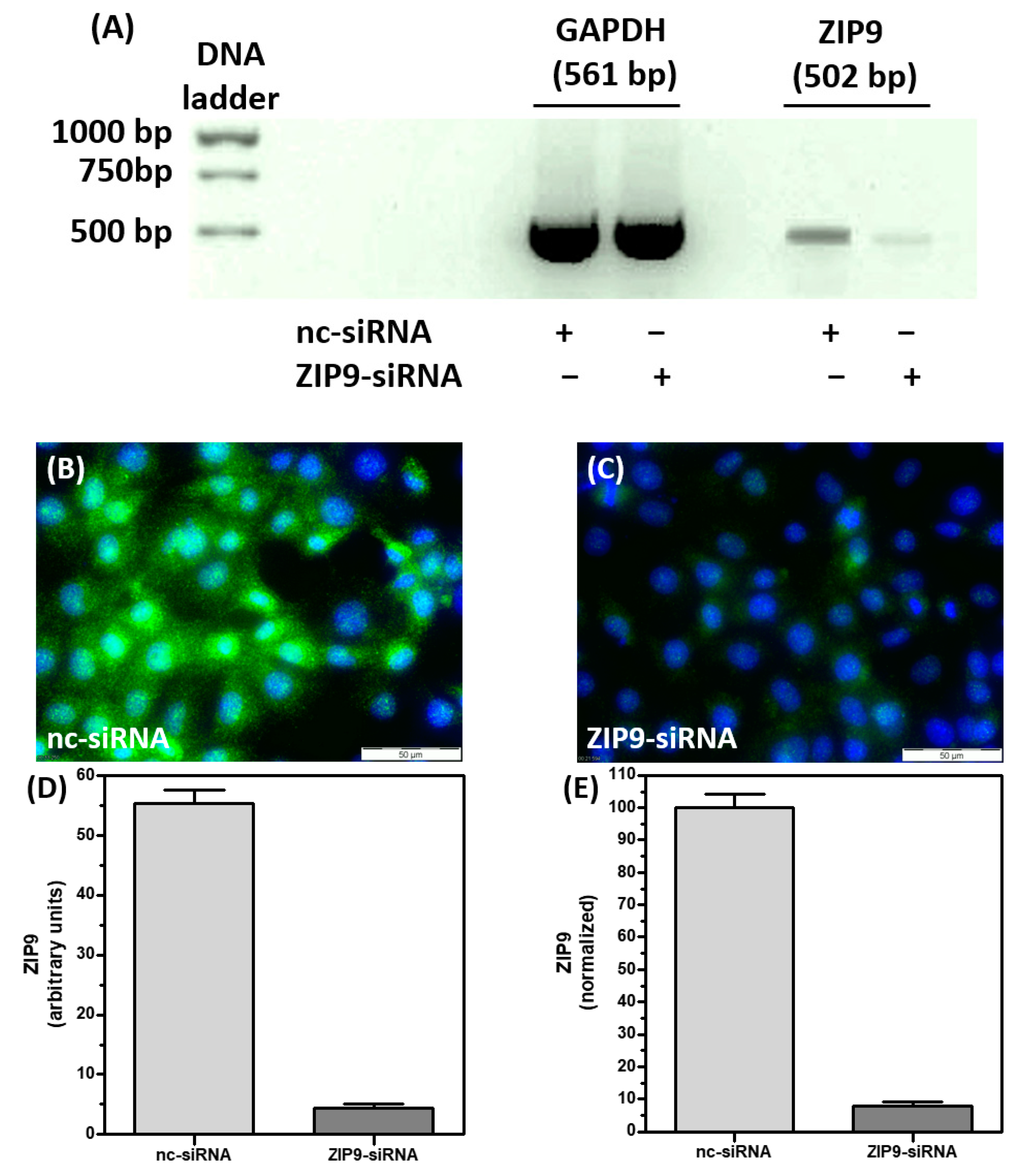
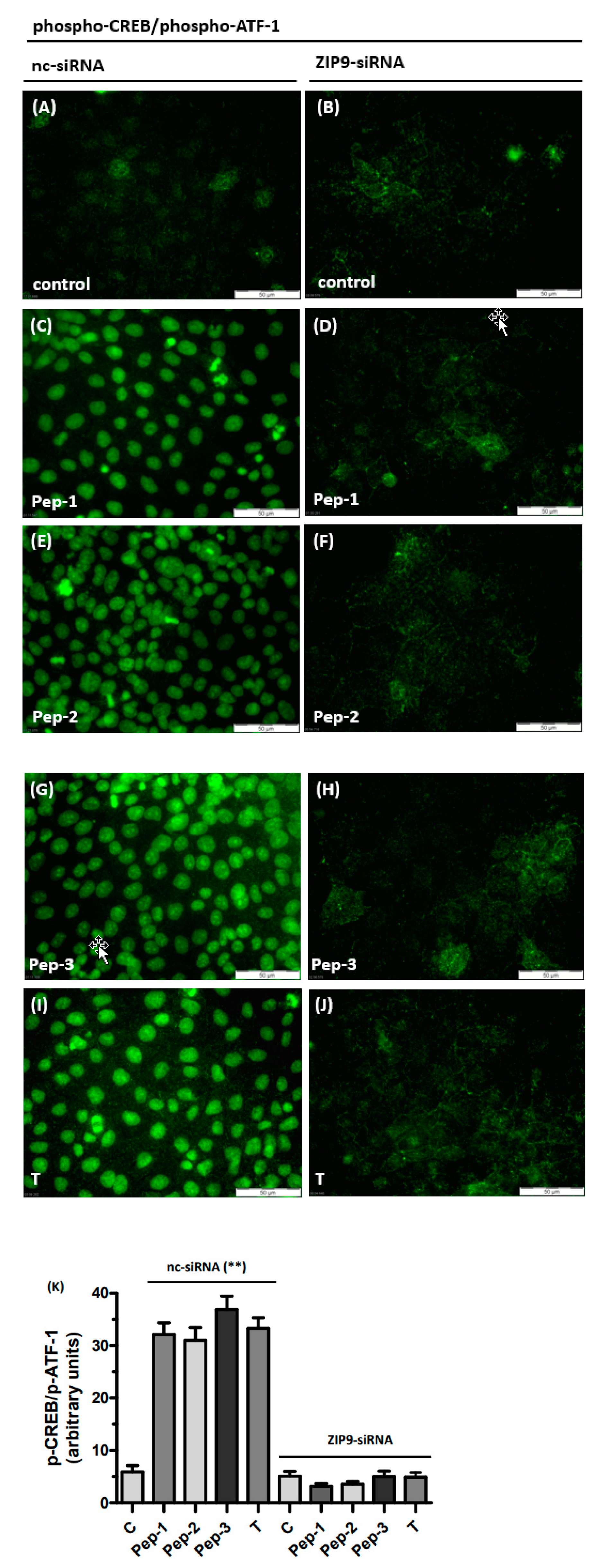
3.1. Silencing ZIP9 Expression by siRNA
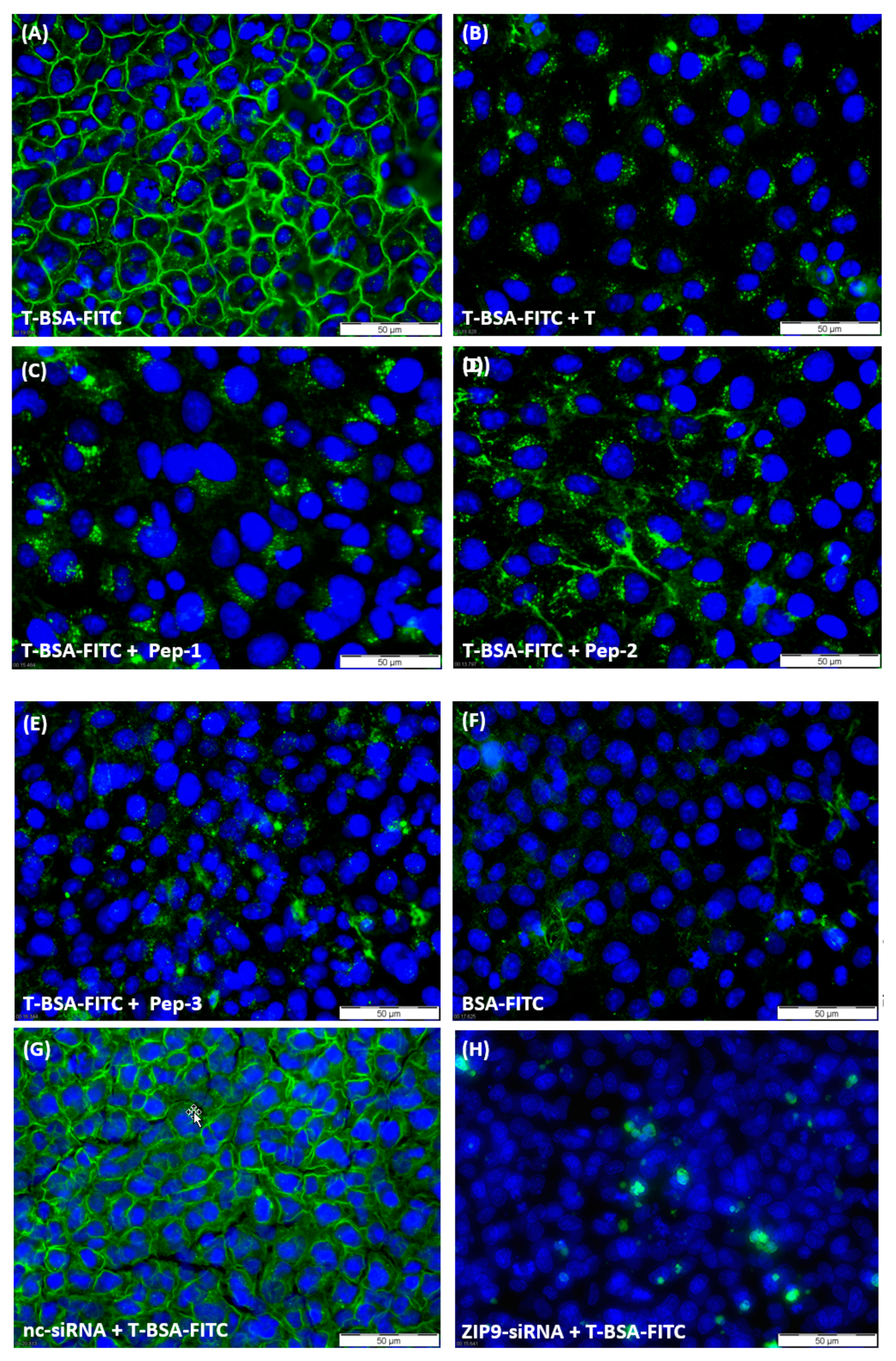
3.2. Tetrapeptides Target the Androgen-Binding Site of ZIP9
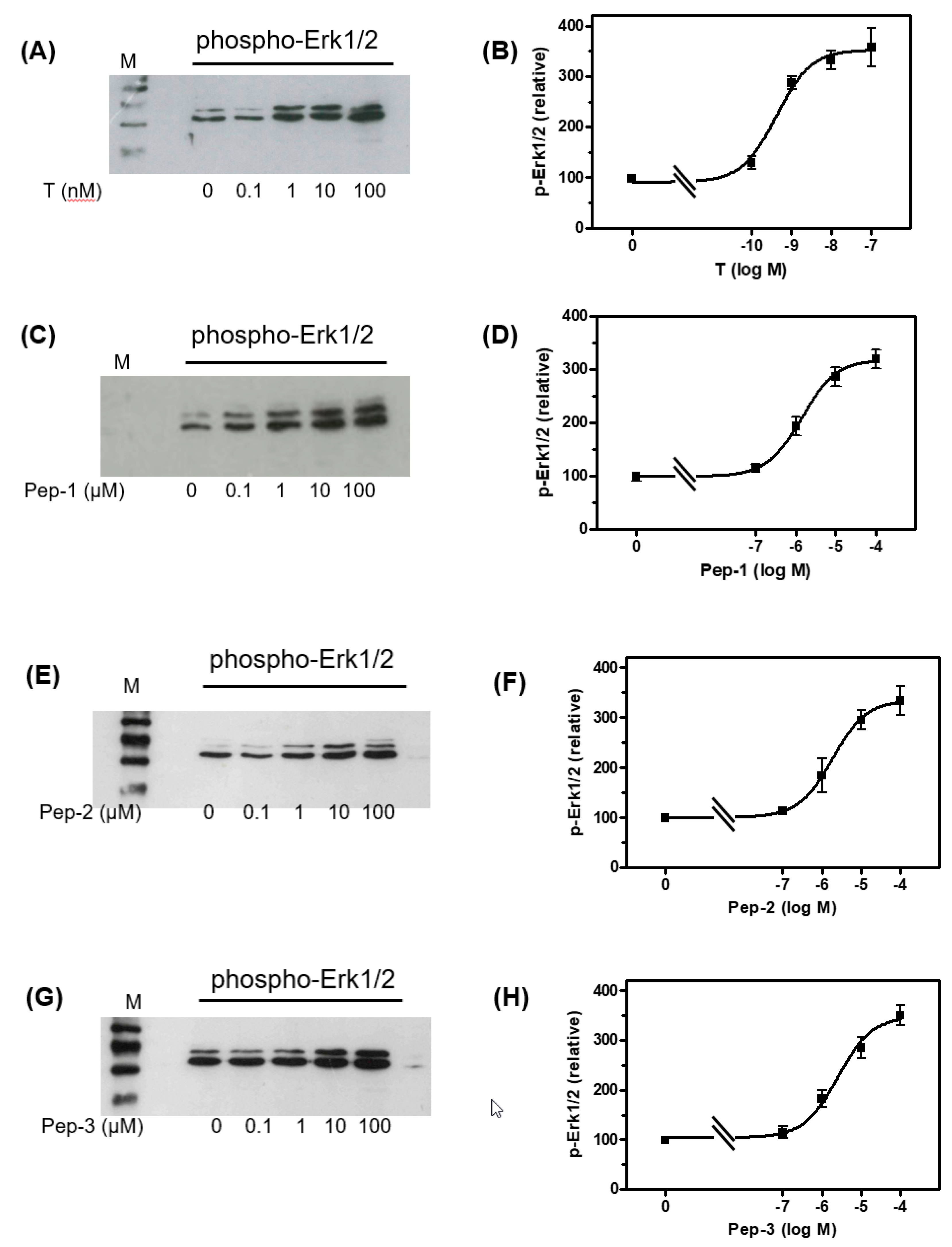
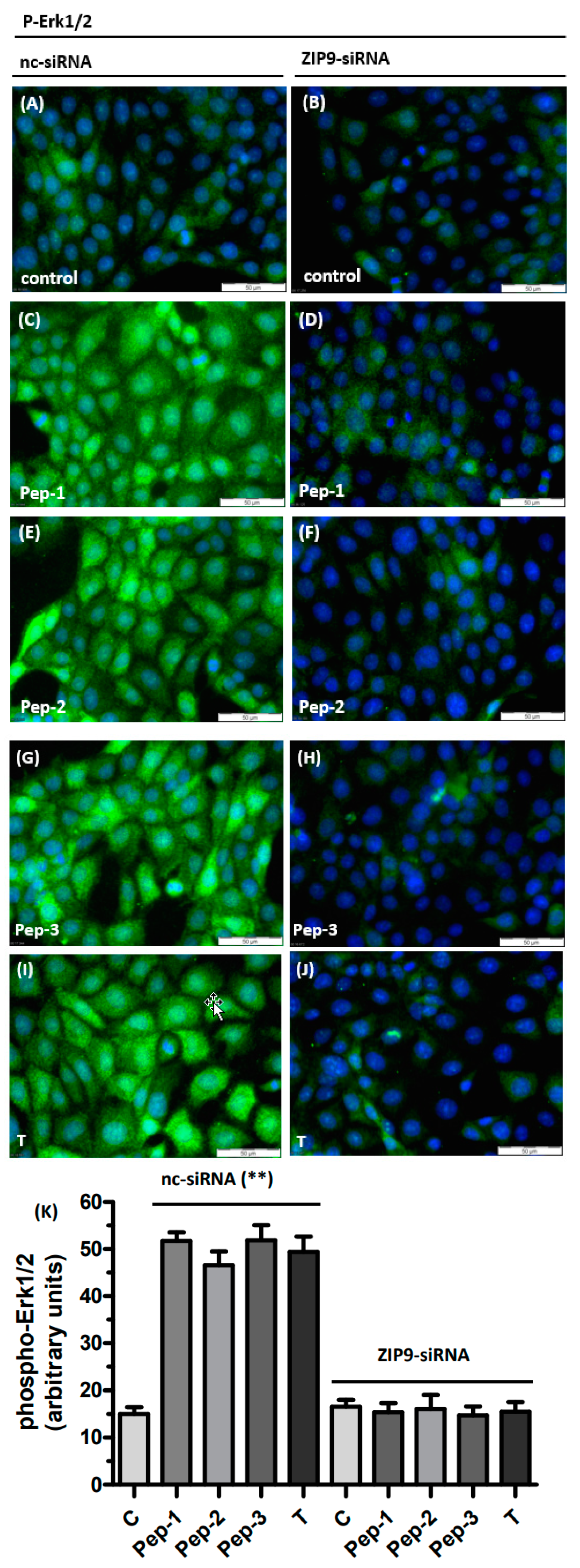
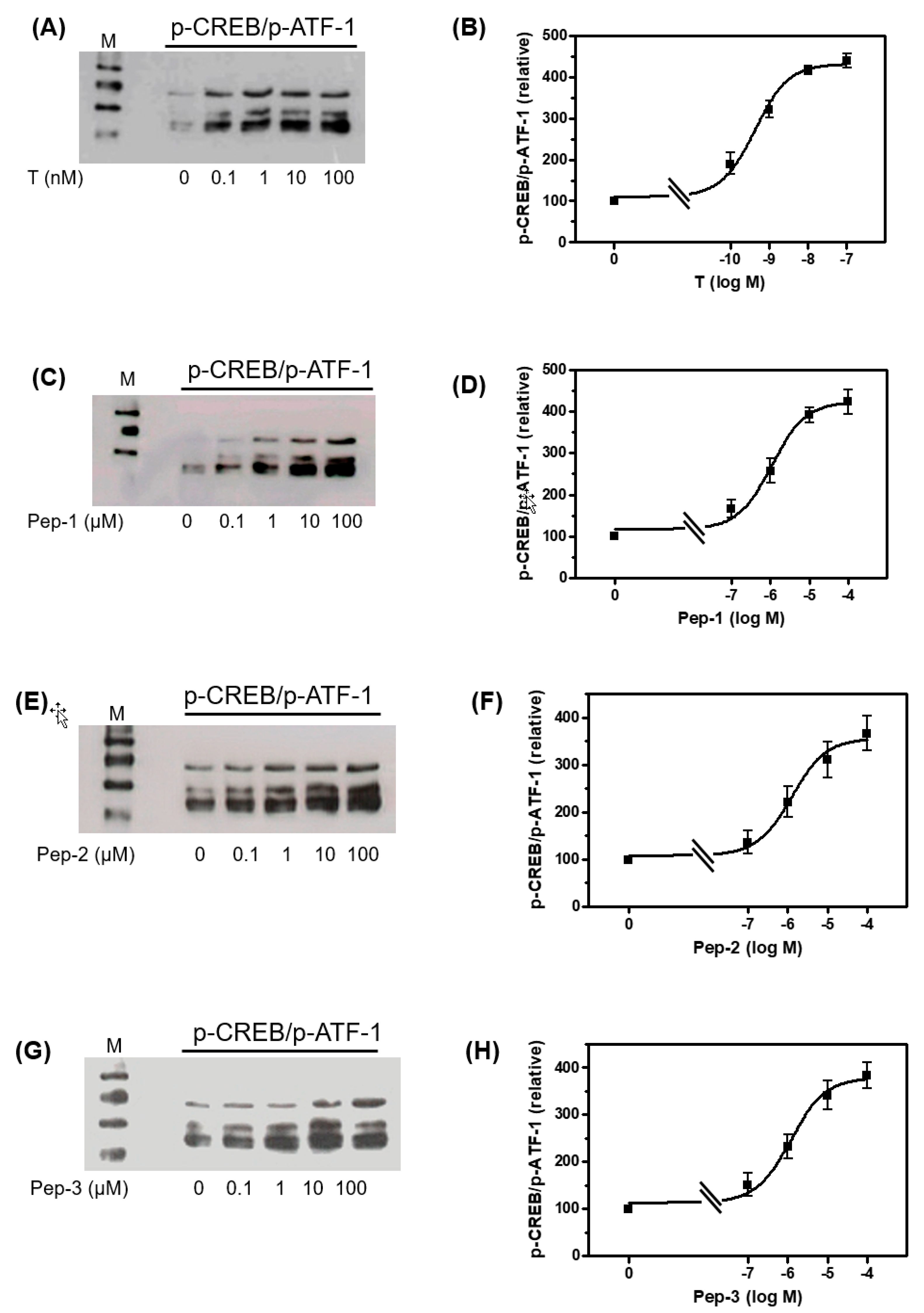
3.3. Tetrapeptides Trigger Phosphorylation of Erk1/2 and CREB/ATF-1
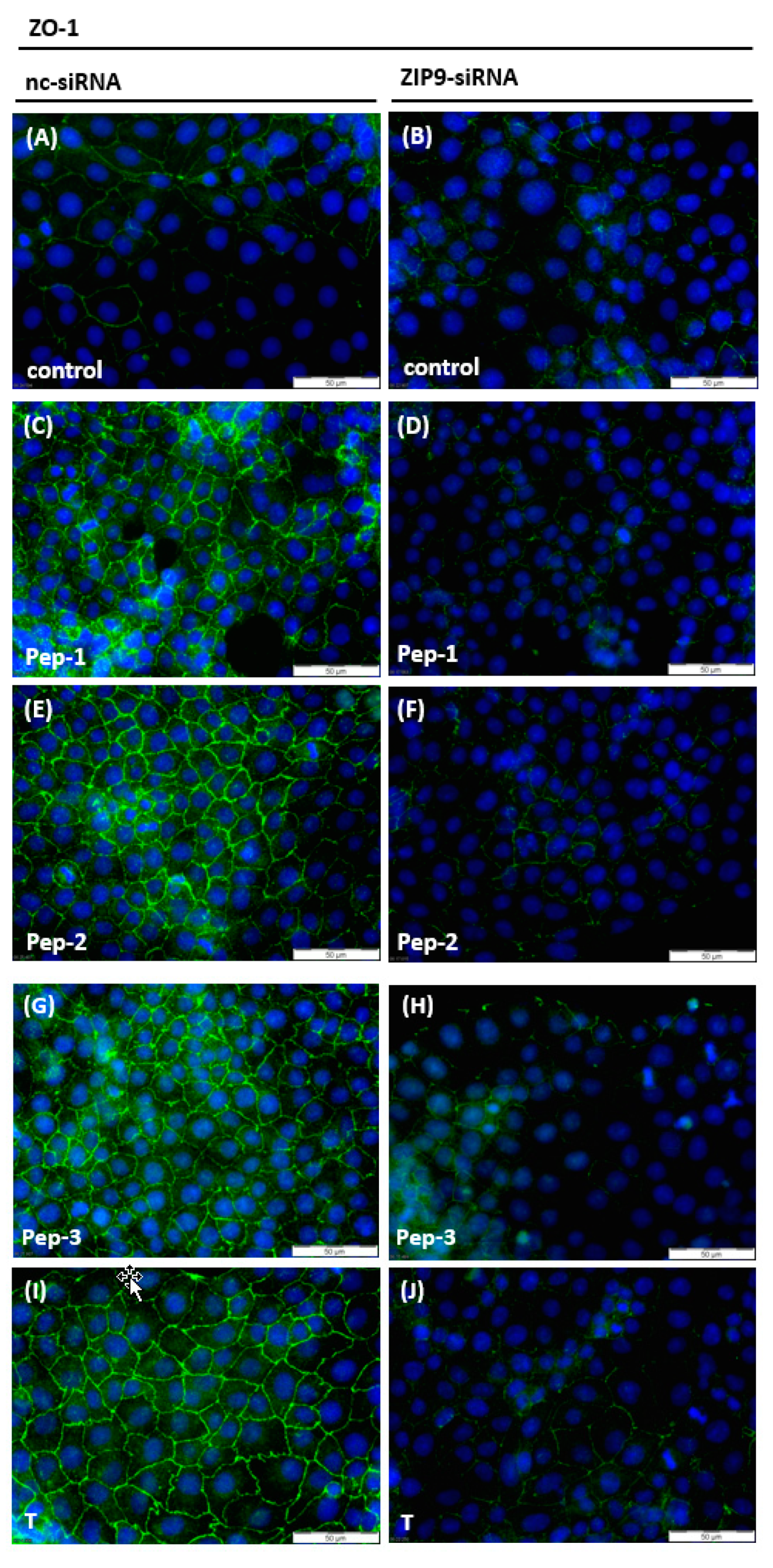
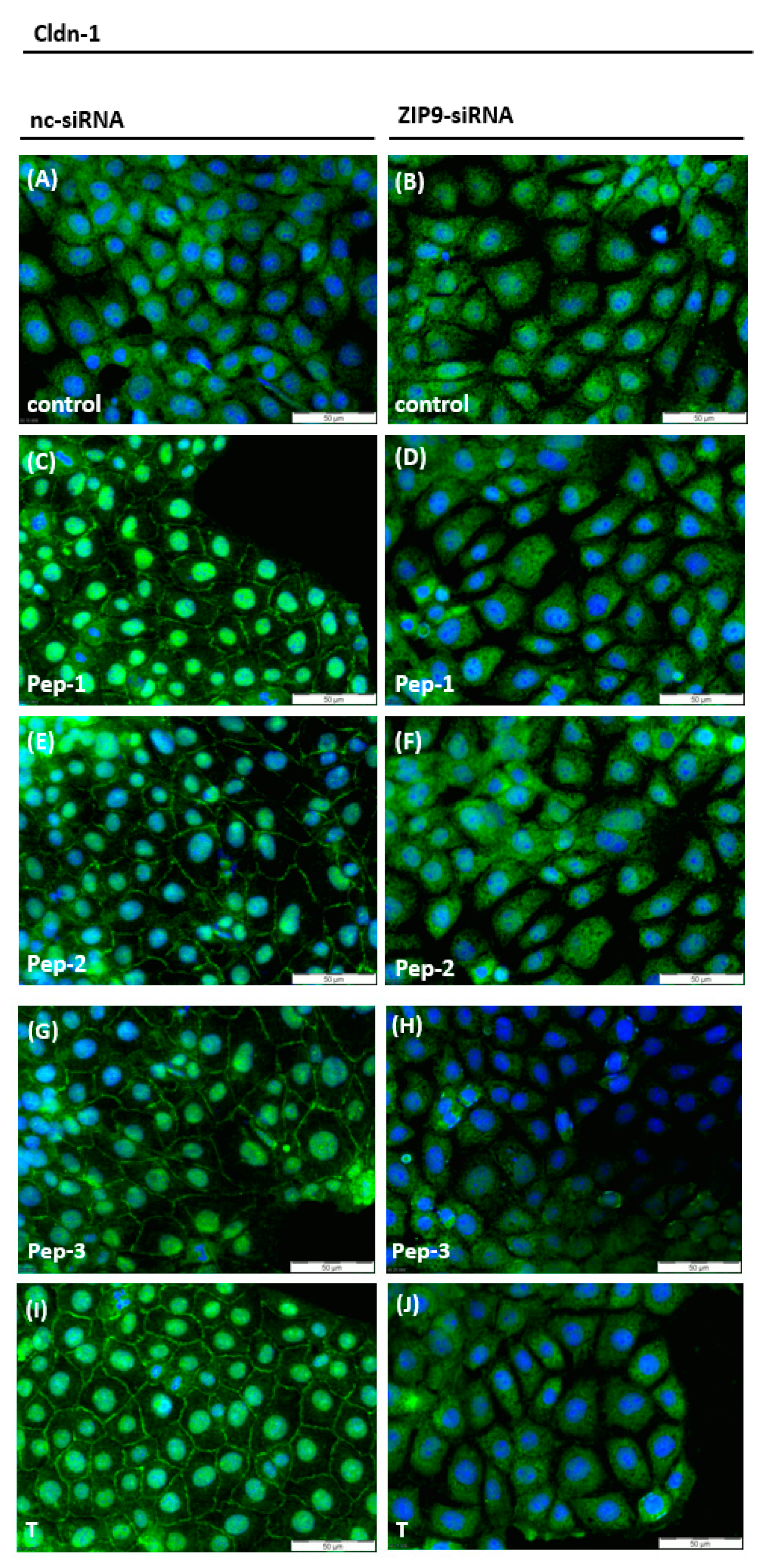
3.4. Stimulation of the Expression of Proteins Involved in Tight Junction Formation
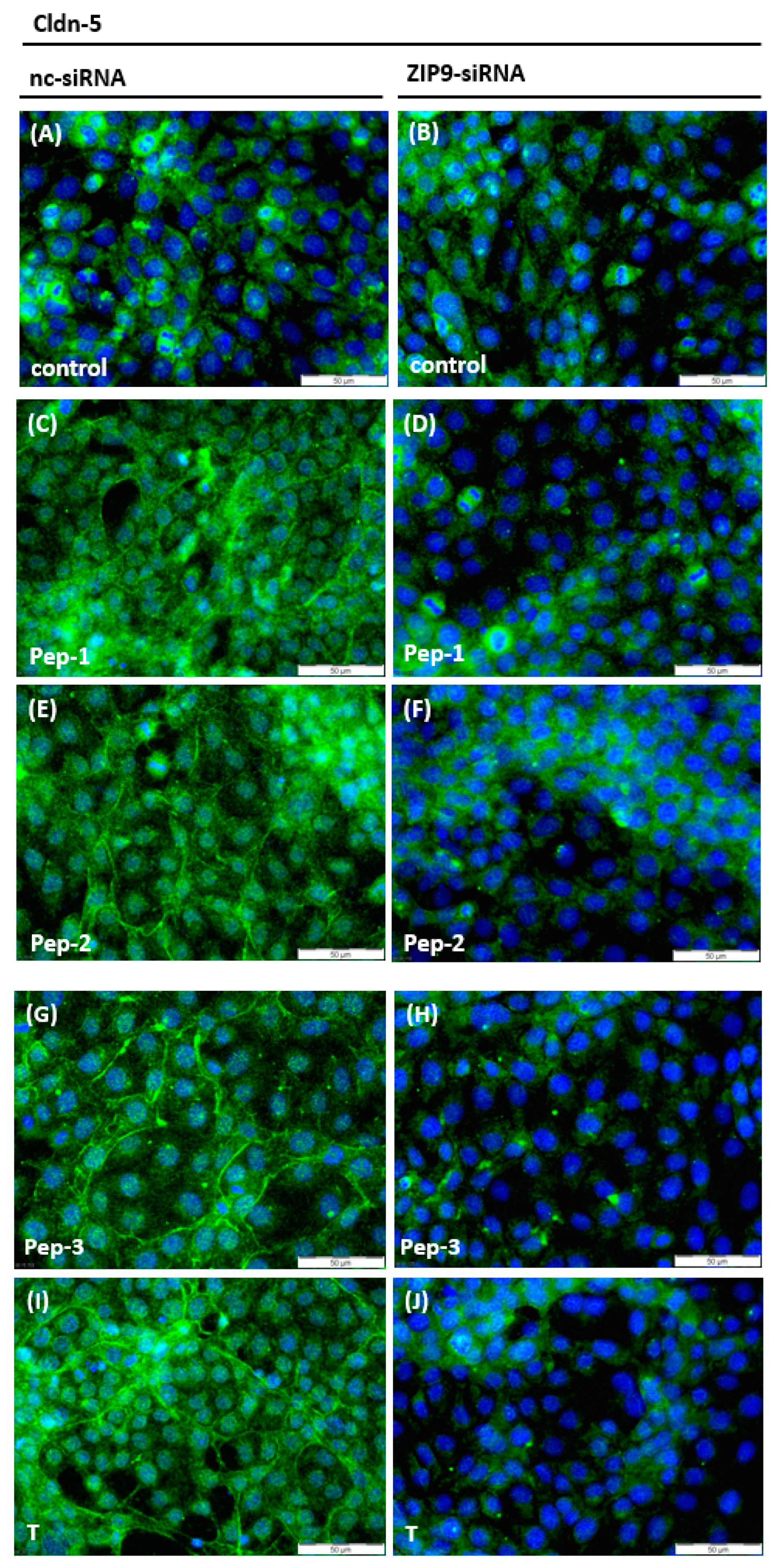
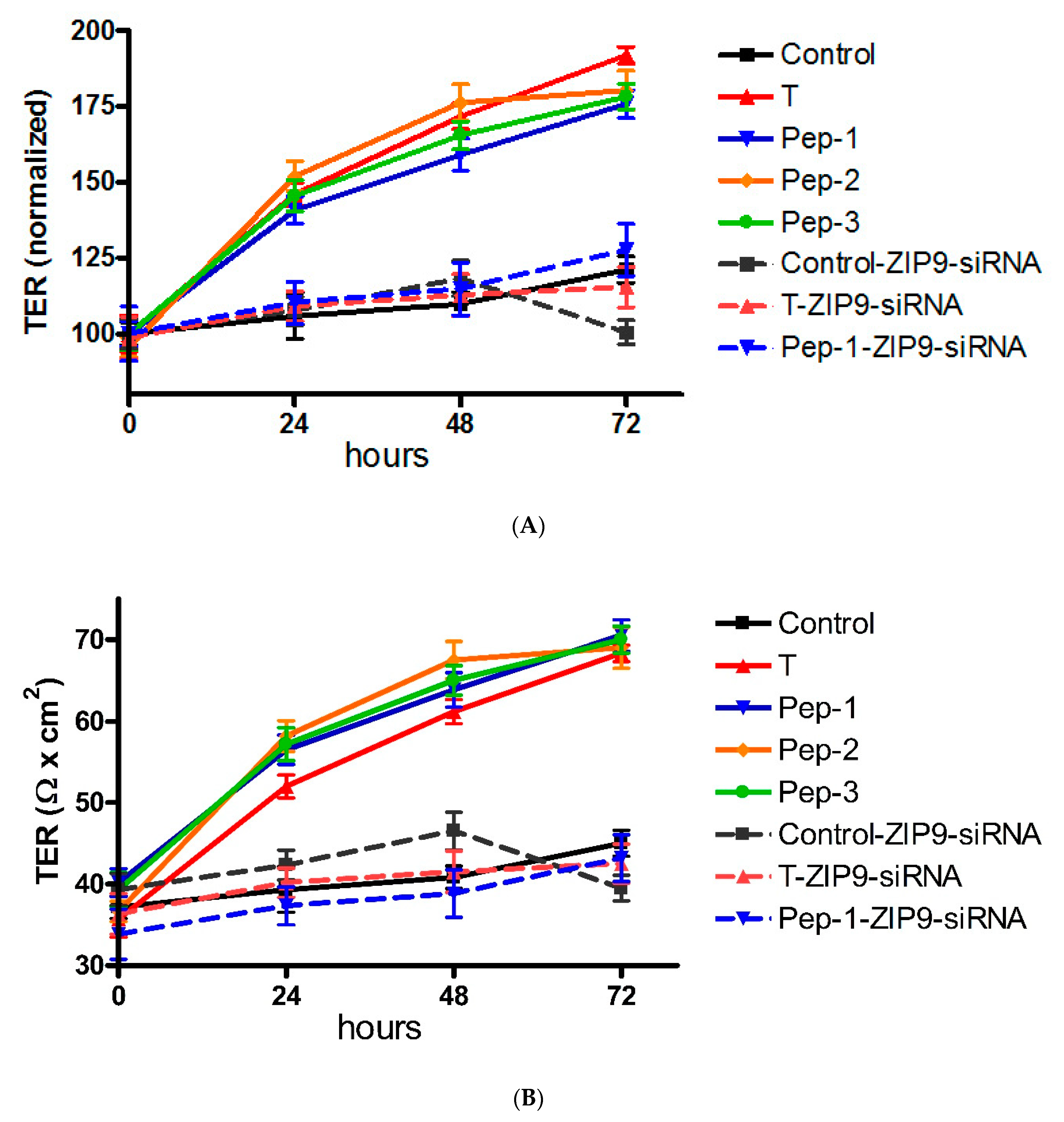
3.5. Stimulation of Tight Junction Formation by the Peptides and Testosterone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X. Roles of androgen receptor in male and female reproduction: Lessons from global and cell-specific androgen receptor knockout (ARKO) mice. J. Androl. 2010, 31, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef] [Green Version]
- Berg, A.H.; Rice, C.D.; Rahman, M.S.; Dong, J.; Thomas, P. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology 2014, 155, 4237–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shihan, M.; Chan, K.H.; Konrad, L.; Scheiner-Bobis, G. Non-classical testosterone signaling in spermatogenic GC-2 cells is mediated through ZIP9 interacting with Gnalpha11. Cell. Signal. 