Precision Preventive Medicine of Relapse in Smoking Cessation: Can MRI Inform the Search of Intermediate Phenotypes?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Anatomical
3.2. Restings State (RS)
3.3. Task-Based Studies
3.3.1. Functional Activation
Inhibition
Drug Cues or Healthy Messages
Smoking Cues vs. Neutral
Health Messages
Reward
3.3.2. Functional Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | anterior cingular cortex |
| DMN | Default Mode Network |
| FTND | Fagerstrom Test for Nicotine Dependence |
| GMV | grey matter volume |
| IC | inhibitory control |
| IFG | inferior frontal gyrus |
| MFG | middle frontal gyrus |
| MRI | Magnetic Resonance Imaging |
| MTG | middle temporal gyrus |
| NRT | nicotine replacement therapies |
| PCC | posterior cingulate cortex |
| PCS | psychological counselling sessions |
| PFC | prefrontal cortex |
| ReHo | regional homogeneity |
| ROIs | regions of interests |
| RS | resting-state |
| SFG | superior frontal gyrus |
| STG | superior temporal gyrus |
| STG | superior temporal gyrus |
| tbFC | task-based functional connectivity |
| VBM | voxel-based morphometry |
| WB | whole brain |
References
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- Ribassin-Majed, L.; Hill, C. Trends in Tobacco-Attributable Mortality in France. Eur. J. Public Health 2015, 25, 824–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hughes, J.R.; Keely, J.; Naud, S. Shape of the Relapse Curve and Long-Term Abstinence among Untreated Smokers. Addiction 2004, 99, 29–38. [Google Scholar] [CrossRef]
- Livingstone-Banks, J.; Norris, E.; Hartmann-Boyce, J.; West, R.; Jarvis, M.; Hajek, P. Relapse Prevention Interventions for Smoking Cessation. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- Piazza, P.V.; Deroche-Gamonet, V. A Multistep General Theory of Transition to Addiction. Psychopharmacology 2013, 229, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.R.; Hogarth, L.; Leventhal, A.M.; Cook, J.W.; Hitsman, B. Cigarette Smoking and Depression Comorbidity: Systematic Review & Proposed Theoretical Model. Addiction 2017, 112, 401–412. [Google Scholar] [PubMed]
- Fatseas, M.; Serre, F.; Alexandre, J.-M.; Debrabant, R.; Auriacombe, M.; Swendsen, J. Craving and Substance Use among Patients with Alcohol, Tobacco, Cannabis or Heroin Addiction: A Comparison of Substance- and Person-Specific Cues: Cues, Craving and Substance Use. Addiction 2015, 110, 1035–1042. [Google Scholar] [CrossRef]
- Cahill, K.; Stevens, S.; Perera, R.; Lancaster, T. Pharmacological Interventions for Smoking Cessation: An Overview and Network Meta-Analysis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Garcia-Rivas, V.; Deroche-Gamonet, V. Not All Smokers Appear to Seek Nicotine for the Same Reasons: Implications for Preclinical Research in Nicotine Dependence. Addict. Biol. 2019, 24, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, X.; Qian, W.; Shen, Z.; Zhang, M. Altered Human Brain Anatomy in Chronic Smokers: A Review of Magnetic Resonance Imaging Studies. Neurol. Sci. 2015, 36, 497–504. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, D.; Peng, P.; Wang, S.-K.; Liu, Z.; Qin, H.-Y.; Li, S.-S.; Wang, C. Effect of Smoking on Resting-State Functional Connectivity in Smokers: An FMRI Study: Effects of Smoking on Brain Function. Respirology 2017, 22, 1118–1124. [Google Scholar] [CrossRef]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.-O. The Fagerstrom Test for Nicotine Dependence: A Revision of the Fagerstrom Tolerance Questionnaire. Addiction 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease; National Academies Press: Washington, DC, USA, 2011; p. 13284. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Rudrauf, D.; Damasio, H.; Bechara, A. Damage to the Insula Disrupts Addiction to Cigarette Smoking. Science 2007, 315, 531–534. [Google Scholar] [CrossRef]
- Froeliger, B.; McConnell, P.A.; Bell, S.; Sweitzer, M.; Kozink, R.V.; Eichberg, C.; Hallyburton, M.; Kaiser, N.; Gray, K.M.; McClernon, F.J. Association Between Baseline Corticothalamic-Mediated Inhibitory Control and Smoking Relapse Vulnerability. JAMA Psychiatry 2017, 74, 379. [Google Scholar] [CrossRef]
- Froeliger, B.; Kozink, R.V.; Rose, J.E.; Behm, F.M.; Salley, A.N.; McClernon, F.J. Hippocampal and Striatal Gray Matter Volume Are Associated with a Smoking Cessation Treatment Outcome: Results of an Exploratory Voxel-Based Morphometric Analysis. Psychopharmacology 2010, 210, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, P.; Shen, Z.; Qian, W.; Li, K.; Luo, X.; Zeng, Q.; Guo, T.; Yu, H.; Yang, Y.; et al. Gray Matter Volumes of Insular Subregions Are Not Correlated with Smoking Cessation Outcomes but Negatively Correlated with Nicotine Dependence Severity in Chronic Smokers. Neurosci. Lett. 2019, 696, 7–12. [Google Scholar] [CrossRef]
