Cortisol and Dexamethasone Mediate Glucocorticoid Actions in the Lesser Spotted Catshark (Scyliorhinus canicula)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Maintenance
2.3. Long-Term In Vivo Responses
2.4. Acute Ex Vivo Responses
2.5. Sampling
2.6. Plasma Hormones Determination
2.7. Tissue Metabolite Content
2.8. Enzyme Activities
2.9. Plasma Metabolites and pH
2.10. Statistics
3. Results
3.1. Long-Term In Vivo Effects of a Corticosteroid IP-Implant
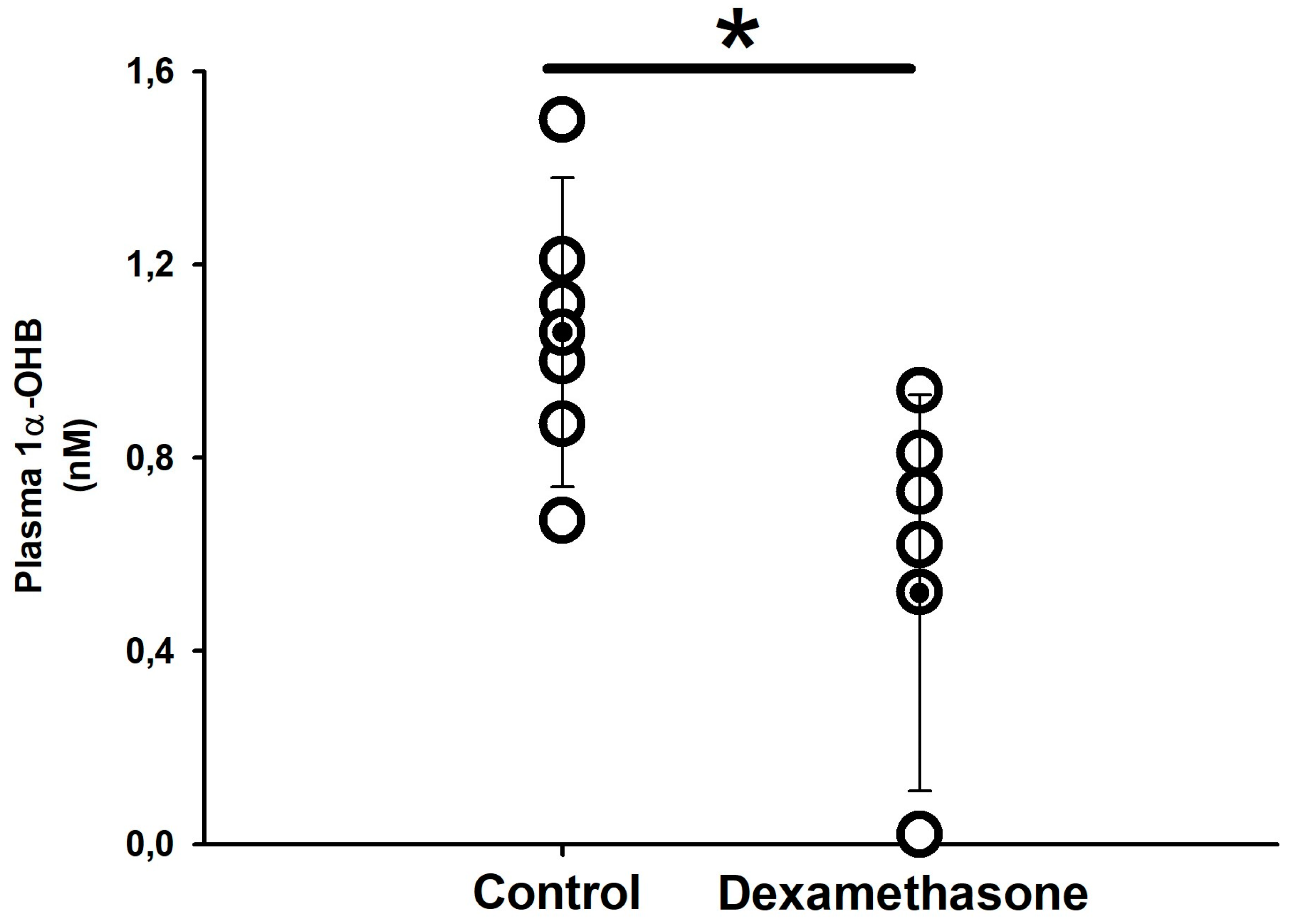
3.1.1. Plasma Hormones
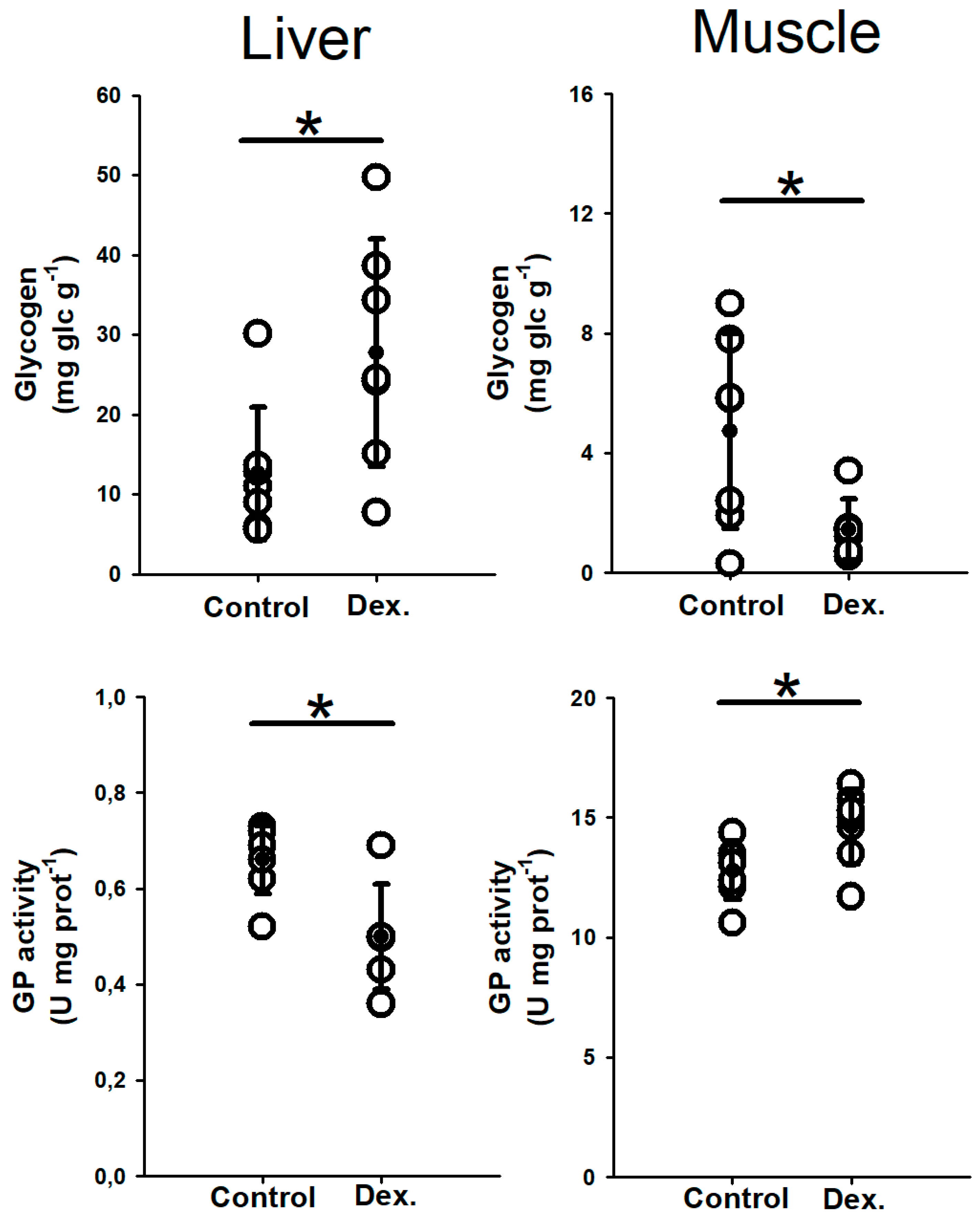
3.1.2. Tissue Metabolites and Enzyme Activities
3.2. Short-Term Ex Vivo Glucocorticoid Actions of Corticosteroids
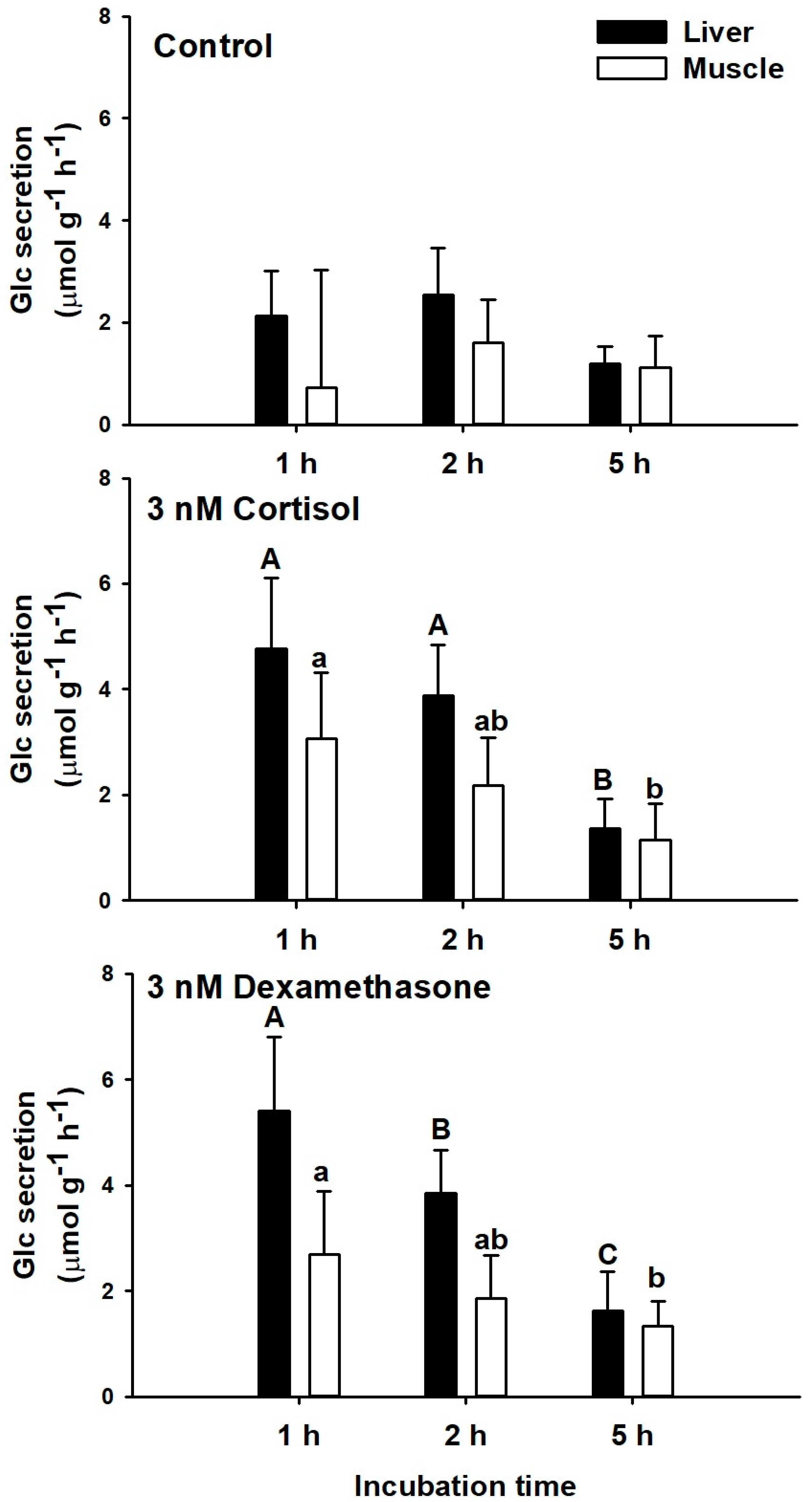
3.2.1. Incubation Time
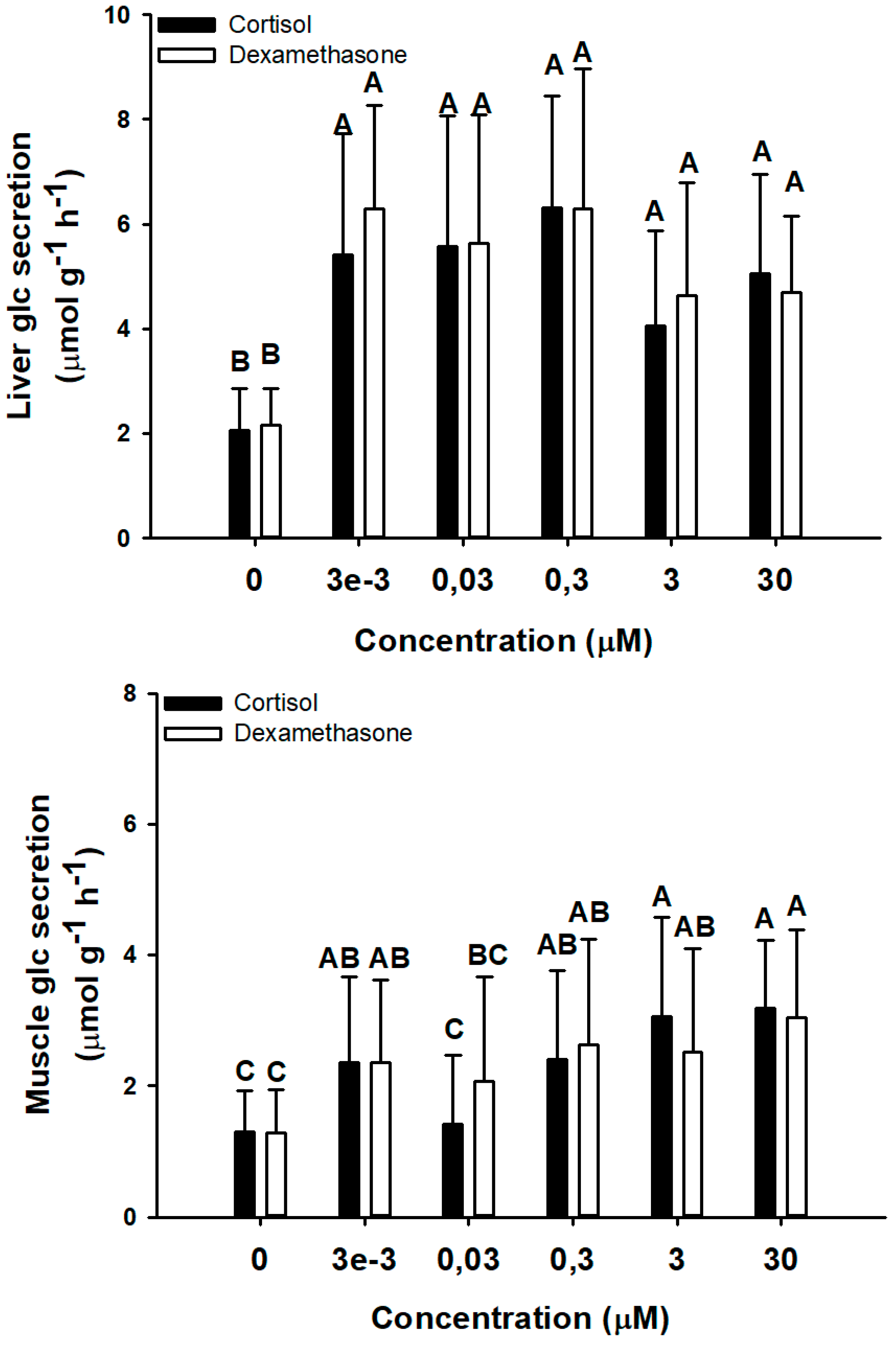
3.2.2. Effect of Corticosteroids Concentration
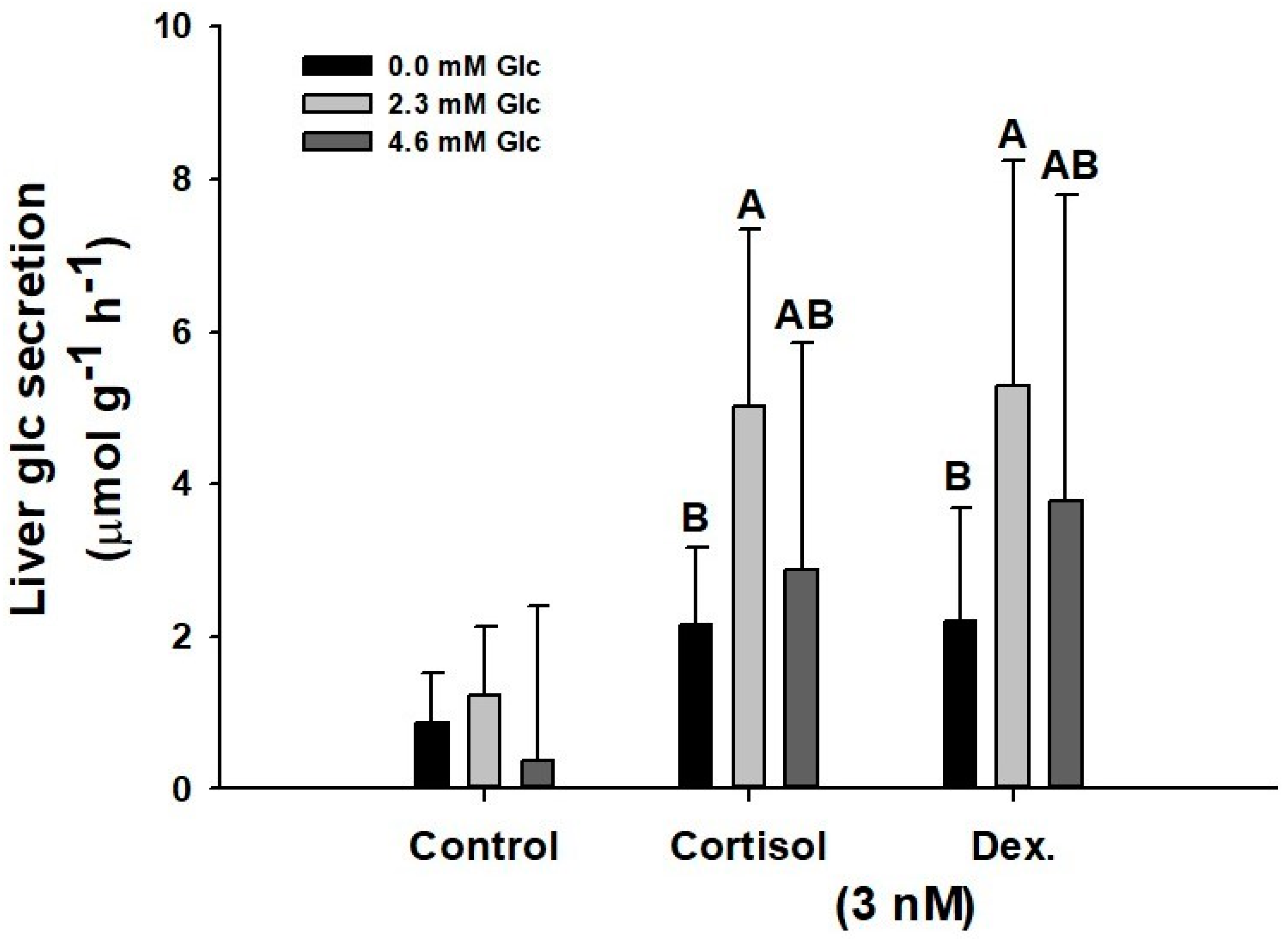
3.2.3. Effect of Initial Glucose Concentration
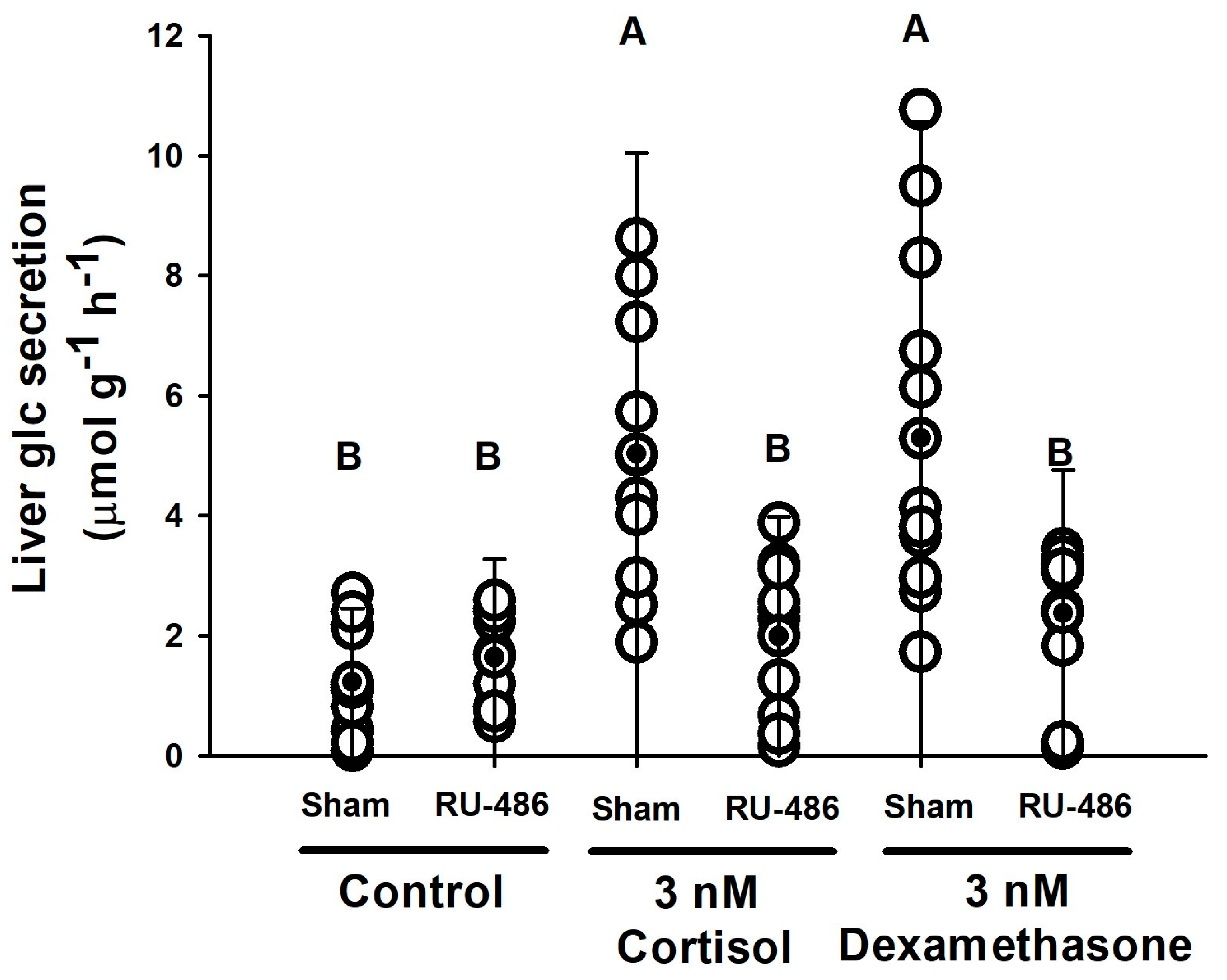
3.2.4. Glucocorticoid Receptors
4. Discussion
4.1. Effect of Long-Term In Vivo Dexamethasone Treatment in Plasma Corticosteroids
4.2. Glycolytic Effects of Long-Term Dexamethasone Treatment in Catsharks
4.3. Acute Ex Vivo Glucocorticoid Actions of Corticosteroids
4.4. Glucocorticoid Actions Mediated by GR in Catsharks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Analysis of Plasma Parameters and Development of the Ex Vivo Incubation Medium for S. canicula
References
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; Taylor, M.L.; Regish, A.M. Cortisol is an osmoregulatory and glucose-regulating hormone in Atlantic sturgeon, a basal ray-finned fish. J. Exp. Biol. 2020, 223, jeb220251. [Google Scholar] [CrossRef] [PubMed]
- Close, D.A.; Yun, S.S.; McCormick, S.D.; Wildbill, A.J.; Li, W. 11-deoxycortisol is a corticosteroid hormone in the lamprey. Proc. Natl. Acad. Sci. USA 2010, 107, 13942–13947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.G. The endocrinology of 1alpha-hydroxycorticosterone in elasmobranch fish: A review. Comp. Biochem. Physiol. A 2012, 162, 73–80. [Google Scholar] [CrossRef]
- Rister, A.L.; Dodds, E.D. Steroid analysis by ion mobility spectrometry. Steroids 2020, 153, 108531. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Funder, J.W.; Pearce, P.T.; Smith, R.; Smith, A.I. Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science 1988, 242, 583–585. [Google Scholar] [CrossRef]
- Prunet, P.; Sturm, A.; Milla, S. Multiple corticosteroid receptors in fish: From old ideas to new concepts. Gen. Comp. Endocrinol. 2006, 147, 17–23. [Google Scholar] [CrossRef]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joels, M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol. Cell. Endocrinol. 2012, 350, 299–309. [Google Scholar] [CrossRef]
