Unexpected Terrain Induced Changes in Cortical Activity in Bipedal-Walking Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
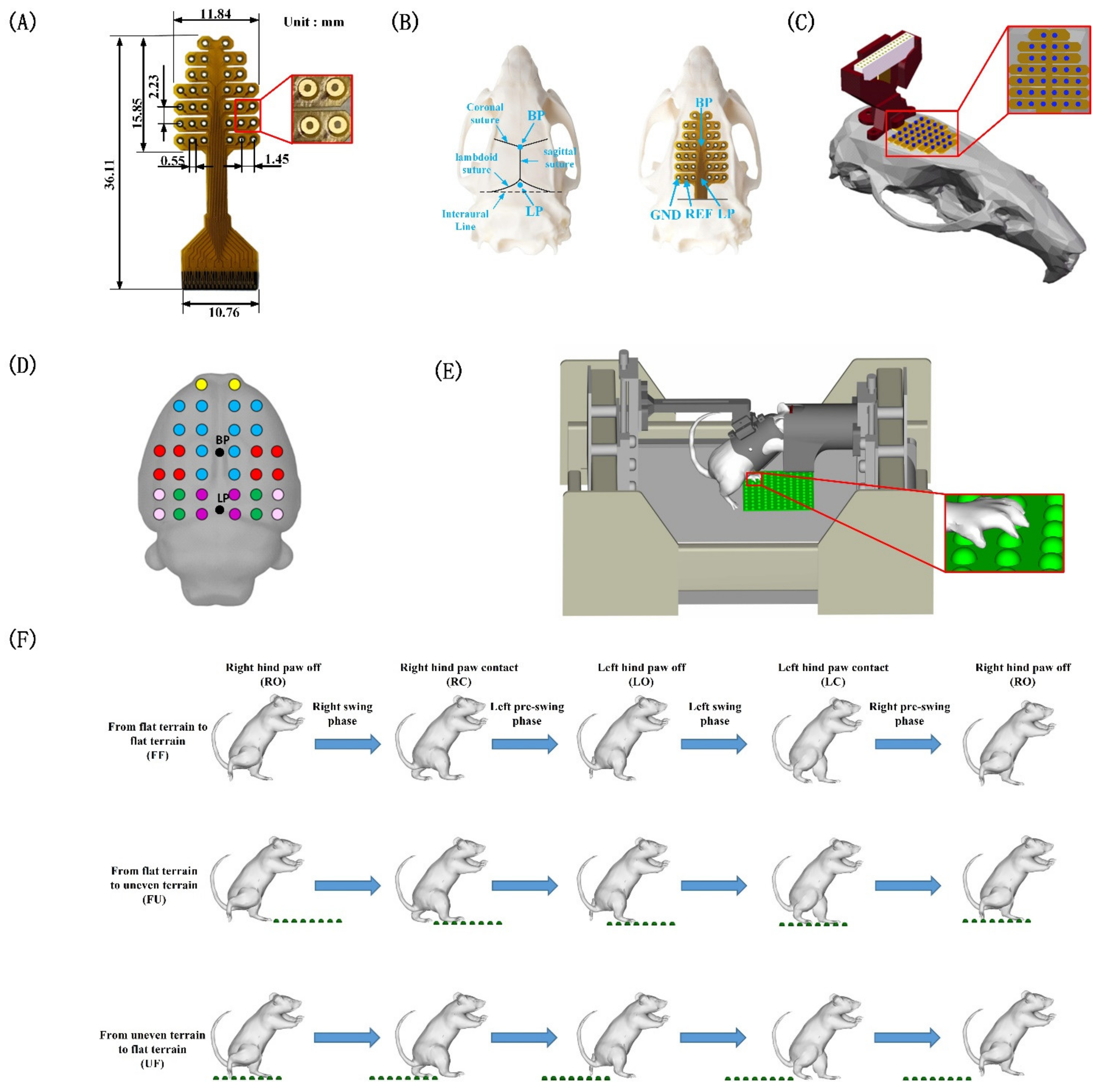
2.2. Experimental Design
2.3. Surgical Procedures
2.4. Data Acquisition
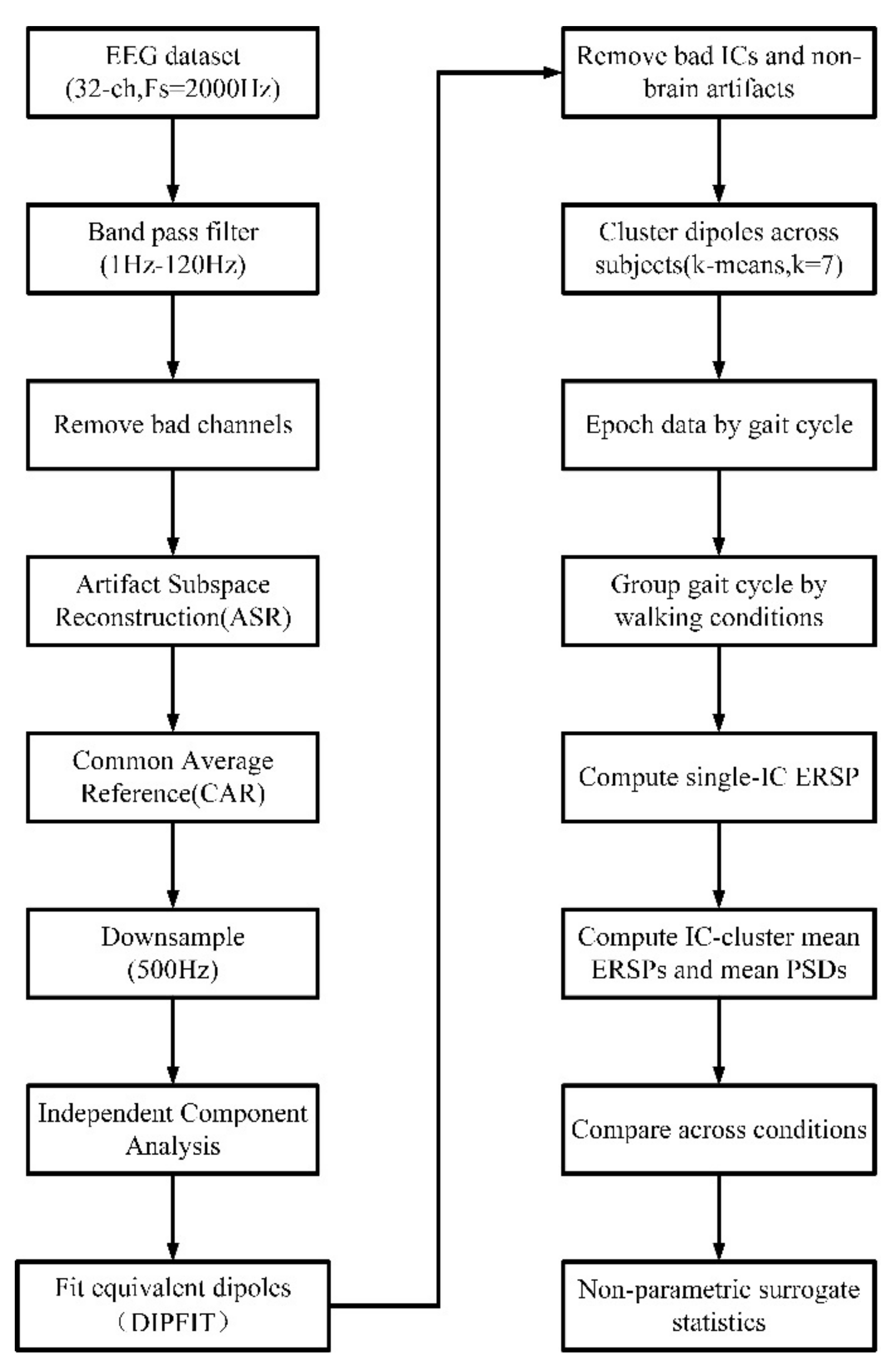
2.5. EEG Analysis
3. Results
3.1. Behavioral Analysis
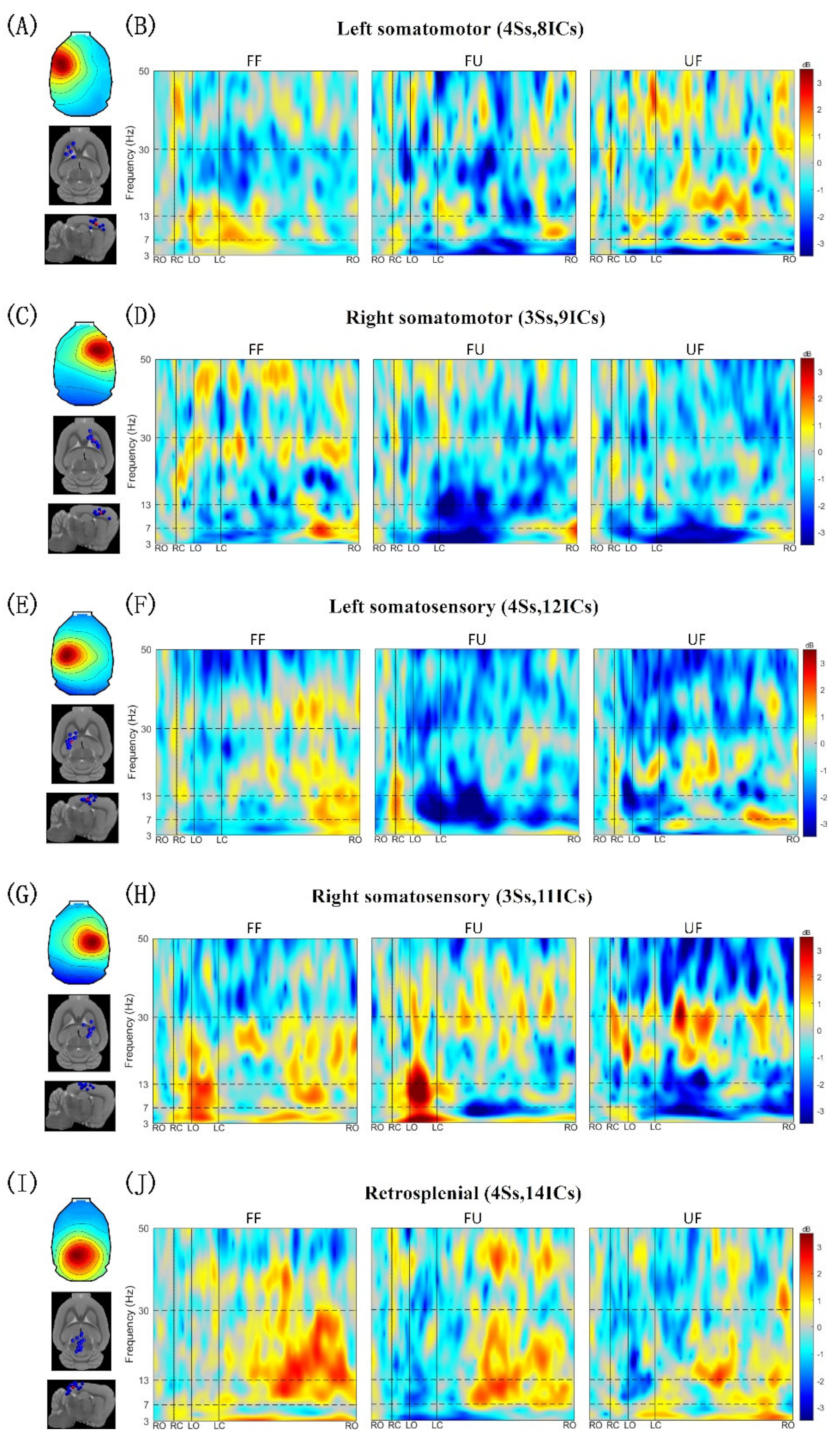
3.2. Clusters of Independent Components
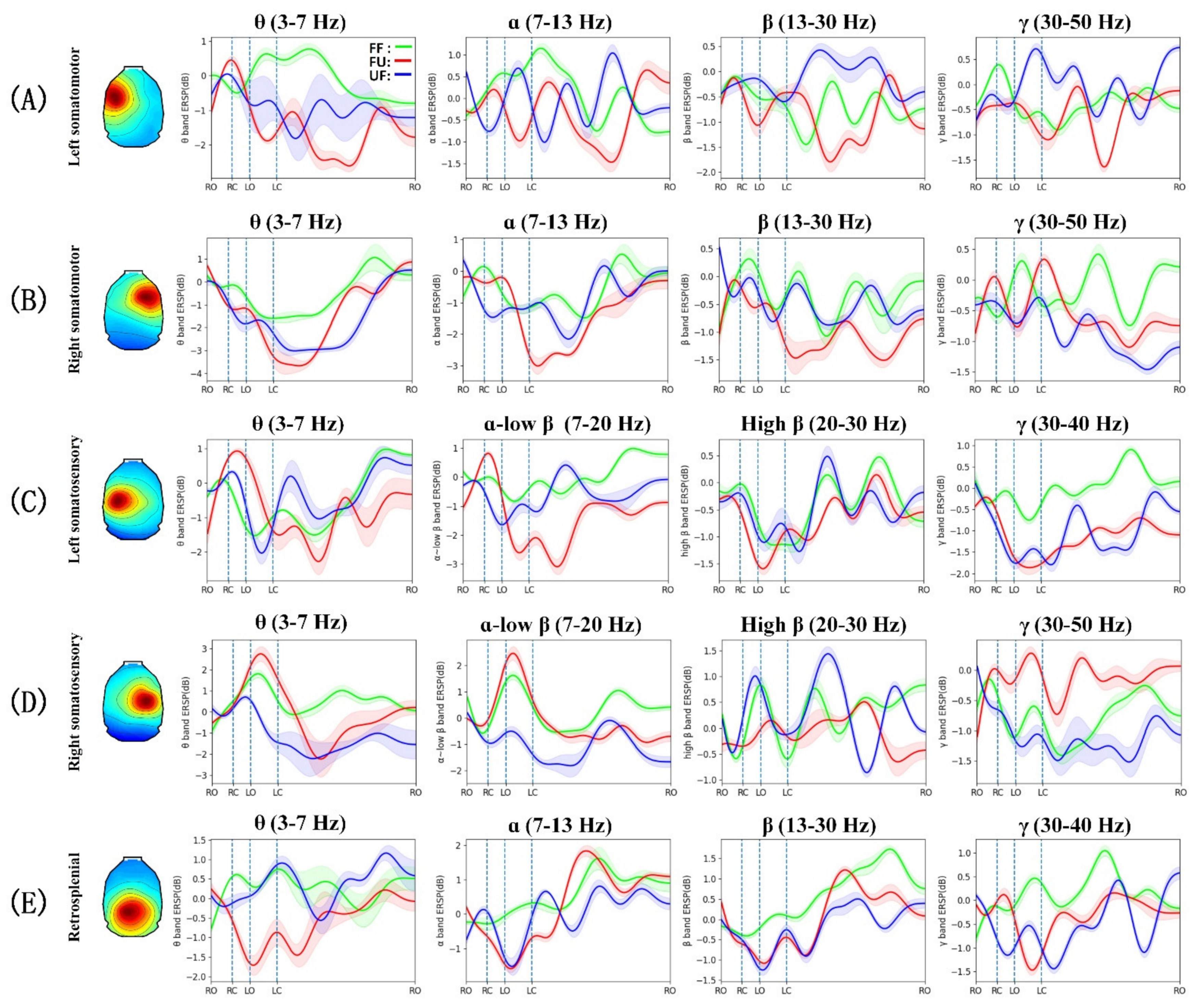
3.3. Event-Related Spectral Perturbation
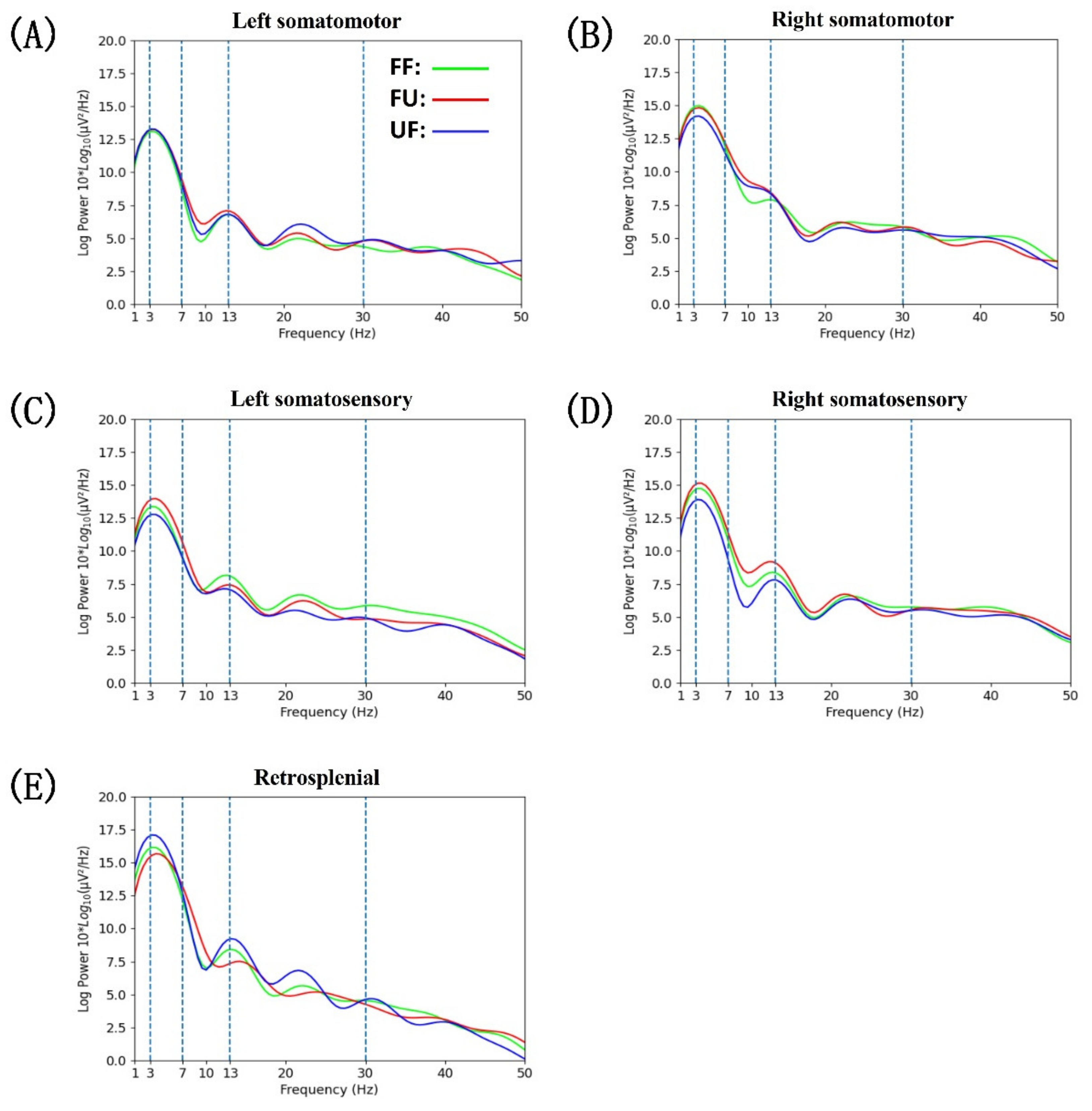
3.4. Power Spectral Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marigold, D.S.; Patla, A.E. Strategies for dynamic stability during locomotion on a slippery surface: Effects of prior experience and knowledge. J. Neurophysiol. 2002, 88, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Thies, S.B.; Richardson, J.K.; Ashton-Miller, J.A. Effects of surface irregularity and lighting on step variability during gait: A study in healthy young and older women. Gait Posture 2005, 22, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Daley, M.A.; Usherwood, J.R.; Felix, G.; Biewener, A.A. Running over rough terrain: Guinea fowl maintain dynamic stability despite a large unexpected change in substrate height. J. Exp. Biol. 2006, 209, 171–187. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical Walking Pattern Changes in the Fit and Healthy Elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, M.H.; Marigold, D.S.; Gabreëls, F.J.M.; Duysens, J. Muscle reflexes and synergies triggered by an unexpected support surface height during walking. J. Neurophysiol. 2007, 97, 3639–3650. [Google Scholar] [CrossRef]
- Shinya, M.; Fujii, S.; Oda, S. Corrective postural responses evoked by completely unexpected loss of ground support during human walking. Gait Posture 2009, 29, 483–487. [Google Scholar] [CrossRef]
- Stuart, D.G.; Hultborn, H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res. Rev. 2008, 59, 74–95. [Google Scholar] [CrossRef]
- Armstrong, B.Y.D.M. The supraspinal control of mammalian locomotion. J. Physiol. 1988, 405, 1–37. [Google Scholar] [CrossRef]
- Grillner, S. Neurobiological bases of rhythmic motor acts in vertebrates. Science 1985, 228, 143–149. [Google Scholar] [CrossRef]