- Qian, W.; Huang, P.; Shen, Z.; Wang, C.; Yang, Y.; Zhang, M. Brain Gray Matter Volume and Functional Connectivity Are Associated With Smoking Cessation Outcomes. Front. Hum. Neurosci. 2019, 13, 361. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Shen, Z.; Qian, W.; Jiaerken, Y.; Luo, X.; Li, K.; Zeng, Q.; Gu, Q.; Yang, Y.; et al. Increased Thalamic Volume and Decreased Thalamo-Precuneus Functional Connectivity Are Associated with Smoking Relapse. NeuroImage Clin. 2020, 28, 102451. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Shen, Z.; Wang, C.; Qian, W.; Zhang, H.; Yang, Y.; Zhang, M. Altered White Matter Integrity in Smokers Is Associated with Smoking Cessation Outcomes. Front. Hum. Neurosci. 2017, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.; Huang, P.; Shen, Z.; Qian, W.; Luo, X.; Li, K.; Zeng, Q.; Gu, Q.; Yu, H.; et al. Abnormal White Matter Tracts of Insula in Smokers. Brain Imaging Behav. 2021, 15, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.B. Resting State FMRI: A Personal History. NeuroImage 2012, 62, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Krienen, F.M. The Evolution of Distributed Association Networks in the Human Brain. Trends Cogn. Sci. 2013, 17, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, M.; Geier, C.F.; Denlinger, R.; Forbes, E.E.; Raiff, B.R.; Dallery, J.; McClernon, F.J.; Donny, E.C. Blunted Striatal Response to Monetary Reward Anticipation during Smoking Abstinence Predicts Lapse during a Contingency-Managed Quit Attempt. Psychopharmacology 2016, 233, 751–760. [Google Scholar] [CrossRef]
- Addicott, M.A.; Sweitzer, M.M.; Froeliger, B.; Rose, J.E.; McClernon, F.J. Increased Functional Connectivity in an Insula-Based Network Is Associated with Improved Smoking Cessation Outcomes. Neuropsychopharmacology 2015, 40, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.E.; Calhoun, V.D.; Rachakonda, S.; Claus, E.D.; Littlewood, R.A.; Mickey, J.; Arenella, P.B.; Hutchison, K.E. Functional Network Connectivity Predicts Treatment Outcome during Treatment of Nicotine Use Disorder. Psychiatry Res. Neuroimaging 2017, 265, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Huang, P.; Qian, W.; Zhou, C.; Li, K.; Zeng, Q.; Luo, X.; Gu, Q.; Yu, H.; et al. Increased Interregional Functional Connectivity of Anterior Insula Is Associated with Improved Smoking Cessation Outcome. Brain Imaging Behav. 2019, 14, 408–415. [Google Scholar] [CrossRef]
- Wang, C.; Huang, P.; Shen, Z.; Qian, W.; Wang, S.; Jiaerken, Y.; Luo, X.; Li, K.; Zeng, Q.; Zhou, C.; et al. Increased Striatal Functional Connectivity Is Associated with Improved Smoking Cessation Outcomes: A Preliminary Study. Addict. Biol. 2021, 26, e12919. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional Homogeneity Approach to FMRI Data Analysis. NeuroImage 2004, 22, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Huang, P.; Qian, W.; Yu, X.; Sun, J.; Yu, H.; Yang, Y.; Zhang, M. Altered Spontaneous Activity of Posterior Cingulate Cortex and Superior Temporal Gyrus Are Associated with a Smoking Cessation Treatment Outcome Using Varenicline Revealed by Regional Homogeneity. Brain Imaging Behav. 2017, 11, 611–618. [Google Scholar] [CrossRef]
- Lohmann, G.; Margulies, D.S.; Horstmann, A.; Pleger, B.; Lepsien, J.; Goldhahn, D.; Schloegl, H.; Stumvoll, M.; Villringer, A.; Turner, R. Eigenvector Centrality Mapping for Analyzing Connectivity Patterns in FMRI Data of the Human Brain. PLoS ONE 2010, 5, e10232. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Huang, P.; Wang, C.; Qian, W.; Yang, Y.; Zhang, M. Increased Network Centrality as Markers of Relapse Risk in Nicotine-Dependent Individuals Treated with Varenicline. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 142–147. [Google Scholar] [CrossRef]
- Gilman, J.M.; Radoman, M.; Schuster, R.M.; Pachas, G.; Azzouz, N.; Fava, M.; Evins, A.E. Anterior Insula Activation during Inhibition to Smoking Cues Is Associated with Ability to Maintain Tobacco Abstinence. Addict. Behav. Rep. 2018, 7, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.E.; Schacht, J.P.; Hutchison, K.; Roche, D.J.O.; Ray, L.A. Neural Substrates of Cue Reactivity: Association with Treatment Outcomes and Relapse: Cue Reactivity and Outcomes. Addict. Biol. 2016, 21, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, K.J.; LeMatty, T.; McRae-Clark, A.L.; Gray, K.M.; George, M.S.; Brady, K.T. Resisting the Urge to Smoke and Craving during a Smoking Quit Attempt on Varenicline: Results from a Pilot FMRI Study. Am. J. Drug Alcohol Abus. 2013, 39, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Janes, A.C.; Pizzagalli, D.A.; Richardt, S.; de Frederick, B.B.; Chuzi, S.; Pachas, G.; Culhane, M.A.; Holmes, A.J.; Fava, M.; Evins, A.E.; et al. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biol. Psychiatry 2010, 67, 722–729. [Google Scholar] [CrossRef]
- Allenby, C.; Falcone, M.; Wileyto, E.P.; Cao, W.; Bernardo, L.; Ashare, R.L.; Janes, A.; Loughead, J.; Lerman, C. Neural Cue Reactivity during Acute Abstinence Predicts Short-term Smoking Relapse. Addict. Biol. 2020, 25, e12733. [Google Scholar] [CrossRef] [PubMed]