2015, 27, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Bulldan, A.; Dietze, R.; Shihan, M.; Scheiner-Bobis, G. Non-classical testosterone signaling mediated through ZIP9 stimulates claudin expression and tight junction formation in Sertoli cells. Cell. Signal. 2016, 28, 1075–1085. [Google Scholar] [CrossRef]
- Bulldan, A.; Malviya, V.N.; Upmanyu, N.; Konrad, L.; Scheiner-Bobis, G. Testosterone/bicalutamide antagonism at the predicted extracellular androgen binding site of ZIP9. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 2402–2414. [Google Scholar] [CrossRef]
- Bulldan, A.; Bartsch, J.W.; Konrad, L.; Scheiner-Bobis, G. ZIP9 but not the androgen receptor mediates testosterone-induced migratory activity of metastatic prostate cancer cells. Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J. Membrane androgen receptor characteristics of human ZIP9 (SLC39A) zinc transporter in prostate cancer cells: Androgen-specific activation and involvement of an inhibitory G protein in zinc and MAP kinase signaling. Mol. Cell. Endocrinol. 2017, 447, 23–34. [Google Scholar] [CrossRef]
- Meng, J.; Holdcraft, R.W.; Shima, J.E.; Griswold, M.D.; Braun, R.E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 16696–16700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Mostaghel, E.A.; Vakar-Lopez, F.; Montgomery, B.; True, L.; Nelson, P.S. Testosterone regulates tight junction proteins and influences