- Petersen, T.H.; Willerslev-Olsen, M.; Conway, B.A.; Nielsen, J.B. The motor cortex drives the muscles during walking in human subjects. J. Physiol. 2012, 590, 2443–2452. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Faster Gait Speeds Reduce Alpha and Beta EEG Spectral Power from Human Sensorimotor Cortex. IEEE Trans. Biomed. Eng. 2020, 67, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Roeder, L.; Boonstra, T.W.; Kerr, G.K. Corticomuscular control of walking in older people and people with Parkinson’s disease. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bodda, S.; Maya, S.; Em Potti, M.N.; Sohan, U.; Bhuvaneshwari, Y.; Mathiyoth, R.; Diwakar, S. Computational analysis of EEG activity during stance and swing gait phases. Procedia Comput. Sci. 2020, 171, 1591–1597. [Google Scholar] [CrossRef]
- Roeder, L.; Boonstra, T.W.; Smith, S.S.; Kerr, G.K. Dynamics of corticospinal motor control during overground and treadmill walking in humans. J. Neurophysiol. 2018, 120, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage 2011, 54, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. Neuroimage 2012, 63, 1203–1211. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Restricted vision increases sensorimotor cortex involvement in human walking. J. Neurophysiol. 2017, 118, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Bradford, J.C.; Lukos, J.R.; Ferris, D.P. Electrocortical activity distinguishes between uphill and level walking in humans. J. Neurophysiol. 2016, 115, 958–966. [Google Scholar] [CrossRef]
- Drew, T. Motor cortical cell discharge during voluntary gait modification. Brain Res. 1988, 457, 181–187. [Google Scholar] [CrossRef]
- Drew, T.; Andujar, J.E.; Lajoie, K.; Yakovenko, S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res. Rev. 2008, 57, 199–211. [Google Scholar] [CrossRef]
- DiGiovanna, J.; Dominici, N.; Friedli, L.; Rigosa, J.; Duis, S.; Kreider, J.; Beauparlant, J.; van den Brand, R.; Schieppati, M.; Micera, S.; et al. Engagement of the rat hindlimb motor cortex across natural locomotor behaviors. J. Neurosci. 2016, 36, 10440–10455. [Google Scholar] [CrossRef]
- Li, B.; Zhang, M.; Liu, Y.; Hu, D.; Zhao, J.; Tang, R.; Lang, Y.; He, J. Rat locomotion detection based on brain functional directed connectivity from implanted electroencephalography signals. Brain Sci. 2021, 11, 345. [Google Scholar] [CrossRef]