- Bridgham, J.T.; Carroll, S.M.; Thornton, J.W. Evolution of hormone-receptor complexity by molecular exploitation. Science 2006, 312, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, S.M.; Bridgham, J.T.; Thornton, J.W. Evolution of hormone signaling in elasmobranchs by exploitation of promiscuous receptors. Mol. Biol. Evol. 2008, 25, 2643–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beato, M.; Herrlich, P.; Schutz, G. Steroid hormone receptors: Many actors in search of a plot. Cell 1995, 83, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Furtbauer, I.; Heistermann, M. Cortisol coregulation in fish. Sci. Rep. 2016, 6, 30334. [Google Scholar] [CrossRef] [Green Version]
- Idler, D.R.; Sangalang, G.B.; Truscott, B. Corticosteroids in the South American lungfish. Gen. Comp. Endocrinol. 1972, 3, 238–244. [Google Scholar] [CrossRef]
- Idler, D.R.; Freeman, H.C.; Truscott, B. Biological activity and protein-binding of 1 alpha-hydroxycorticosterone: An interrenal steroid in elasmobranch fish. Gen. Comp. Endocrinol. 1967, 9, 207–213. [Google Scholar] [CrossRef]
- Hazon, N.; Henderson, I.W. Factors affecting the secretory dynamics of 1 alpha-hydroxycorticosterone in the dogfish, Scyliorhinus canicula. Gen. Comp. Endocrinol. 1985, 59, 50–55. [Google Scholar] [CrossRef]
- Katsu, Y.; Oana, S.; Lin, X.; Hyodo, S.; Baker, M.E. Aldosterone and dexamethasone activate African lungfish mineralocorticoid receptor: Increased activation after removal of the amino-terminal domain. J. Steroid Biochem. Mol. Biol. 2021, 106024. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Barragán-Méndez, C.; Jerez-Cepa, I.; Fernandez-Castro, M.; Sobrino, I.; Mancera, J.M.; Aerts, J. Plasma 1alpha-hydroxycorticosterone as biomarker for acute stress in catsharks (Scyliorhinus canicula). Front. Physiol. 2019, 10, 1217. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Amanajas, R.D.; Baldisserotto, B.; Mancera, J.M.; Val, A.L. Tambaqui (Colossoma macropomum) acclimated to different tropical waters from the Amazon basin shows specific acute-stress responses. Comp. Biochem. Physiol. A 2020, 245, 110706. [Google Scholar] [CrossRef]
- Iki, A.; Anderson, W.G.; Deck, C.A.; Ogihara, M.H.; Ikeba, K.; Kataoka, H.; Hyodo, S. Measurement of 1alpha hydroxycorticosterone in the Japanese banded houndshark, Triakis scyllium, following exposure to a series of stressors. Gen. Comp. Endocrinol. 2020, 292, 113440. [Google Scholar] [CrossRef]