prostatic autoimmune responses. Horm. Cancer 2011, 2, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.S.; Yeh, S.; Chen, L.M.; Lin, H.Y.; Zhang, C.; Ni, J.; Wu, C.C.; di Sant’Agnese, P.A.; deMesy-Bentley, K.L.; Tzeng, C.R.; et al. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 2006, 147, 5624–5633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinhardt, A.; Hedger, M.P. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol. Cell. Endocrinol. 2011, 335, 60–68. [Google Scholar] [CrossRef]
- Fijak, M.; Bhushan, S.; Meinhardt, A. Immunoprivileged sites: The testis. Methods Mol. Biol. 2011, 677, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.R.; Kotwinski, P.J.; Montgomery, H.E. Cardiac effects of anabolic steroids. Heart 2004, 90, 473–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, J.H.; Medei, E. Cardiac effects of anabolic steroids: Hypertrophy, ischemia and electrical remodelling as potential triggers of sudden death. Mini Rev. Med. Chem. 2011, 11, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S. Male hypogonadism. Lancet 2014, 383, 1250–1263. [Google Scholar] [CrossRef]
- Patel, A.S.; Leong, J.Y.; Ramos, L.; Ramasamy, R. Testosterone Is a Contraceptive and Should Not Be Used in Men Who Desire Fertility. World J. Men’s Health 2019, 37, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wong, C.H.; Lee, N.P.; Lee, W.M.; Cheng, C.Y. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: An in vivo study using an androgen suppression model. J. Cell. Physiol. 2005, 205, 141–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malviya, V.N.; Bulldan, A.; Wende, R.C.; Kabbesh, H.; Möller, M.-L.; Schreiner, P.R.; Scheiner-Bobis, G. The Effects of Tetrapeptides Designed to Fit the Androgen Binding Site of ZIP9 on Myogenic and Osteogenic Cells. Biology 2022, 11, 19. [Google Scholar] [CrossRef]
- Jiang, C.; Hall, S.J.; Boekelheide, K. Development and characterization of a prepubertal rat Sertoli cell line, 93RS2. J. Androl. 1997, 18, 393–399. [Google Scholar]
- Hemendinger, R.A.; Gores, P.; Blacksten, L.; Harley, V.; Halberstadt, C. Identification of a specific Sertoli cell marker, Sox9, for use in transplantation. Cell Transplant. 2002, 11, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Mruk, D.D.; Cheng, C.Y. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis 2011, 1, 121–122. [Google Scholar] [CrossRef] [Green Version]
- Gaetjens, E.; Pertschuk, L.P. Synthesis of fluorescein labelled steroid hormone-albumin conjugates for the fluorescent histochemical detection of hormone receptors. J. Steroid Biochem. 1980, 13, 1001–1003. [Google Scholar] [CrossRef]
- Benten, W.P.; Lieberherr, M.; Giese, G.; Wrehlke, C.; Stamm, O.; Sekeris, C.E.; Mossmann, H.; Wunderlich, F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999, 13, 123–133. [Google Scholar] [CrossRef]
