Peer Presence Effect on Numerosity and Phonological Comparisons in 4th Graders: When Working with a SchoolMate Makes Children More Adult-like
Abstract
:Simple Summary
Abstract
1. Introduction
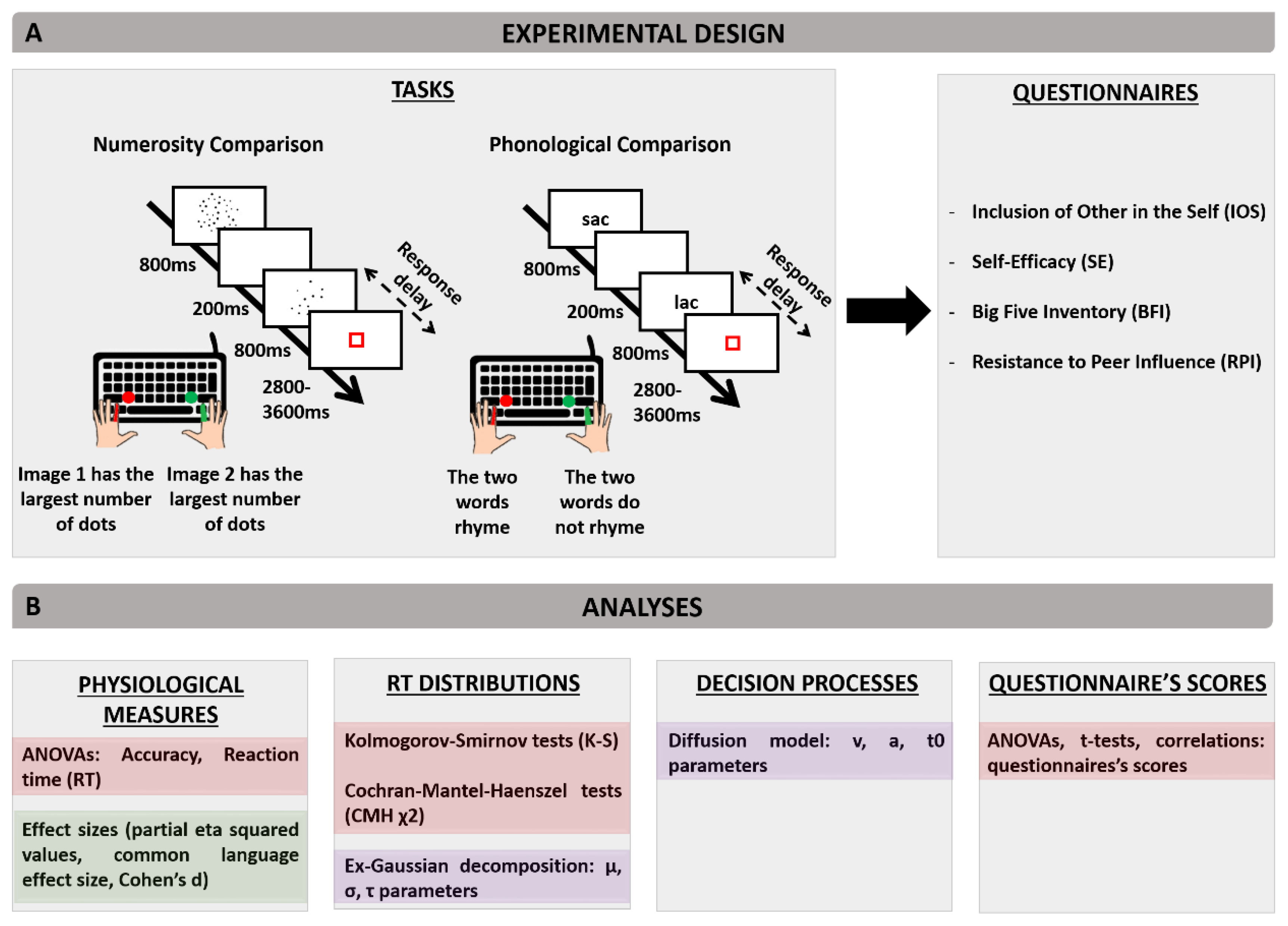
2. Materials and Methods
2.1. Participants
2.2. Solitary Versus Social Testing
2.3. Task
2.4. Stimuli
2.5. Accuracy, Reaction Times, and Effect Sizes