- Rigosa, J.; Panarese, A.; Dominici, N.; Friedli, L.; Van Den Brand, R.; Carpaneto, J.; Digiovanna, J.; Courtine, G.; Micera, S. Decoding bipedal locomotion from the rat sensorimotor cortex. J. Neural Eng. 2015, 12, 056014. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, G.; Aprigliano, F.; Micera, S.; Monaco, V.; De Venuto, D. EEG/EMG based Architecture for the Early Detection of Slip-induced Lack of Balance. In Proceedings of the 2019 IEEE 8th International Workshop on Advances in Sensors and Interfaces (IWASI), Otranto, Italy, 13–14 June 2019; pp. 9–14. [Google Scholar] [CrossRef]
- Tse, Y.Y.F.; Petrofsky, J.S.; Berk, L.; Daher, N.; Lohman, E.; Laymon, M.S.; Cavalcanti, P. Postural sway and Rhythmic Electroencephalography analysis of cortical activation during eight balance training tasks. Med. Sci. Monit. 2013, 19, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Büchel, D.; Lehmann, T.; Ullrich, S.; Cockcroft, J.; Louw, Q.; Baumeister, J. Stance leg and surface stability modulate cortical activity during human single leg stance. Exp. Brain Res. 2021, 239, 1193–1202. [Google Scholar] [CrossRef]
- Mierau, A.; Pester, B.; Hülsdünker, T.; Schiecke, K.; Strüder, H.K.; Witte, H. Cortical Correlates of Human Balance Control. Brain Topogr. 2017, 30, 434–446. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Human electrocortical dynamics while stepping over obstacles. Sci. Rep. 2019, 9, 4693. [Google Scholar] [CrossRef]
- Sipp, A.R.; Gwin, J.T.; Makeig, S.; Ferris, D.P. Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J. Neurophysiol. 2013, 110, 2050–2060. [Google Scholar] [CrossRef]
- Solis-escalante, T.; Van Der Cruijsen, J.; De Kam, D. Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. Neuroimage 2019, 188, 557–571. [Google Scholar] [CrossRef]
- Beloozerova, I.N.; Sirota, M.G.; Swadlow, H.A.; Orlovsky, G.N.; Popova, L.B.; Deliagina, T.G. Activity of different classes of neurons of the motor cortex during postural corrections. J. Neurosci. 2003, 23, 7844–7853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beloozerova, I.N.; Sirota, M.G.; Orlovsky, G.N.; Deliagina, T.G. Activity of pyramidal tract neurons in the cat during postural corrections. J. Neurophysiol. 2005, 93, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Brower, M.; Grace, M.; Kotz, C.M.; Koya, V. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Lab. Anim. Res. 2015, 31, 166. [Google Scholar] [CrossRef]