- Kampa, M.; Papakonstanti, E.A.; Hatzoglou, A.; Stathopoulos, E.N.; Stournaras, C.; Castanas, E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002, 16, 1429–1431. [Google Scholar] [CrossRef]
- Wen-Hua, Z.; Quirion, R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Masson, N.; John, J.; Lee, K.A.W. In vitro phosphorylation studies of a conserved region of the transcription factor ATF1. Nucleic Acids Res. 1993, 21, 4166–4173. [Google Scholar] [CrossRef]
- Gye, M.C. Expression of claudin-1 in mouse testis. Arch Androl. 2003, 49, 271–279. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Sluka, P.; Foo, C.F.; Stanton, P.G. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 2007, 133, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- McCabe, M.J.; Allan, C.M.; Foo, C.F.; Nicholls, P.K.; McTavish, K.J.; Stanton, P.G. Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol. Reprod. 2012, 87, 38. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, B.R.; Heintzelman, M.B.; Anderson, J.M.; Citi, S.; Mooseker, M.S. ZO-1 and cingulin: Tight junction proteins with distinct identities and localizations. Am. J. Physiol. 1989, 257, C621–C628. [Google Scholar] [CrossRef]
- Wong, E.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc. Natl. Acad. Sci. USA 2010, 107, 11399–11404. [Google Scholar] [CrossRef] [Green Version]