2.6. RT Distributions
2.7. Diffusion Modeling
2.8. Questionnaires
2.8.1. Pairs’ Relationship Quality
2.8.2. Personality and Self-Efficacy
2.8.3. Resistance to Peer Influence
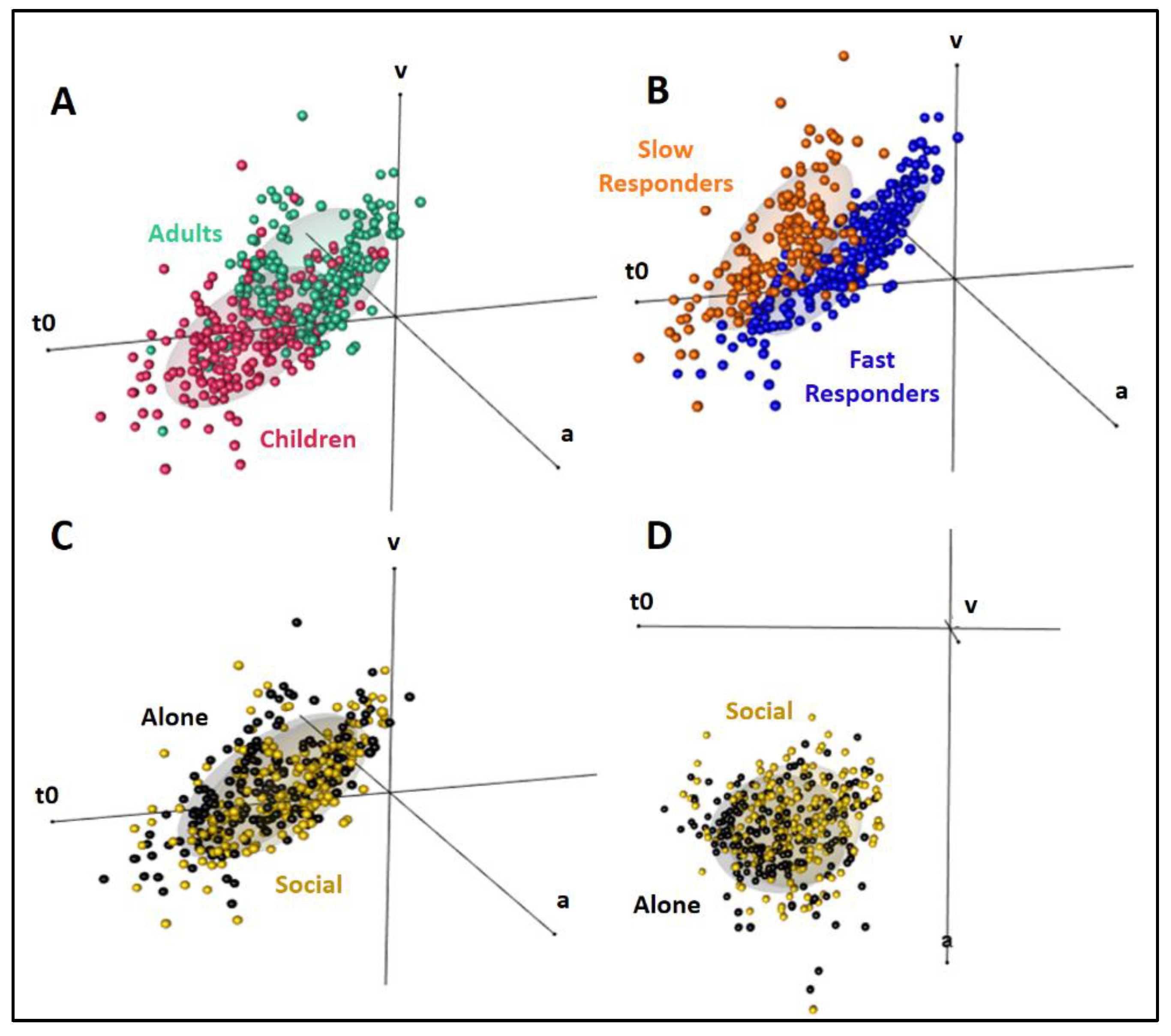