- Langer, M.; Brandt, C.; Löscher, W. Marked strain and substrain differences in induction of status epilepticus and subsequent development of neurodegeneration, epilepsy, and behavioral alterations in rats. Strain and substrain differences in an epilepsy model in rats. Epilepsy Res. 2011, 96, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Havnes, M.B.; Widerøe, M.; Thuen, M.; Torp, S.H.; Brubakk, A.O.; Møllerløkken, A. Simulated dive in rats lead to acute changes in cerebral blood flow on MRI, but no cerebral injuries to grey or white matter. Eur. J. Appl. Physiol. 2013, 113, 1405–1414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Golozoubova, V.; Brodersen, T.K.; Klastrup, S.; Oksama, M.; Løgsted, J.; Makin, A. Repeated measurements of motor activity in rats in long-term toxicity studies. J. Pharmacol. Toxicol. Methods 2014, 70, 241–245. [Google Scholar] [CrossRef]
- Hestehave, S.; Abelson, K.S.; Brønnum Pedersen, T.; Munro, G. Stress sensitivity and cutaneous sensory thresholds before and after neuropathic injury in various inbred and outbred rat strains. Behav. Brain Res. 2019, 375, 112149. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Mullen, T.; Kothe, C.; Chi, Y.M.; Ojeda, A.; Kerth, T.; Makeig, S.; Cauwenberghs, G.; Jung, T.P. Real-time modeling and 3D visualization of source dynamics and connectivity using wearable EEG. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2184–2187. [Google Scholar] [CrossRef]
- MacQueen, J. Some methods for classification and analysis of multivariate observations. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Los Angeles, CA, USA, 21 June–18 July 1965; University of California: Los Angeles, CA, USA, 1967; Volume 1, pp. 281–297. [Google Scholar]
- Makeig, S. Auditor-Event-Related Dynamics of the EEG Spectrum. Electroencephalogr. Clin. Neurophysiol. 1993, 86, 20. [Google Scholar] [CrossRef]
- Dempster, J. Signal Analysis and Measurement. In The Laboratory Computer; Academic Press: Cambridge, MA, USA, 2001; pp. 136–171. [Google Scholar] [CrossRef]