- Morrow, C.M.; Mruk, D.; Cheng, C.Y.; Hess, R.A. Claudin and occludin expression and function in the seminiferous epithelium. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1679–1696. [Google Scholar] [CrossRef]
- Hess, R.A.; Morrow, C.M.K. Claudin 5, Germ Cells, and the Blood-Testis Barrier. Biol. Reprod. 2010, 83, 109. [Google Scholar] [CrossRef]
- Rahman, F.; Christian, H.C. Non-classical actions of testosterone: An update. Trends Endocrinol. Metab. 2007, 18, 371–378. [Google Scholar] [CrossRef]
- Walker, W.H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Scobey, M.; Bertera, S.; Somers, J.; Watkins, S.; Zeleznik, A.; Walker, W. Delivery of a cyclic adenosine 3’,5’-monophosphate response element-binding protein (creb) mutant to seminiferous tubules results in impaired spermatogenesis. Endocrinology 2001, 142, 948–954. [Google Scholar] [CrossRef]
- Servillo, G.; Della Fazia, M.A.; Sassone-Corsi, P. Coupling cAMP signaling to transcription in the liver: Pivotal role of CREB and CREM. Exp. Cell Res. 2002, 275, 143–154. [Google Scholar] [CrossRef]
- Su, L.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Differential effects of testosterone and TGF-beta3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp. Cell Res. 2010, 316, 2945–2960. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.H.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008, 22, 1945–1959. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [Green Version]