- Speers-Roesch, B.; Treberg, J.R. The unusual energy metabolism of elasmobranch fishes. Comp. Biochem. Physiol. A 2010, 155, 417–434. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Gorissen, M.; Mancera, J.M.; Ruiz-Jarabo, I. What can we learn from glucocorticoid administration in fish? Effects of cortisol and dexamethasone on intermediary metabolism of gilthead seabream (Sparus aurata L.). Comp. Biochem. Physiol. A 2019, 231, 1–10. [Google Scholar] [CrossRef]
- Skrzynska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Miguez, J.M.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of air exposure on vasotocinergic and isotocinergic systems in gilthead sea bream (Sparus aurata): New insights on fish stress response. Front. Physiol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadoul, B.; Vijayan, M.M. Stress and growth. In Biology of Stress in Fish; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; Volume 35, pp. 35–75. [Google Scholar]
- Skomal, G.B.; Mandelman, J.W. The physiological response to anthropogenic stressors in marine elasmobranch fishes: A review with a focus on the secondary response. Comp. Biochem. Physiol. A 2012, 162, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, J.S. Jaws: The inside story. The metabolism of elasmobranch fishes. Comp. Biochem. Physiol. B 1997, 118, 703–742. [Google Scholar] [CrossRef]
- Milligan, C.L. A regulatory role for cortisol in muscle glycogen metabolism in rainbow trout Oncorhynchus mykiss Walbaum. J. Exp. Biol. 2003, 206, 3167–3173. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, M.M.; Raptis, S.; Sathiyaa, R. Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. Gen. Comp. Endocrinol. 2003, 132, 256–263. [Google Scholar] [CrossRef]
- Polakof, S.; Mommsen, T.P.; Soengas, J.L. Glucosensing and glucose homeostasis: From fish to mammals. Comp. Biochem. Physiol. B 2011, 160, 123–149. [Google Scholar] [CrossRef]
- Laiz-Carrion, R.; Martin Del Rio, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Influence of cortisol on osmoregulation and energy metabolism in gilthead seabream Sparus aurata. J. Exp. Zool. 2003, 298A, 105–118. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Deck, C.A.; Anderson, W.G.; Conlon, J.M.; Walsh, P.J. The activity of the rectal gland of the North Pacific spiny dogfish Squalus suckleyi is glucose dependent and stimulated by glucagon-like peptide-1. J. Comp. Physiol. B 2017, 187, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Aedo, J.E.; Ruiz-Jarabo, I.; Martinez-Rodriguez, G.; Boltana, S.; Molina, A.; Valdes, J.A.; Mancera, J.M. Contribution of non-canonical cortisol actions in the early modulation of glucose metabolism of gilthead sea bream (Sparus aurata). Front. Endocrinol. 2019, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Aedo, J.E.; Zuloaga, R.; Boltana, S.; Molina, A.; Valdes, J.A. Membrane-initiated cortisol action modulates early pyruvate dehydrogenase kinase 2 (pdk2) expression in fish skeletal muscle. Comp. Biochem. Physiol. A 2019, 233, 24–29. [Google Scholar] [CrossRef]