- Widajewicz, W.; Kably, B.; Drew, T. Motor cortical activity during voluntary gait modifications in the cat. II. Cells related to the hindlimbs. J. Neurophysiol. 1994, 72, 2070–2089. [Google Scholar] [CrossRef]
- Beloozerova, I.N.; Sirota, M.G. Cortically controlled gait adjustments in the cat. Ann. N. Y. Acad. Sci. 1998, 860, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Karayannidou, A.; Beloozerova, I.N.; Zelenin, P.V.; Stout, E.E.; Sirota, M.G.; Orlovsky, G.N.; Deliagina, T.G. Activity of pyramidal tract neurons in the cat during standing and walking on an inclined plane. J. Physiol. 2009, 587, 3795–3811. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Hansen, E.; Beyer, L.; Conradi, M.L.; Merten, F.; Nichelmann, C.; Rost, R.; Zippel, C. Activation processes during mental practice in stroke patients. Int. J. Psychophysiol. 1994, 17, 91–100. [Google Scholar] [CrossRef]
- Autret, A.; Auvert, L.; Laffont, F.; Larmande, P. Electroencephalographic spectral power and lateralized motor activities. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 228–236. [Google Scholar] [CrossRef]
- Weiss, T.; Sust, M.; Beyer, L.; Hansen, E.; Rost, R.; Schmalz, T. Theta power decreases in preparation for voluntary isometric contractions performed with maximal subjective effort. Neurosci. Lett. 1995, 193, 153–156. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Brzezicka, A.; Jan Kamiński1, C.M.R.; Chung, J.M.; Mamelak, A.N. Working Memory Load-related Theta Power Decreases in Dorsolateral Prefrontal Cortex Predict Individual Differences in Performance. J. Cogn. Neurosci. 2019, 31, 1290–1307. [Google Scholar] [CrossRef]
- Gorassini, M.A.; Prochazka, A.; Hiebert, G.W.; Gauthier, M.J.A. Corrective responses to loss of ground support during walking I. Intact cats. J. Neurophysiol. 1994, 71, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Makeig, S.; Gola, M.; Neuper, C.; Müller-Putz, G. Distinct β band oscillatory networks subserving motor and cognitive control during gait adaptation. J. Neurosci. 2016, 36, 2212–2226. [Google Scholar] [CrossRef]
- Bulea, T.C.; Kim, J.; Damiano, D.L.; Stanley, C.J.; Park, H.S. Prefrontal, posterior parietal and sensorimotor network activity underlying speed control during walking. Front. Hum. Neurosci. 2015, 9, 247. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003, 18, 35–46. [Google Scholar] [CrossRef]