- McClernon, F.J.; Hiott, F.B.; Liu, J.; Salley, A.N.; Behm, F.M.; Rose, J.E. Selectively Reduced Responses to Smoking Cues in Amygdala Following Extinction-Based Smoking Cessation: Results of a Preliminary Functional Magnetic Resonance Imaging Study. Addict. Biol. 2007, 12, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.M.; MacKillop, J.; Gray, J.C.; Beach, S.R.H.; Stein, M.D.; Niaura, R.S.; Sweet, L.H. Neural Correlates of Tobacco Cue Reactivity Predict Duration to Lapse and Continuous Abstinence in Smoking Cessation Treatment: Neural Correlates of Tobacco Cue Reactivity. Addict. Biol. 2018, 23, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Janes, A.C.; Gilman, J.M.; Radoman, M.; Pachas, G.; Fava, M.; Evins, A.E. Revisiting the Role of the Insula and Smoking Cue-Reactivity in Relapse: A Replication and Extension of Neuroimaging Findings. Drug Alcohol Depend. 2017, 179, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.F.; Ho, S.S.; Jasinska, A.J.; Polk, T.A.; Welsh, R.C.; Liberzon, I.; Strecher, V.J. Self-Related Neural Response to Tailored Smoking-Cessation Messages Predicts Quitting. Nat. Neurosci. 2011, 14, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.M.; MacKillop, J.; Gray, J.C.; Hawkshead, B.E.; Murphy, C.M.; Sweet, L.H. Neural Correlates of Graphic Cigarette Warning Labels Predict Smoking Cessation Relapse. Psychiatry Res. Neuroimaging 2017, 262, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, M.M.; Geier, C.F.; Addicott, M.A.; Denlinger, R.; Raiff, B.R.; Dallery, J.; McClernon, F.J.; Donny, E.C. Smoking Abstinence-Induced Changes in Resting State Functional Connectivity with Ventral Striatum Predict Lapse During a Quit Attempt. Neuropsychopharmacology 2016, 41, 2521–2529. [Google Scholar] [CrossRef]
- Grosskopf, C.M.; Kroemer, N.B.; Pooseh, S.; Böhme, F.; Smolka, M.N. Temporal Discounting and Smoking Cessation: Choice Consistency Predicts Nicotine Abstinence in Treatment-Seeking Smokers. Psychopharmacology 2021, 238, 399–410. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Bechara, A. The Role of the Insula in Goal-Directed Drug Seeking and Choice in Addiction. In Addiction and Choice; Heather, N., Segal, G., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 205–224. [Google Scholar] [CrossRef]
- Abdolahi, A.; Williams, G.C.; Benesch, C.G.; Wang, H.Z.; Spitzer, E.M.; Scott, B.E.; Block, R.C.; van Wijngaarden, E. Smoking Cessation Behaviors Three Months Following Acute Insular Damage from Stroke. Addict. Behav. 2015, 51, 24–30. [Google Scholar] [CrossRef]
- Suñer-Soler, R.; Grau, A.; Gras, M.E.; Font-Mayolas, S.; Silva, Y.; Dávalos, A.; Cruz, V.; Rodrigo, J.; Serena, J. Smoking Cessation 1 Year Poststroke and Damage to the Insular Cortex. Stroke 2012, 43, 131–136. [Google Scholar] [CrossRef]
- Abdolahi, A.; Williams, G.C.; Benesch, C.G.; Wang, H.Z.; Spitzer, E.M.; Scott, B.E.; Block, R.C.; van Wijngaarden, E. Damage to the Insula Leads to Decreased Nicotine Withdrawal during Abstinence: Insular Damage and Withdrawal during Abstinence. Addiction 2015, 110, 1994–2003. [Google Scholar] [CrossRef]
- Abdolahi, A.; Williams, G.C.; Benesch, C.G.; Wang, H.Z.; Spitzer, E.M.; Scott, B.E.; Block, R.C.; van Wijngaarden, E. Immediate and Sustained Decrease in Smoking Urges After Acute Insular Cortex Damage. Nicotine Tob. Res. 2017, 19, 756–762. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct Funct 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Hariri, A.R.; Brown, S.M.; Williamson, D.E.; Flory, J.D.; de Wit, H.; Manuck, S.B. Preference for Immediate over Delayed Rewards is Associated with Magnitude of Ventral Striatal Activity. J. Neurosci. 2006, 26, 13213–13217. [Google Scholar] [CrossRef]
- Luijten, M.; Schellekens, A.F.; Kühn, S.; Machielse, M.W.J.; Sescousse, G. Disruption of Reward Processing in Addiction: An Image-Based Meta-Analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 2017, 74, 387. [Google Scholar] [CrossRef] [PubMed]
- David, S.P.; Munafò, M.R.; Johansen-Berg, H.; Smith, S.M.; Rogers, R.D.; Matthews, P.M.; Walton, R.T. Ventral Striatum/Nucleus Accumbens Activation to Smoking-Related Pictorial Cues in Smokers and Nonsmokers: A Functional Magnetic Resonance Imaging Study. Biol. Psychiatry 2005, 58, 488–494. [Google Scholar] [CrossRef]
- Franklin, T.R.; Wang, Z.; Wang, J.; Sciortino, N.; Harper, D.; Li, Y.; Ehrman, R.; Kampman, K.; O’Brien, C.P.; Detre, J.A.; et al. Limbic Activation to Cigarette Smoking Cues Independent of Nicotine Withdrawal: A Perfusion FMRI Study. Neuropsychopharmacol 2007, 32, 2301–2309. [Google Scholar] [CrossRef]
- Wang, Z.; Faith, M.; Patterson, F.; Tang, K.; Kerrin, K.; Wileyto, E.P.; Detre, J.A.; Lerman, C. Neural Substrates of Abstinence-Induced Cigarette Cravings in Chronic Smokers. J. Neurosci. 2007, 27, 14035–14040. [Google Scholar] [CrossRef]
- Jing, C.; Jing, C.; Zheng, L.; Hong, G.; Zheng, J.; Yu, L.; Song, N.; Zhang, T.; Ma, Q.; Fang, J. Disruption of Cigarette Smoking Addiction After Dorsal Striatum Damage. Front. Behav. Neurosci. 2021, 15, 646337. [Google Scholar] [CrossRef]