- Aedo, J.; Aravena-Canales, D.; Ruiz-Jarabo, I.; Oyarzún, R.; Molina, A.; Martínez-Rodríguez, G.; Valdés, J.A.; Mancera, J.M. Differential metabolic and transcriptional responses of gilthead seabream (Sparus aurata) administered with cortisol or cortisol-BSA. Animals 2021, 11, 3310. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; Sánchez-García, F.; Sobrino, I.; Mancera, J.M.; Ruiz-Jarabo, I. Air exposure in catshark (Scyliorhinus canicula) modify muscle texture properties: A pilot study. Fishes 2018, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Barragán-Méndez, C.; Ruiz-Jarabo, I.; Fuentes, J.; Mancera, J.M.; Sobrino, I. Survival rates and physiological recovery responses in the lesser-spotted catshark (Scyliorhinus canicula) after bottom-trawling. Comp. Biochem. Physiol. A 2019, 233, 1–9. [Google Scholar] [CrossRef]
- Deck, C.A.; Bockus, A.B.; Seibel, B.A.; Walsh, P.J. Effects of short-term hyper- and hypo-osmotic exposure on the osmoregulatory strategy of unfed North Pacific spiny dogfish (Squalus suckleyi). Comp. Biochem. Physiol. A 2016, 193, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.M.; Walsh, P.J.; Kajimura, M.; McClelland, G.B.; Chew, S.F. The influence of feeding and fasting on plasma metabolites in the dogfish shark (Squalus acanthias). Comp. Biochem. Physiol. A 2010, 155, 435–444. [Google Scholar] [CrossRef]
- Wood, C.M.; Kajimura, M.; Bucking, C.; Walsh, P.J. Osmoregulation, ionoregulation and acid-base regulation by the gastrointestinal tract after feeding in the elasmobranch (Squalus acanthias). J. Exp. Biol. 2007, 210, 1335–1349. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Arjona, F.J.; Laiz-Carrion, R.; Flik, G.; Klaren, P.H.M.; Mancera, J.M. Energy metabolism of hyperthyroid gilthead sea bream Sparus aurata L. Comp. Biochem. Physiol. A 2016, 191, 25–34. [Google Scholar] [CrossRef]
- Arjona, F.J.; Vargas-Chacoff, L.; Martin Del Rio, M.P.; Flik, G.; Mancera, J.M.; Klaren, P.H.M. Effects of cortisol and thyroid hormone on peripheral outer ring deiodination and osmoregulatory parameters in the Senegalese sole (Solea senegalensis). J. Endocrinol. 2011, 208, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.; Brinca, L.; Guerreiro, P.M.; Power, D.M. PRL and GH synthesis and release from the sea bream (Sparus auratus L.) pituitary gland in vitro in response to osmotic challenge. Gen. Comp. Endocrinol. 2010, 168, 95–102. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, I.A.; Olinga, P.; de Jager, M.H.; Merema, M.T.; de Kanter, R.; van de Kerkhof, E.G.; Groothuis, G.M. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010, 5, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, A.; Ranganathan, S.; Baskar, K.; Bhuvaneswari, S.; Skandaprasad, S.; Gajalakshmi, P.; Chatterjee, S. Developing a partially perfused liver model using caprine liver explant conditioned to the chicken extra-embryonic perfusion system. Tissue Cell 2020, 62, 101308. [Google Scholar] [CrossRef]
- Cadepond, F.; Ulmann, A.; Baulieu, E.E. RU486 (mifepristone): Mechanisms of action and clinical uses. Annu. Rev. Med. 1997, 48, 129–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, M.; Guennoun, R.; Ghoumari, A.; Massaad, C.; Robert, F.; El-Etr, M.; Akwa, Y.; Rajkowski, K.; Baulieu, E.E. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr. Rev. 2007, 28, 387–439. [Google Scholar] [CrossRef] [Green Version]
- Katsu, Y.; Shariful, I.M.D.; Lin, X.; Takagi, W.; Urushitani, H.; Kohno, S.; Hyodo, S.; Baker, M.E. N-terminal domain regulates steroid activation of elephant shark glucocorticoid and mineralocorticoid receptors. J. Steroid Biochem. Mol. Biol. 2021, 210, 105845. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Klaren, P.H.M.; Louro, B.; Martos-Sitcha, J.A.; Pinto, P.I.S.; Vargas-Chacoff, L.; Flik, G.; Martínez-Rodríguez, G.; Power, D.M.; Mancera, J.M.; et al. Characterization of the peripheral thyroid system of gilthead seabream acclimated to different ambient salinities. Comp. Biochem. Physiol. A 2017, 203, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Costas, B.; Conceicao, L.; Aragao, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Jorgensen, C.; Brande-Lavridsen, N.; Engelund, M.; Olesen, J.H.; Madsen, S.S. Effects of cortisol, growth hormone and prolactin on gill claudin expression in Atlantic salmon. Gen. Comp. Endocrinol. 2009, 163, 270–277. [Google Scholar] [CrossRef]
- Kiilerich, P.; Kristiansen, K.; Madsen, S.S. Cortisol regulation of ion transporter mRNA in Atlantic salmon gill and the effect of salinity on the signaling pathway. J. Endocrinol. 2007, 194, 417–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Joy, K.P. Stress hormones modulate lipopolysaccharide stimulation of head kidney interleukin-6 production in the catfish Heteropneustes fossilis: In vivo and in vitro studies. Gen. Comp. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Bottino, M.C.; Cerliani, J.P.; Rojas, P.; Giulianelli, S.; Soldati, R.; Mondillo, C.; Gorostiaga, M.A.; Pignataro, O.P.; Calvo, J.C.; Gutkind, J.S.; et al. Classical membrane progesterone receptors in murine mammary carcinomas: Agonistic effects of progestins and RU-486 mediating rapid non-genomic effects. Breast Cancer Res. Treat. 