3. Results
3.1. Age, Trial Type, and Difficulty Effects
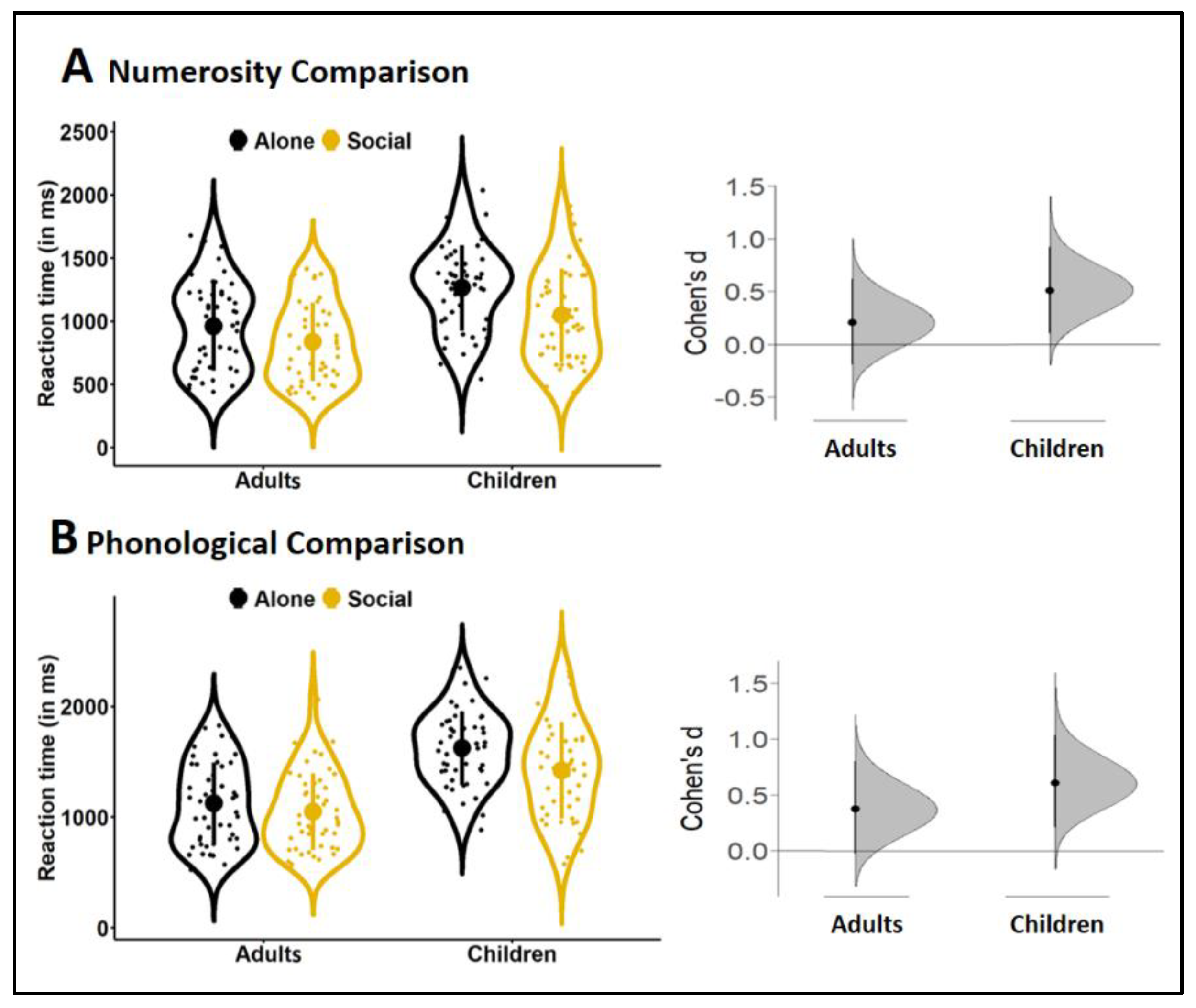
3.2. Peer Presence Effect
3.3. Effect Sizes
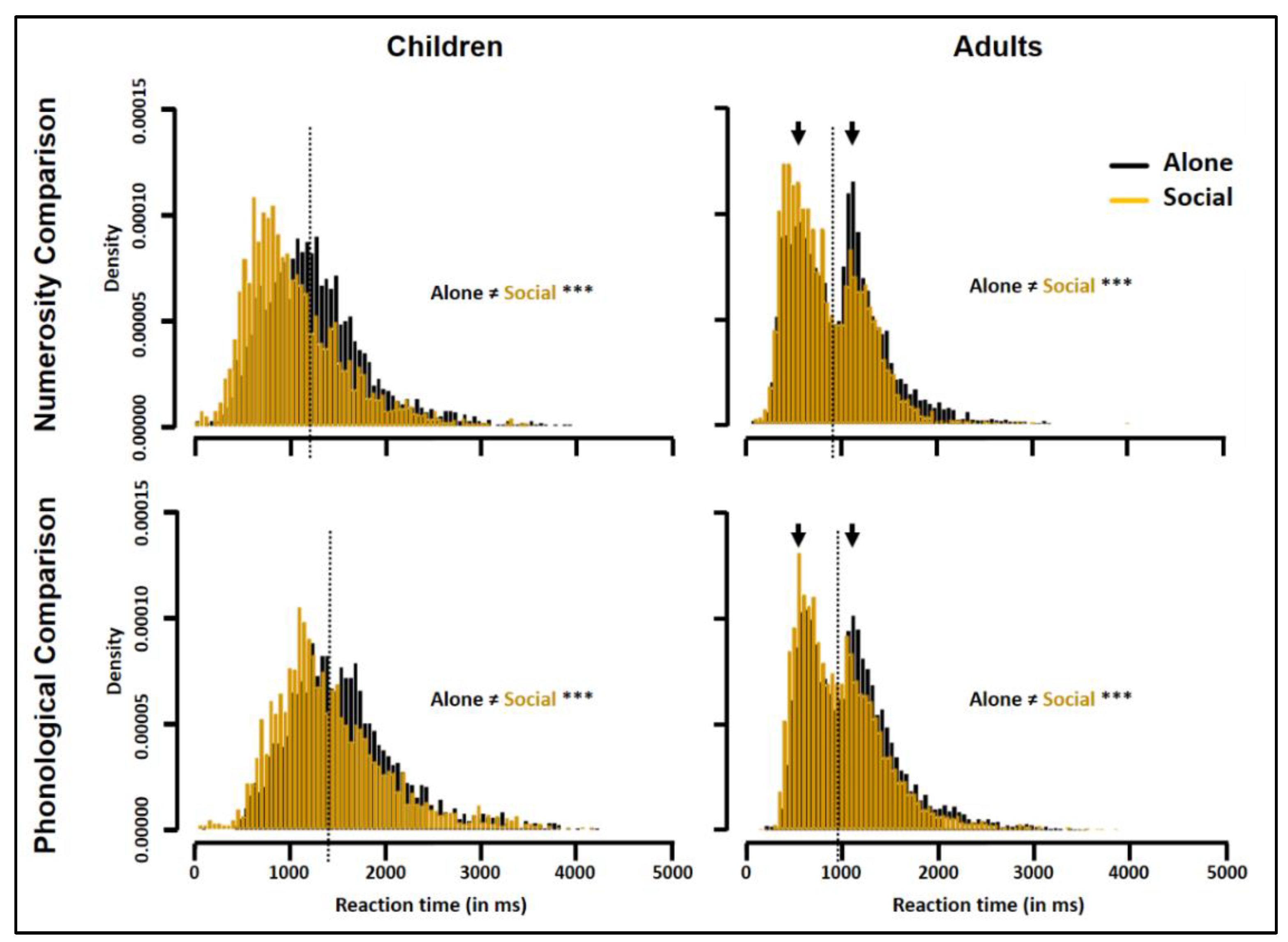