- Gaznick, N.; Tranel, D.; McNutt, A.; Bechara, A. Basal Ganglia plus Insula Damage Yields Stronger Disruption of Smoking Addiction than Basal Ganglia Damage Alone. Nicotine Tob. Res. 2014, 16, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Noël, X.; Brevers, D.; Bechara, A. A Triadic Neurocognitive Approach to Addiction for Clinical Interventions. Front. Psychiatry 2013, 4, 179. [Google Scholar] [CrossRef]
- Piasecki, T.M. Relapse to Smoking. Clin. Psychol. Rev. 2006, 26, 196–215. [Google Scholar] [CrossRef]
- Alboksmaty, A.; Agaku, I.T.; Odani, S.; Filippidis, F.T. Prevalence and Determinants of Cigarette Smoking Relapse among US Adult Smokers: A Longitudinal Study. BMJ Open 2019, 9, e031676. [Google Scholar] [CrossRef]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafò, M.R. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. 2017, 19, 3–13. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stewart, J.L. Interoception and Drug Addiction. Neuropharmacology 2014, 76, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Spada, M.M.; Caselli, G.; Nikčević, A.V.; Wells, A. Metacognition in Addictive Behaviors. Addict. Behav. 2015, 44, 9–15. [Google Scholar] [CrossRef]
- Flaudias, V.; Heeren, A.; Brousse, G.; Maurage, P. Toward a Triadic Approach to Craving in Addictive Disorders: The Metacognitive Hub Model. Harv. Rev. Psychiatry 2019, 27, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.; Meyerhoff, D.; Murray, D. Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults. Int. J. Environ. Res. Public Health 2015, 12, 8198–8213. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Baldoni, P.L.; Strizich, G.M.; Pérez-Stable, E.J.; Saccone, N.L.; Peralta, C.A.; Perreira, K.M.; Gellman, M.D.; Williams-Nguyen, J.S.; Rodriguez, C.J.; et al. Current Smoking Raises Risk of Incident Hypertension: Hispanic Community Health Study–Study of Latinos. Am. J. Hypertens. 2021, 34, 190–197. [Google Scholar] [CrossRef]
- Haight, T.J.; Bryan, R.N.; Erus, G.; Davatzikos, C.; Jacobs, D.R.; D’Esposito, M.; Lewis, C.E.; Launer, L.J. Vascular Risk Factors, Cerebrovascular Reactivity, and the Default-Mode Brain Network. NeuroImage 2015, 115, 7–16. [Google Scholar] [CrossRef]
- Gray, J.C.; Thompson, M.; Bachman, C.; Owens, M.M.; Murphy, M.; Palmer, R. Associations of Cigarette Smoking with Gray and White Matter in the UK Biobank. Neuropsychopharmacol 2020, 45, 1215–1222. [Google Scholar] [CrossRef]
- Sutherland, M.T.; Riedel, M.C.; Flannery, J.S.; Yanes, J.A.; Fox, P.T.; Stein, E.A.; Laird, A.R. Chronic Cigarette Smoking Is Linked with Structural Alterations in Brain Regions Showing Acute Nicotinic Drug-Induced Functional Modulations. Behav. Brain Funct. 2016, 12, 16. [Google Scholar] [CrossRef]
- Kim, S.H.; Yun, C.-H.; Lee, S.-Y.; Choi, K.; Kim, M.B.; Park, H.-K. Age-Dependent Association between Cigarette Smoking on White Matter Hyperintensities. Neurol. Sci. 2012, 33, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Deal, J.A.; Sharrett, A.R.; Jack, C.R.; Knopman, D.; Mosley, T.H.; Gottesman, R.F. Smoking and White Matter Hyperintensity Progression: The ARIC-MRI Study. Neurology 2015, 84, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Zubieta, J.-K.; Heitzeg, M.M.; Xu, Y.; Koeppe, R.A.; Ni, L.; Guthrie, S.; Domino, E.F. Regional Cerebral Blood Flow Responses to Smoking in Tobacco Smokers After Overnight Abstinence. Am. J. Psychiatry 2005, 162, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Nomi, J.S.; Farrant, K.; Damaraju, E.; Rachakonda, S.; Calhoun, V.D.; Uddin, L.Q. Dynamic Functional Network Connectivity Reveals Unique and Overlapping Profiles of Insula Subdivisions: Dynamic Connections of Insula Subdivisions. Hum. Brain Mapp. 2016, 37, 1770–1787. [Google Scholar] [CrossRef] [PubMed]
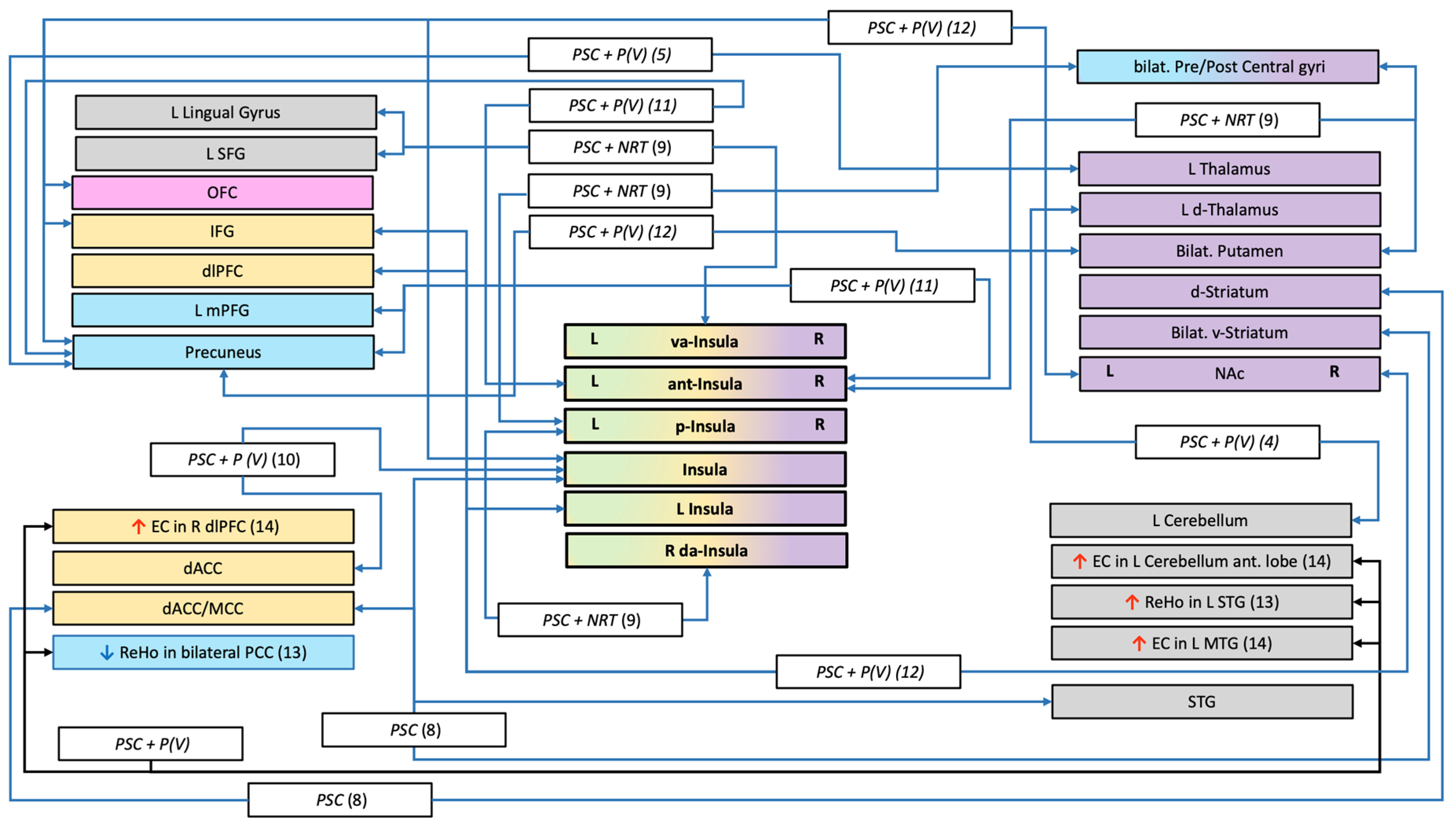
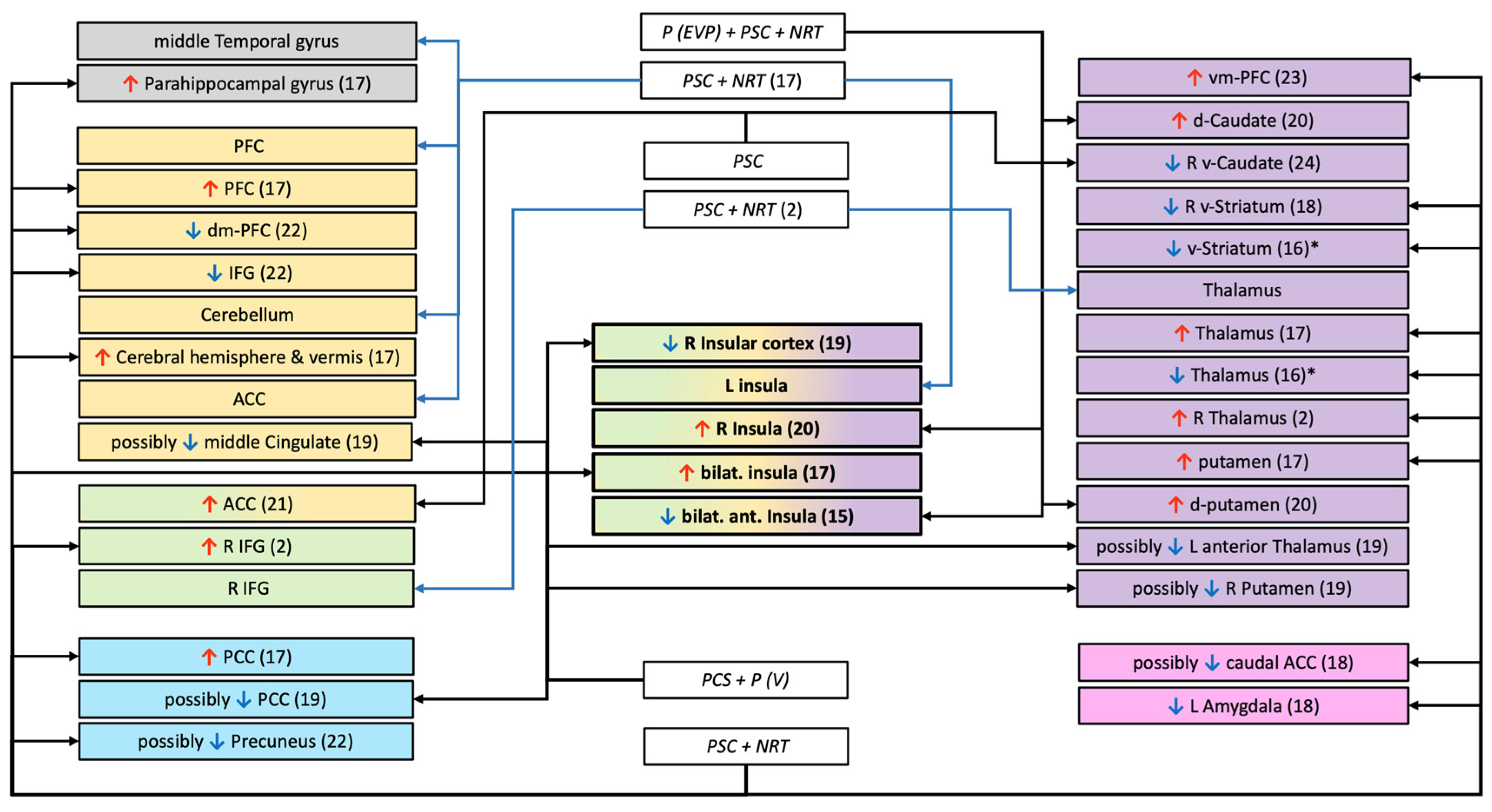
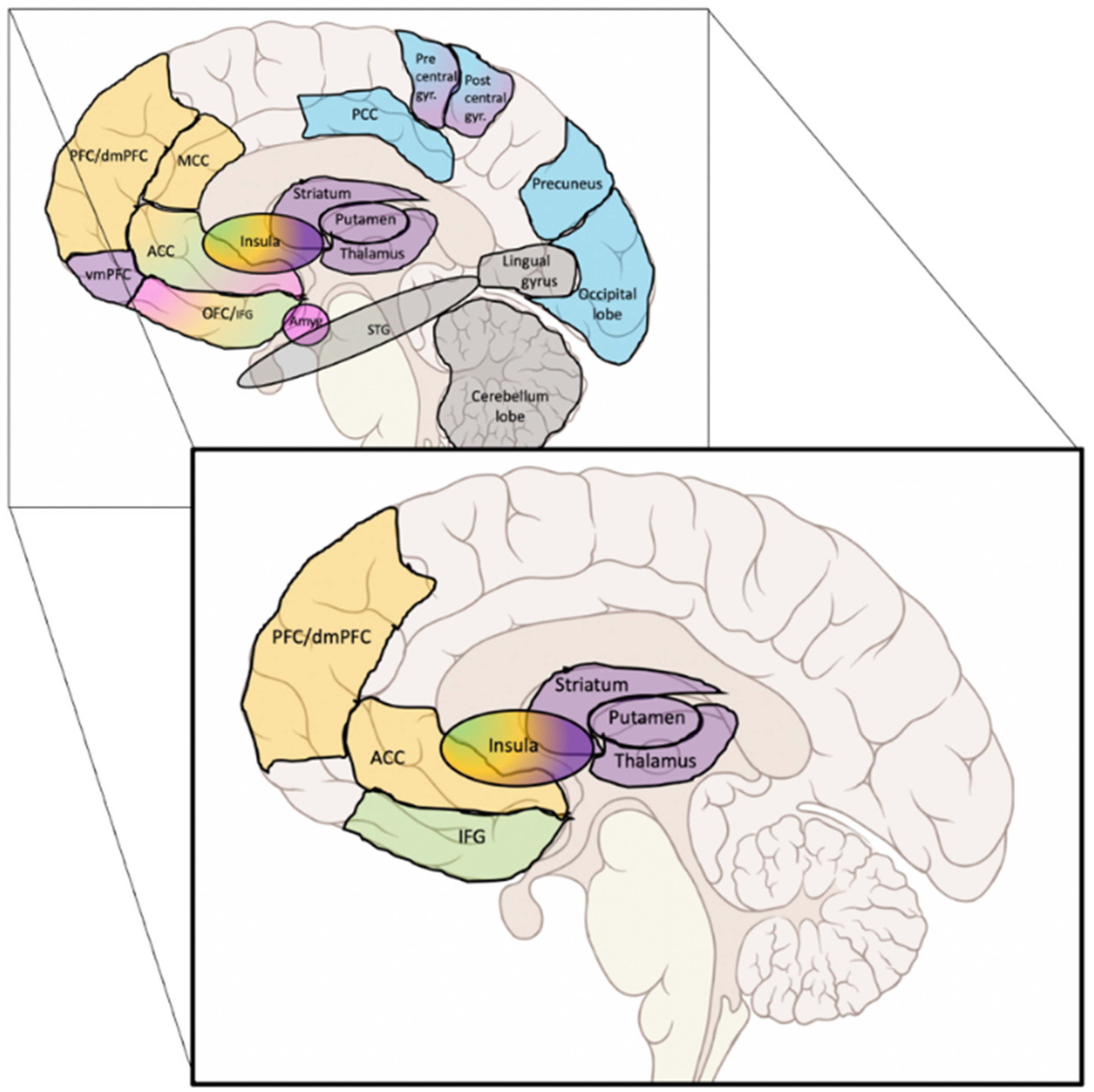
| Authors and N° | Population; % of Abstainers | Treatment | Duration of ttt; End Point; Abstinence Criteria | Scans: All Had at Least One MRI Before the Treatment | Interpretation of the Authors | ||
|---|---|---|---|---|---|---|---|
| A N A T O M I C A L | Froeliger-2010 1 | N = 18 (16w; 4 m) 39.06 yo (9.37) 10R/8A 44.44% | Single arm study NRT (RNC + Patch) | Treatment: 10 weeks End point: 4 weeks point prevalence abstinence Criteria: - Self-reported abstinence in the 21 days leading up to the 4 weeks clinic visit - CO ≤ 8 ppm | Whole Brain 1.5 T GE NVi SIGNA scanner | Results: Relapse was associated with lower GMV in the left putamen and right occipital lobe and greater GMV in bilateral hippocampus and right cuneus. Maintaining smoking abstinence is associated with higher prequit brain volume in regions that subserve habit learning and visual processing, and lower brain volume in regions that subserve long-term memory processes and visual information processing. Covariates: years smoked and cigarettes per day as covariates, Total Intracranial Volume TIV and sex. | |