2011, 126, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Prange-Kiel, J.; Rune, G.M.; Wallwiener, D.; Kiesel, L. Inhibition of proliferation and differentiation by RU 486 in human endometrial stromal and epithelial cells in vitro. Exp. Clin. Endocrinol. Diabetes 2000, 108, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Dasmahapatra, A.K.; Lee, P.C. Down regulation of CYP 1A1 by glucocorticoids in trout hepatocytes in vitro. In Vitro Cell. Dev. Biol. Anim. 1993, 29A, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Jain-Schlaepfer, S.; Zolderdo, A.J.; Algera, D.A.; Gilour, K.M.; Gallagher, A.J.; Cooke, S.J. Are 3-minutes good enough for obtaining baseline physiological samples from teleost fish? Can. J. Zool. 2018, 96, 774–786. [Google Scholar] [CrossRef]
- Keppler, D.; Decker, K. Glycogen determination with amyloglucosidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; Volume 3, pp. 1127–1131. [Google Scholar]
- Keevil, B.G. LC-MS/MS analysis of steroids in the clinical laboratory. Clin. Biochem. 2016, 49, 989–997. [Google Scholar] [CrossRef]
- Szeitz, A.; Manji, J.; Riggs, K.W.; Thamboo, A.; Javer, A.R. Validated assay for the simultaneous determination of cortisol and budesonide in human plasma using ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharmacol. Biomed. Anal. 2014, 90, 198–206. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Armour, K.J.; O’Toole, L.B.; Hazon, N. The effect of dietary protein restriction on the secretory dynamics of 1 alpha-hydroxycorticosterone and urea in the dogfish, Scyliorhinus canicula: A possible role for 1 alpha-hydroxycorticosterone in sodium retention. J. Endocrinol. 1993, 138, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hazon, N.; Henderson, I.W. Secretory dynamics of 1 alpha-hydroxycorticosterone in the elasmobranch fish, Scyliorhinus canicula. J. Endocrinol. 1984, 103, 205–211. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Gonçalves, O.; Pâscoa, I.; Martín del Río, M.P.; Mancera, J.M. Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 2009, 287, 419–426. [Google Scholar] [CrossRef]
- Shadwick, R.E.; Farrell, A.P.; Brauner, C. Physiology of Elasmobranch Fishes: Internal Processes; Academic Press: London, UK, 2015; Volume 34B, p. 580. [Google Scholar]
- Gomez-Munoz, A.; Hales, P.; Brindley, D.N.; Sancho, M.J. Rapid activation of glycogen phosphorylase by steroid hormones in cultured rat hepatocytes. Biochem. J. 1989, 262, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cliff, G.; Thurman, G.D. Pathological effects of stress during capture and transport in the juvenile dusky shark, Carcharhinus obscurus. Comp. Biochem. Physiol. A 1984, 78, 167–173. [Google Scholar] [CrossRef]