- Landon, G.V.; Pryor, J.P. The blood-testis barrier in men of diverse fertility status: An ultrastructural study. Virchows Arch. A Pathol. Anat. Histol. 1981, 392, 355–364. [Google Scholar] [CrossRef]
- Cavicchia, J.C.; Sacerdote, F.L.; Ortiz, L. The human blood-testis barrier in impaired spermatogenesis. Ultrastruct. Pathol. 1996, 20, 211–218. [Google Scholar] [CrossRef]
- Jiang, X.H.; Bukhari, I.; Zheng, W.; Yin, S.; Wang, Z.; Cooke, H.J.; Shi, Q.H. Blood-testis barrier and spermatogenesis: Lessons from genetically-modified mice. Asian J. Androl. 2014, 16, 572–580. [Google Scholar] [CrossRef]
- Islam, R.; Yoon, H.; Kim, B.-S.; Bae, H.-S.; Shin, H.-R.; Kim, W.-J.; Yoon, W.-J.; Lee, Y.-S.; Woo, K.M.; Baek, J.-H.; et al. Blood-testis barrier integrity depends on Pin1 expression in Sertoli cells. Sci. Rep. 2017, 7, 6977. [Google Scholar] [CrossRef]
- McCabe, M.J.; Tarulli, G.A.; Meachem, S.J.; Robertson, D.M.; Smooker, P.M.; Stanton, P.G. Gonadotropins regulate rat testicular tight junctions in vivo. Endocrinology 2010, 151, 2911–2922. [Google Scholar] [CrossRef] [Green Version]
- Bulldan, A.; Shihan, M.; Goericke-Pesch, S.; Scheiner-Bobis, G. Signaling events associated with gonadotropin releasing hormone-agonist-induced hormonal castration and its reversal in canines. Mol. Reprod. Dev. 2016, 83, 1092–1101. [Google Scholar] [CrossRef]