| Froeliger-2017 2 | N = 81 40R (21 w;19 m) 41A (24 w;17 m) 50.62% | Randomized control trial Before the quit date: Two-arms 30 days smoking cessation program Gp1: continue smoking Gp2: low nicotine cigarettes + NRT (patch) After the quit date: NRT (patch) + PCS both groups | Treatment: 10 weeks End point: 10 weeks Criteria: - Daily diaries of cigarette use - CO < 8 ppm - relapse was defined as 7 days consecutive of smoking at least 1 cig/day | Whole Brain 3T scanners (Signa Excite HD and MR750; GE Healthcare). Contingency management has been used during the post-scan behavioral task. | Results: Relapsers had lower GMV in right IFG. Together, these findings suggest that reduced prefrontal gray matter volume may reduce inhibitory control over behavioral response to conditioned drug-cues. This finding is consistent with the extant literature implicating drug addiction-related neuroplasticity in fronto-striatal circuitry, which mediates cue-induced relapse as being associated with frontally mediated behavior inhibition. Covariates: FTND (nuisance covariate) | ||
| Wang–2019a 3 | N = 74 All men 46R/28A 37.83% | Single arm study Pharm (Varenicline) +PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: - weekly self-reports of smoking behavior - CO ≤ 6 ppm | ROI: Insula (anterior and posterior) 3.0 T GE Signa MR scanner GMV + SCN | Results: No association Smoking cessation outcomes showed no correlations with the gray matter volume and seed-based structural covariance network of insular subregions prior to smoking cessation. Covariates: Not reported | ||
| Qian–2019 4 | N = 73 All men 44R/29A 39.73% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | Whole brain + ROI (OFC, dorsal striatum, postcentral gyrus, thalamus) 3.0 T GE Signa MR scanner | Results: Relapsers had lower GMV in the right post-central gyrus, right dorsal striatum, and left OFC. Structural integrity of OFC is important for quitting smoking. Smaller dorsal striatum grey matter volume may be associated with a disturbance of DA functions, and it may contribute to the neurobiology of nicotine abstinence symptomatology. Covariates: Age and education years. | ||
| Wang–2020 5 | N = 74 All men 47R/27A 36.49% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | ROI: Thalamus 3.0 T GE Signa MR scanner | Results: Relapsers had greater left thalamic GMV. Pre-existing abnormalities in the thalamus may potentially predispose individuals to the initiation of smoking and the development of nicotine dependence, such as the genetic factor. Covariates: Not reported. | ||
| Huang–2017 6 | N = 66 All men 38R/28A 42.42% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | Whole Brain 3.0 T GE Signa MR scanner | Results: Relapsers had higher fractional anisotropy in the right cerebellum and in post-central gyrus. Considering the general function of these two structures and the related evidence, we suggested that the higher FA may indicate the formation of habitual/automatic smoking behaviors that promote smoking relapse. Covariates: Age and education years. | ||
| Wang–2021a 7 | N = 58 All men 38R/20A 34.48% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6ppm | ROI: Insular seed regions and OFC and NAc targets 3.0 T GE Signa MR scanner | Results: No association Cessation likelihood may be more susceptible to insula-related functional connectivity than insula-related structural connectivity. Covariates: Not reported | ||
| R E S T I N G - S T A T E | Sweitzer–2016 (b) 8 | N = 37 23R (13 w;10 m) 14A (6 w; 8 m) 37.84% | Single arm study PCS (Contingency management) | Treatment: 3 weeks Endpoint: 18 days of abstinence Criteria: - Any smoking after achieving an initial 24 h abstinence (ie, two consecutive abstinent samples), - Self-report - CO ≤ 8 ppm or a 50% reduction from baseline | 2 randomized MRI (condition: satiety and 24 h of abstinence) Whole brain: connectivity with ventral and dorsal striatum seed regions | Modulation of striatal connectivity with the cingulo insula network during early withdrawal may be associated with smoking cessation outcomes. Covariates: Age, sex, cigarettes smoked per day, and session order (i.e., abstinent or satiated condition first). | |
| Addicott–2015 9 | N = 85 41R (23 w;18 m) 44A (23 w;22 m) 51.76% | Randomized control trial Before the quit date: Two-arms 30 days smoking cessation program Gp1: continue smoking Gp2: low nicotine cigarettes + NRT (patch) After the quit date: NRT (patch) + PCS both groups | Treatment: 10 weeks Endpoint: 10 weeks Criteria: - relapse = 7 days consecutive of smoking cigarettes (at least one per/day) following the quit day or lost to follow-up (N = 14 *) - Daily diaries of cigarette and NRT use + expired CO on four occasions spaced across the 10 weeks of post-quit date treatment | 1 MRI in satiated state Whole brain: connectivity with bilateral posterior, ventroanterior and dorsoanterior insula seed regions. | Relapse vulnerability is associated with weaker connectivity between the posterior insula and primary sensorimotor cortices. Perhaps greater connectivity in this network improves the ability to inhibit a motor response to cigarette cravings when those cravings conflict with a goal to remain abstinent. Covariates: Treatment group and FTND scores. | ||