- Hoffmayer, E.R.; Parsons, G.R. The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiol. Biochem. 2001, 25, 277–285. [Google Scholar] [CrossRef]
- Martins, C.L.; Walker, T.I.; Reina, R.D. Stress-related physiological changes and post-release survival of elephant fish (Callorhinchus milii) after longlining, gillnetting, angling and handling in a controlled setting. Fish. Res. 2018, 204, 116–124. [Google Scholar] [CrossRef]
- Kneebone, J.; Chisholm, J.; Bernal, D.; Skomal, G. The physiological effects of capture stress, recovery, and post-release survivorship of juvenile sand tigers (Carcharias taurus) caught on rod and reel. Fish. Res. 2013, 147, 103–114. [Google Scholar] [CrossRef]
- Brill, R.; Bushnell, P.; Schroff, S.; Seifert, R.; Galvin, M. Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J. Exp. Mar. Biol. Ecol. 2008, 354, 132–143. [Google Scholar] [CrossRef]
- Ellis, J.R.; McCully Phillips, S.R.; Poisson, F. A review of capture and post-release mortality of elasmobranchs. J. Fish. Biol. 2017, 90, 653–722. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.M.; Ortlund, E.A.; Thornton, J.W. Mechanisms for the evolution of a derived function in the ancestral glucocorticoid receptor. PLoS Genet. 2011, 7, e1002117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bury, N.R.; Sturm, A.; Le Rouzic, P.; Lethimonier, C.; Ducouret, B.; Guiguen, Y.; Robinson-Rechavi, M.; Laudet, V.; Rafestin-Oblin, M.E.; Prunet, P. Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 2003, 31, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiilerich, P.; Triqueneaux, G.; Christensen, N.M.; Trayer, V.; Terrien, X.; Lombes, M.; Prunet, P. Interaction between the trout mineralocorticoid and glucocorticoid receptors in vitro. J. Mol. Endocrinol. 2015, 55, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Iwama, G.K. Stress in fish. Ann. N. Y. Acad. Sci. 1998, 851, 304–310. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Madsen, S.S. Distinct hormonal regulation of Na(+),K(+)-atpase genes in the gill of Atlantic salmon (Salmo salar L.). J. Endocrinol. 2009, 203, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Deck, C.A.; LeMoine, C.M.; Walsh, P.J. Phylogenetic analysis and tissue distribution of elasmobranch glucose transporters and their response to feeding. Biol. Open 2016, 5, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.G.; Nawata, C.M.; Wood, C.M.; Piercey-Normore, M.D.; Weihrauch, D. Body fluid osmolytes and urea and ammonia flux in the colon of two chondrichthyan fishes, the ratfish, Hydrolagus colliei, and spiny dogfish, Squalus acanthias. Comp. Biochem. Physiol. A 2012, 161, 27–35. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; Sobrino, I.; Marin-Rincon, A.; Fernandez-Boo, S.; Costas, B.; Mancera, J.M.; Ruiz-Jarabo, I. Acute-stress biomarkers in three Octopodidae species after bottom trawling. Front. Physiol. 2019, 10, 784. [Google Scholar] [CrossRef] [Green Version]