- Luu, T.P.; Brantley, J.A.; Nakagome, S.; Zhu, F.; Contreras-Vidal, J.L. Electrocortical correlates of human level-ground, slope, and stair walking. PLoS ONE 2017, 12, e0188500. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, S.; McCrea, D.A.; Stecina, K.; Prochazka, A. Control of locomotor cycle durations. J. Neurophysiol. 2005, 94, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E. Gait Changes in Older Adults: Indicators of Fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Ferrandez, A.M.; Pailhous, J.; Durup, M. Slowness in elderly gait. Exp. Aging Res. 1990, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Ikeda, A.; Kunieda, T.; Yazawa, S.; Baba, K.; Nagamine, T.; Taki, W.; Hashimoto, N.; Mihara, T.; Shibasaki, H. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain 2000, 123, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B. How we walk: Central control of muscle activity during human walking. Neuroscientist 2003, 9, 195–204. [Google Scholar] [CrossRef]
- Chapin, J.K.; Woodward, D.J. Somatic sensory transmission to the cortex during movement: Gating of single cell responses to touch. Exp. Neurol. 1982, 78, 654–669. [Google Scholar] [CrossRef]
- Chapin, J.K.; Woodward, D.J. Modulation of sensory responsiveness of single somatosensory cortical cells during movement and arousal behaviors. Exp. Neurol. 1981, 72, 164–178. [Google Scholar] [CrossRef]
- Slobounov, S.; Cao, C.; Jaiswal, N.; Newell, K.M. Neural basis of postural instability identified by VTC and EEG. Exp. Brain Res. 2009, 199, 1–16. [Google Scholar] [CrossRef]
- Hülsdünker, T.; Mierau, A.; Neeb, C.; Kleinöder, H.; Strüder, H.K. Cortical processes associated with continuous balance control as revealed by EEG spectral power. Neurosci. Lett. 2015, 592, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, A.; Gritsenko, V.; Yakovenko, S. Sensory control of locomotion: Reflexes versus higher-level control. Adv. Exp. Med. Biol. 2002, 508, 357–367. [Google Scholar] [CrossRef]
- Pearson, K.G. Generating the walking gait: Role of sensory feedback. Prog. Brain Res. 2004, 143, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Gossard, J.; Rossignol, S. Dynamic Sensorimotor Interactions in Locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef]