| Wilcox–2017 10 | N = 144 (53 w;91 m) N = 82 varenicline treatment, N = 62 placebo 120R/24A 16.67% | Randomized control trial Pharm (Varenicline) Gp1: Varenicline + PCS Gp2: Placebo + PCS | Treatment: 12 weeks End point: 12 weeks Criteria: - Number of cigarettes in previous 30 days | 1 MRI in abstinence state (N = 8 were not) ROI network: - 6 networks utilized Dorsolateral PFC, dorsal ACC, rostral ACC, Insula, Caudate, Putamen | No main effect of treatment group. Covariates: Treatment group and baseline smoking. | ||
| Qian–2019 4 | N = 73 44R/29A 39.73% | Single arm study Pharm (Varenicline)+ PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | 1 MRI in satiated state Whole brain connectivity with OFC, dorsal striatum, postcentral gyrus and thalamic seed regions | Decreased thalamus-cerebellar FC may hinder the communication between the frontal lobe and the cerebellum, invalidating the top-down regulations. Therefore, the relapsers may experience difficulties in utilizing cognitive abilities to reverse habitual behaviors. Covariates: Age and education years. | ||
| Wang–2020 5 | N = 74 All men 47R/27A(#) 36.49% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | 1 MRI in satiated state Whole brain: connectivity with thalamic seed regions | Relapsers showed lower left thalamo-precuneus functional connectivity. Thalamo-precuneus functional connectivity degradation may be associated with both the maintenance of smoking behavior and smoking relapse. Covariates: White matter signal and corticospinal fluid signal in addition to head motion (nuisance covariates). | ||
| Wang–2019b 11 | N = 30 All men 14R/16A 53.33% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | 2 MRI scanning sessions in satiated state: - at baseline - after treatment Whole brain: connectivity with insula seed regions. | Altered interregional functional connectivity but not regional activity of insular subregions is associated with smoking cessation outcome. Increased FC network of the anterior insula could help resist relapse to improve smoking cessation likelihood. Covariates: White matter signal and corticospinal fluid signal in addition to head motion (nuisance covariates). Age was added in the fALFF analysis. | ||
| Wang–2021b 12 | N = 30 All men 14R/16A 53.33% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | 2 MRI scanning sessions in satiated state: - at baseline - after treatment Whole brain: connectivity with striatum (NAc, caudate, putamen) seed regions. | Lower NAc-based functional connectivity with the frontoinsular areas may reflect both a lower awareness of subjective urge to smoke and a lower ability of cognitive control for maintaining abstinence. Covariates: White matter signal and corticospinal fluid signal in addition to head motion (nuisance covariates); Age. | ||
| Wang–2017 13 | N = 55 All men 32R/23A 41.82% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12 weeks End point: 12 weeks Criteria: Continuously abstinent for 12 weeks - weekly self-reports of smoking behavior - CO ≤ 6 ppm | 1 MRI in satiated state Whole brain: Reho | Relapsers had decreased ReHo in the bilateral PCC and increased ReHo in the left STG, suggesting that regional brain function variables may be promising predictors of smoking relapse. Covariates: Years of education, years smoked, cigarettes smoked per day and FTND score. | ||
| Shen–2017 14 | N = 57 36R/21A 36.84% | Single arm study Pharm (Varenicline) + PCS | Treatment: 12weeks End point: last 4 weeks Criteria: Continuously abstinent for the last 4 weeks of treatment - Weekly self-reports of smoking behavior - CO ≤ 6 ppm | 1 MRI in satiated state Whole brain: Eigenvector centrality (EC) mapping. | These findings suggest that the dlPFC, MTG, and cerebellum may be important substrates of smoking relapse vulnerability. The data also suggest that relapse-vulnerable smokers can be identified before quit attempts, which could enable personalized treatment and improve smoking cessation outcomes. Covariates: Years smoked, cigarettes per day. | ||
| T A S K - B A S E D A C T I V A T I O N | I N H I B I T I O N | Froeliger–2017 (Study 1) 2 | N = 81 40R (21 w;19 m) 41A (24 w;17 m) 50.62% | Randomized control trial Before the quit date: Two-arms 30 days smoking cessation program Gp1: continue smoking Gp2: low nicotine cigarettes + NRT (patch) After the quit date: NRT (patch) + PCS both groups | Treatment: 10 weeks End point: 10 weeks Criteria: - Daily diaries of cigarette use - CO < 8 ppm - relapse was defined as 7 days consecutive of smoking at least 1 cig/day | 1 MRI in satiated state ROI: Right IFG, bilateral thalamus, subthalamic nucleus, presupplementary motor area and left primary motor cortex Contingency management has been used during the post-scan behavioral task. | Individual differences in corticothalamic circuitry function have important implications for smoking cessation and relapse vulnerability. Covariates: Baseline FTND. |