- Profaska-Szymik, M.; Galuszka, A.; Korzekwa, A.J.; Hejmej, A.; Gorowska-Wojtowicz, E.; Pawlicki, P.; Kotula-Balak, M.; Tarasiuk, K.; Tuz, R. Implication of Membrane Androgen Receptor (ZIP9) in Cell Senescence in Regressed Testes of the Bank Vole. Int. J. Mol. Sci. 2020, 21, 6888. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, M.-L.; Bulldan, A.; Scheiner-Bobis, G. Tetrapeptides Modelled to the Androgen Binding Site of ZIP9 Stimulate Expression of Tight Junction Proteins and Tight Junction Formation in Sertoli Cells. Biology 2022, 11, 55. https://doi.org/10.3390/biology11010055
Möller M-L, Bulldan A, Scheiner-Bobis G. Tetrapeptides Modelled to the Androgen Binding Site of ZIP9 Stimulate Expression of Tight Junction Proteins and Tight Junction Formation in Sertoli Cells. Biology. 2022; 11(1):55. https://doi.org/10.3390/biology11010055
Chicago/Turabian StyleMöller, Marie-Louise, Ahmed Bulldan, and Georgios Scheiner-Bobis. 2022. "Tetrapeptides Modelled to the Androgen Binding Site of ZIP9 Stimulate Expression of Tight Junction Proteins and Tight Junction Formation in Sertoli Cells" Biology 11, no. 1: 55. https://doi.org/10.3390/biology11010055
APA StyleMöller, M.-L., Bulldan, A., & Scheiner-Bobis, G. (2022). Tetrapeptides Modelled to the Androgen Binding Site of ZIP9 Stimulate Expression of Tight Junction Proteins and Tight Junction Formation in Sertoli Cells. Biology, 11(1), 55. https://doi.org/10.3390/biology11010055