- Maki, B.E.; Perry, S.D.; Nome, R.G.; Mcllroy, W.E. Effect of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 1999, 54, 281–287. [Google Scholar] [CrossRef]
- Cevallos, C.; Zarka, D.; Hoellinger, T.; Leroy, A.; Dan, B.; Cheron, G. Oscillations in the human brain during walking execution, imagination and observation. Neuropsychologia 2015, 79, 223–232. [Google Scholar] [CrossRef]
- Braun, C.; Hess, H.; Burkhardt, M.; Wühle, A.; Preissl, H. The right hand knows what the left hand is feeling. Exp. Brain Res. 2005, 162, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hlushchuk, Y.; Hari, R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J. Neurosci. 2006, 26, 5819–5824. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Hu, D.; Li, G.; Tang, R.; Song, D.; Lang, Y.; He, J. Electrocortical activity in freely walking rats varies with environmental conditions. Brain Res. 2021, 1751, 147188. [Google Scholar] [CrossRef]
- Wagner, J.; Solis-Escalante, T.; Scherer, R.; Neuper, C.; Müller-Putz, G. It’s how you get there: Walking down a virtual alley activates premotor and parietal areas. Front. Hum. Neurosci. 2014, 8, 93. [Google Scholar] [CrossRef]
- Jensen, O.; Lisman, J.E. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005, 28, 67–72. [Google Scholar] [CrossRef]
- Winters, B.D.; Reid, J.M. A distributed cortical representation underlies crossmodal object recognition in rats. J. Neurosci. 2010, 30, 6253–6261. [Google Scholar] [CrossRef] [PubMed]
- Hindley, E.L.; Nelson, A.J.D.; Aggleton, J.P.; Vann, S.D. Dysgranular retrosplenial cortex lesions in rats disrupt cross-modal object recognition. Learn. Mem. 2014, 21, 171–179. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Klimesch, W. Functional topography during a visuoverbal judgment task studied with event-related desynchronization mapping. J. Clin. Neurophysiol. 1992, 9, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes Da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Spiers, H.J.; Maguire, E.A. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage 2006, 31, 1826–1840. [Google Scholar] [CrossRef]
- Omlor, W.; Patino, L.; Hepp-Reymond, M.C.; Kristeva, R. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage 2007, 34, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