| Gilman–2018 15 | N = 22 12R (4 w;8 m) 10A (2 w;8 m) 45.45% | Randomized control trial Pharm (EVP-6124 or placebo) + NRT (patch or placebo)+ PCS for all groups Gp1: EVP-6124 + NRT Gp2: EVP-placebo + NRT Gp3: EVP-6124 + NRT-placebo Gp4: EVP-placebo + NRT-placebo | Treatment: 12 weeks End point: 2 weeks Criteria: - Self-report of 2 weeks abstinence - CO < 10 ppm - Cotinine < 50 ng/mL. | 1 MRI in satiated state ROI: bilateral ant insula, right IFG | Results from the current study suggest that while brain activation during inhibition to smoking cues does not significantly differ from inhibition to neutral cues, decreased activation in the anterior insula to inhibition of smoking cues may be associated with relapse among smokers attempting to remain abstinent. Covariates: FTND. | ||
| D R U G C U E S | McClernon–2007 16 | N = 16 (14 w;2 m) 12R/4A 25.00% | Single arm study NRT (RNC + Patch) + PCS | Treatment: 8 to 9 weeks End point: 1 month Criteria: - Self-reports of no cigarette smoking since the quit day - CO < 9 ppm | 3 MRI (at baseline; quit date; 2-4weeks of abstinence) ROI: ventral and dorsal ACG, SFG, MFG, IFG, NAc, thalamus, caudate, putamen, amygdala, hippocampus, insula, ventral striatum (NAc+central caudate and putamen) | An extinction-based smoking cessation treatment could alter brain responses to smoking cues and these changes may be associated with treatment outcome. Covariates: Not reported. | |
| Janes–2010 17 | N = 21 All women 9R/12A 57.14% | Single arm study NRT: (Patch+ nicotine gum (lozenge)) + PCS | Treatment: 8 weeks End point: 8 weeks Criteria: - 7 days or more consecutive or more than once/week for 2 or more consecutive weeks - Self-reported abstinence - CO < 9 ppm | 1 MRI Whole brain | Insula and amygdala activation might imply that smoking-related images are more emotionally salient and may induce interoceptive awareness to a greater extent than neutral images in smokers vulnerable to relapse. (In addition…) vulnerable subjects may be more likely to prepare for, or initiate, motor responses geared toward reducing interoceptive sensations related to craving. Covariates: Not reported | ||
| Owens–2018 18 | N = 32 23R/9A for 49 days of treatment 28.13% ** | Single arm study NRT (Patch) + PCS | Treatment: 9 weeks End point: 49 days Criteria: - Self-reported timeline follow back calendar - CO < 10 ppm At each session Lost of follow-up are in relapser group | 1 MRI (2 h of abstinence) ROI: R&L ventral striatum, caudal and rostral ACC, left amygdala | Greater in ventral striatum, amygdala and ACC was associated with less difficulty quitting, suggesting their activity is an indicator of less severe risk for lapse. Clinically, this implies that for smokers who have transitioned from incentive learning and more deliberative cognitive processing to having habits learning and automatized drug seeking, smoking cessation is more difficult. Covariates: Self-reported craving prior and during the MRI session. | ||
| Hartwell–2013 19 | N = 21 (12 w;9 m) 11R/10A 47.62% | Single arm study Pharm (Varenicline) + PCS | Treatment: 5 weeks End point: 5 weeks Criteria: - 7 days point prevalence abstinence - CO ≤ 3 ppm - Cotinine < 200 ng/mL | 2 MRI: - 1 at baseline (resist and crave during scan) - 1 month after quit date Whole brain *** | Successful smoking cessation with varenicline is associated with increased activation, prior to a quit attempt, in brain areas related to attentiveness and memory while resisting the urge to smoke. Covariates: Not reported. | ||
| Janes–2017 20 | N = 23 (7 w;16 m) 13R/10A 43.48% | Randomized control trial Pharm (EVP-6124 or placebo) + NRT (patch or placebo) + PCS for all groups Gp1: EVP-6124 + NRT Gp2: EVP-placebo + NRT Gp3: EVP-6124 + NRT-placebo Gp4: EVP-placebo + NRT-placebo | Treatment: 12 weeks End point: 2 weeks Criteria: - Self-report of 2 weeks abstinence - CO < 10 ppm, - Cotinine < 50 ng/mL. | 1 MRI in satiated state ROI: Bilate insula, striatum (nucleus accumbens, putamen, caudate), and thalamus. | The current work supports prior results and builds on the concept that the insula and dorsal striatum work in concert to maintain nicotine dependence. Covariates: Not reported | ||
| Allenby–2020 21 | N = 75 (35 w;40 m) 52R/23A 30.67% | Single arm study PCS | Treatment: 1 weeks End point: 1 weeks Criteria: - Self-report abstinence - Urine cotinine < 100 ng/mL - CO ≤ 5 ppm | 2 MRI (satiety and 24 h of abstinence) Whole brain | This study provides the first evidence that changes in smoking cue reactivity in the ACC during acute abstinence predict smoking relapse, thereby improving our understanding of the neurobiology of smoking cessation. Covariates: Abstinence-induced changes in craving and withdrawal, sex, age, baseline smoking (cigarettes per day and CO at intake). | ||