- Roldán, A.; van Muiswinkel, G.C.J.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physico chemical and sensory characteristics. Food Chem. 2011, 126, 574–582. [Google Scholar] [CrossRef]
| Tissue | Parameter | Control | Dexamethasone | p-Value |
|---|---|---|---|---|
| Plasma | Haematocrit (%) | 18.1 ± 3.3 | 17.3 ± 2.5 | 0.593 |
| pH | 7.69 ± 0.05 | 7.66 ± 0.05 | 0.231 | |
| Glucose (mM) | 1.91 ± 0.60 | 2.31 ± 1.10 | 0.421 | |
| Lactate (mM) | 0.85 ± 0.71 | 0.72 ± 0.36 | 0.683 | |
| Liver | HSI | 3.26 ± 0.78 | 3.70 ± 1.09 | 0.401 |
| Glucose (mg g−1) | 2.65 ± 0.69 | 2.45 ± 0.59 | 0.568 | |
| Lactate (mg g−1) | 1.39 ± 1.22 | 1.19 ± 0.52 | 0.697 | |
| HK act. (U mg prot−1) | 0.08 ± 0.04 | 0.07 ± 0.03 | 0.614 | |
| PK act. (U mg prot−1) | 3.20 ± 0.71 | 3.28 ± 0.75 | 0.354 | |
| LDH act. (U mg prot−1) | 0.02 ± 0.02 | 0.01 ± 0.02 | 0.119 | |
| FBP act. (U mg prot−1) | 0.90 ± 0.16 | 0.97 ± 0.15 | 0.437 | |
| Muscle | Water content (%) | 68.7 ± 2.63 | 73.0 ± 2.27 * | 0.006 |
| Glucose (mg g−1) | 3.13 ± 1.3 | 2.88 ± 0.99 | 0.704 | |
| Lactate (mg g−1) | 36.1 ± 7.78 | 42.0 ± 3.80 | 0.099 | |
| HK act. (U mg prot−1) | 0.17 ± 0.06 | 0.33 ± 0.15 * | 0.019 | |
| PK act. (U mg prot−1) | 71.4 ± 17.32 | 108.9 ± 10.45 * | 0.000 | |
| LDH act. (U mg prot−1) | 0.37 ± 0.11 | 0.60 ± 0.17 * | 0.014 | |
| FBP act. (U mg prot−1) | 0.39 ± 0.20 | 0.29 ± 0.12 | 0.304 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Busto, J.; Mancera, J.M.; Ruiz-Jarabo, I. Cortisol and Dexamethasone Mediate Glucocorticoid Actions in the Lesser Spotted Catshark (Scyliorhinus canicula). Biology 2022, 11, 56. https://doi.org/10.3390/biology11010056
Cabrera-Busto J, Mancera JM, Ruiz-Jarabo I. Cortisol and Dexamethasone Mediate Glucocorticoid Actions in the Lesser Spotted Catshark (Scyliorhinus canicula). Biology. 2022; 11(1):56. https://doi.org/10.3390/biology11010056
Chicago/Turabian StyleCabrera-Busto, Juncal, Juan M. Mancera, and Ignacio Ruiz-Jarabo. 2022. "Cortisol and Dexamethasone Mediate Glucocorticoid Actions in the Lesser Spotted Catshark (Scyliorhinus canicula)" Biology 11, no. 1: 56. https://doi.org/10.3390/biology11010056
APA StyleCabrera-Busto, J., Mancera, J. M., & Ruiz-Jarabo, I. (2022). Cortisol and Dexamethasone Mediate Glucocorticoid Actions in the Lesser Spotted Catshark (Scyliorhinus canicula). Biology, 11(1), 56. https://doi.org/10.3390/biology11010056