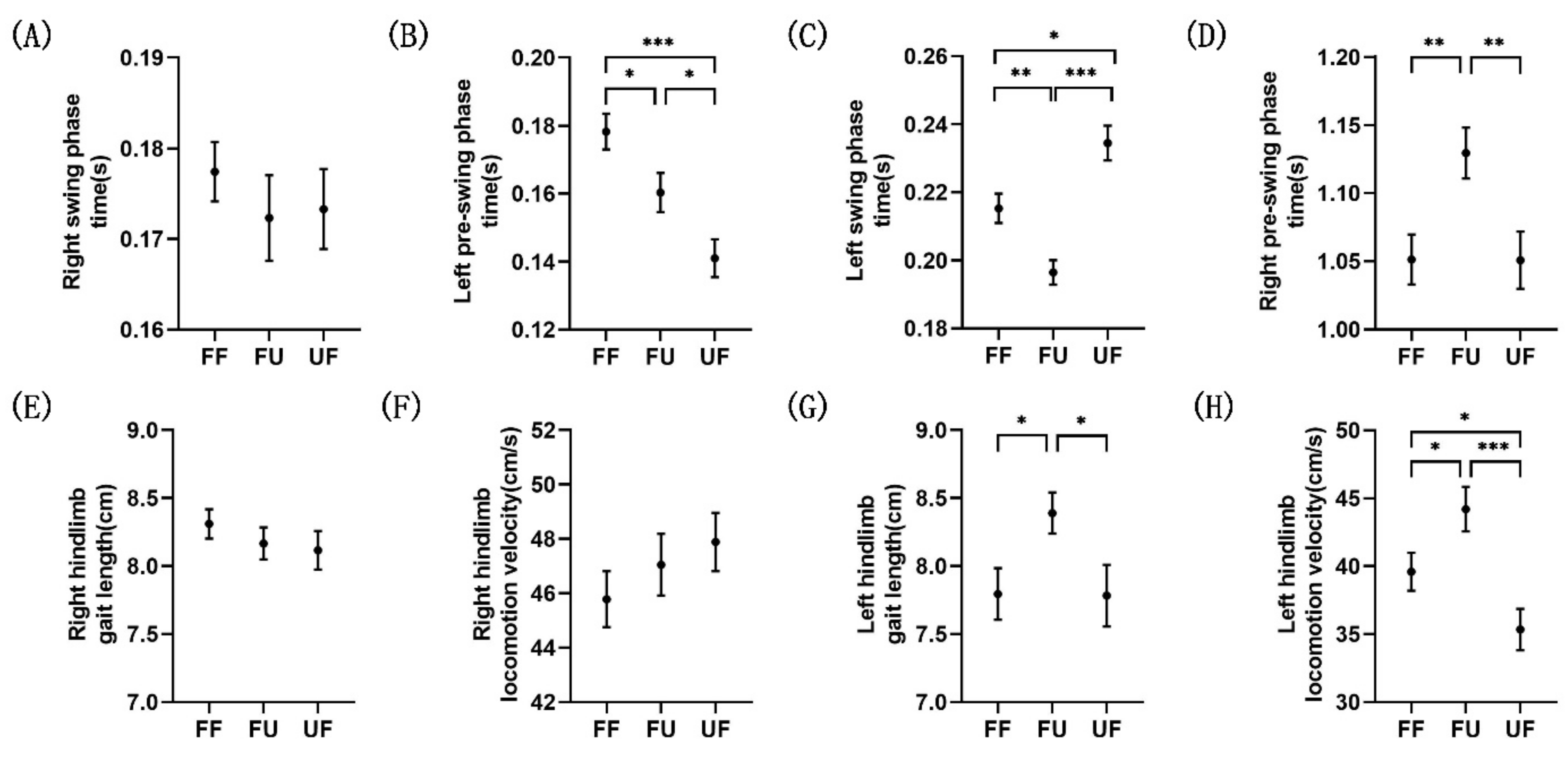
| Right Swing Phase | Left Pre-Swing Phase | Left Swing Phase | Right Pre-Swing Phase | ||
|---|---|---|---|---|---|
| Time (s) | FF | 0.1774 ± 0.0033 | 0.1782 ± 0.0053 | 0.2153 ± 0.0043 | 1.0513 ± 0.0183 |
| FU | 0.1723 ± 0.0047 | 0.1603 ± 0.0058 | 0.1964 ± 0.0036 | 1.1294 ± 0.0187 | |
| UF | 0.1733 ± 0.0044 | 0.1410 ± 0.0056 | 0.2345 ± 0.0051 | 1.0508 ± 0.0210 | |
| Gait length (cm) | FF | 8.31 ± 0.11 | 7.79 ± 0.19 | ||
| FU | 8.17 ± 0.12 | 8.39 ± 0.15 | |||
| UF | 8.12 ± 0.14 | 7.71 ± 0.22 | |||
| Locomotion velocity (cm/s) | FF | 45.78 ± 1.03 | 39.59 ± 1.39 | ||
| FU | 47.05 ± 1.14 | 44.20 ± 1.64 | |||
| UF | 47.88 ± 1.07 | 35.33 ± 1.53 |
| Cluster | Talairach Coordinates | Cortical Location | Number of Subjects and ICs |
|---|---|---|---|
| Left somatomotor | −26, 28, 52 | Left primary motor area | 4Ss, 8ICs |
| Right somatomotor | 24, 26, 51 | Right primary motor area | 3Ss, 9ICs |
| Left somatosensory | −32, −5, 60 | Left primary somatosensory area | 4Ss, 12ICs |
| Right somatosensory | 27, −7, 64 | Right primary somatosensory area | 3Ss, 11ICs |
| Retrosplenial | −3, −46, 62 | Retrosplenial area | 4Ss, 14ICs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, B.; Zhang, M.; Dai, C.; Xi, P.; Liu, Y.; Huang, Q.; He, J.; Lang, Y.; Tang, R. Unexpected Terrain Induced Changes in Cortical Activity in Bipedal-Walking Rats. Biology 2022, 11, 36. https://doi.org/10.3390/biology11010036
Liu H, Li B, Zhang M, Dai C, Xi P, Liu Y, Huang Q, He J, Lang Y, Tang R. Unexpected Terrain Induced Changes in Cortical Activity in Bipedal-Walking Rats. Biology. 2022; 11(1):36. https://doi.org/10.3390/biology11010036
Chicago/Turabian StyleLiu, Honghao, Bo Li, Minjian Zhang, Chuankai Dai, Pengcheng Xi, Yafei Liu, Qiang Huang, Jiping He, Yiran Lang, and Rongyu Tang. 2022. "Unexpected Terrain Induced Changes in Cortical Activity in Bipedal-Walking Rats" Biology 11, no. 1: 36. https://doi.org/10.3390/biology11010036
APA StyleLiu, H., Li, B., Zhang, M., Dai, C., Xi, P., Liu, Y., Huang, Q., He, J., Lang, Y., & Tang, R. (2022). Unexpected Terrain Induced Changes in Cortical Activity in Bipedal-Walking Rats. Biology, 11(1), 36. https://doi.org/10.3390/biology11010036