| H E A L T H Y M E S S A G E | Chua–2011 22 | N = 91 (44 w,47 m) 45A/42R 4 lost 51.72% | Single arm study NRT (Patch) + PCS | Treatment: 10 weeks End point: 4 months after the intervention Criteria: - Self-reported 7 days point prevalence abstinence | 2 MRI (two tasks) Whole brain + ROI: (dorsomedial PFC, precuneus/PCC, angular gyrus) | The dmPFC region has been associated with the evaluative and decision-making aspect of self-related processing which could underlie the efficacy of tailored message interventions. Covariates: Number of cigarettes per day at baseline. | |
| Owens–2017 23 | N = 48 (17 w;31 m) 24R/24A 50.00% | Single arm study NRT (Patch) + PCS | Treatment: 9 weeks End point: 30 days Criteria: - Self reported - CO ≤ 10 ppm | 1 MRI (2 h of abstinence) ROI: vmPFC, Right and Left amygdala | Neurocognitive processes in the vmPFC may be critical to understanding how Graphic Warning Labels on cigarette packaging induce behavior changes and may be useful as a predictor of smoking cessation treatment prognosis. Covariates: FTND | ||
| R E W A R D | Sweitzer–2016 (a) 24 | N = 36 (19 w;17 m) 23R/13A 36.11% | Single arm study PCS Contingency management during treatment | Treatment: 3 weeks End point: 18 days of abstinence Criteria: - Self-reported abstinence - CO < 8 ppm or a 50% reduction from baseline | 2 randomized MRI (condition: satiety and 24 h of abstinence) ROI: Right and Left striatum (head of the caudate) | Results support the importance of blunted reward sensitivity as a risk factor for relapse and highlight the moderating effect of abstinence state. Early abstinence may be a particular period of vulnerability. Covariates: Age, sex, abstinence-induced craving, and baseline cigarette per day | |
| Grosskopf–2020 25 | N = 44 (21 w;23 m) 19R/25A 56.82% | Single arm study PCS | Treatment: length not reported End point: 30 days Criteria: - Smoking less than 1 day over a 30-day period - CO verified | 2 MRI: -1 at baseline in satiated state - 2 to 5 weeks after quit date Whole brain: | Tobacco abstinence did not affect discounting behavior as well as related fMRI brain activity in smokers. Covariates: FNTD and discounting behavior. | ||
| F U N C T I O N A L C O N N E C T I V I T Y | Janes–2010 17 | N = 21 All women9R/12A 57.14% | Single arm study NRT: (Patch+ nicotine gum (lozenge)) + PCS | Treatment: 8 weeks End point: 8 weeks Criteria: - 7 days or more consecutive or more than once/week for 2 or more consecutive weeks - Self-reported abstinence - CO < 9 ppm | 1 MRI ROI: ICA defined: Bilateral insula et ACC | Relapsers may have decreased top-down control of emotion regulation. This could result in increased interoceptive awareness of smoking-related cues, leading to enhanced smoking cue reactivity, interference effects, and relapse vulnerability. Covariates: Not reported. | |
| Froeliger–2017 2 | N = 81 40R (21 w;19 m) 41A (24w;17m) 50.62% | Randomized control trial Before the quit date: Two-arms 30 days smoking cessation program Gp1: continue smoking Gp2: low nicotine cigarettes + NRT (patch) After the quit date: NRT (patch) + PCS both groups | Treatment: 10 weeks End point: 10 weeks Criteria: - Daily diaries of cigarette use - CO < 8 ppm - Relapse was defined as 7 days consecutive of smoking at least 1 cigarette/day | 1 MRI ROI: Thalamus Inferior frontal gyrus Contingency management has been used during the post-scan behavioral task. | Baseline differences in corticothalamic circuitry function were associated with mediated IC and smoking relapse vulnerability. These findings warrant further examination of interventions for augmenting corticothalamic neurotransmission and enhancing inhibitory control during the course of tobacco use disorder treatment. Covariates: FTND (nuisance covariate). | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabat, Y.; Chanraud, S.; Abdallah, M.; Sibon, I.; Berthoz, S. Precision Preventive Medicine of Relapse in Smoking Cessation: Can MRI Inform the Search of Intermediate Phenotypes? Biology 2022, 11, 35. https://doi.org/10.3390/biology11010035
Rabat Y, Chanraud S, Abdallah M, Sibon I, Berthoz S. Precision Preventive Medicine of Relapse in Smoking Cessation: Can MRI Inform the Search of Intermediate Phenotypes? Biology. 2022; 11(1):35. https://doi.org/10.3390/biology11010035
Chicago/Turabian StyleRabat, Yolaine, Sandra Chanraud, Majd Abdallah, Igor Sibon, and Sylvie Berthoz. 2022. "Precision Preventive Medicine of Relapse in Smoking Cessation: Can MRI Inform the Search of Intermediate Phenotypes?" Biology 11, no. 1: 35. https://doi.org/10.3390/biology11010035
APA StyleRabat, Y., Chanraud, S., Abdallah, M., Sibon, I., & Berthoz, S. (2022). Precision Preventive Medicine of Relapse in Smoking Cessation: Can MRI Inform the Search of Intermediate Phenotypes? Biology, 11(1), 35. https://doi.org/10.3390/biology11010035