3.4. Group RT Distributions
3.5. Individual RT Distributions
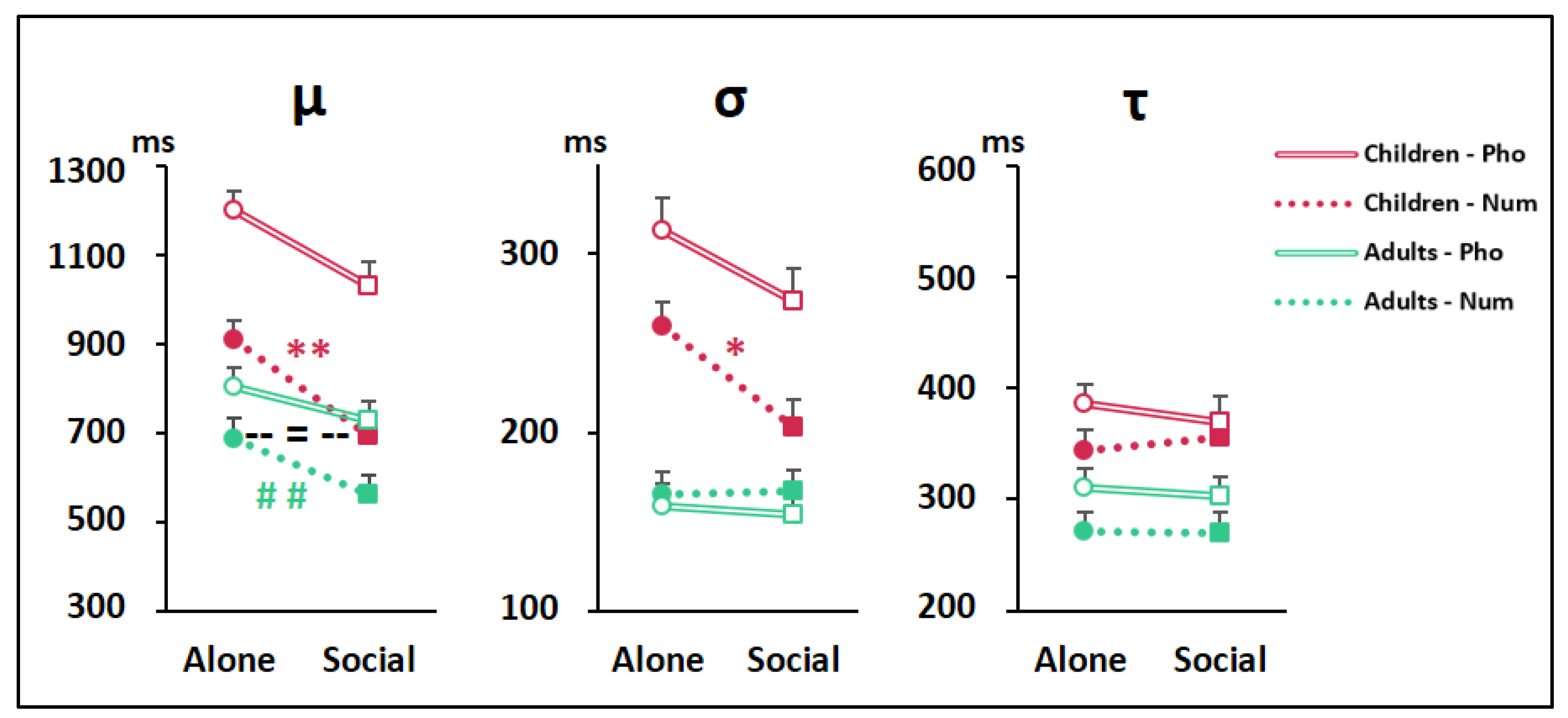
3.6. Diffusion Modeling
3.7. Questionnaires
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahanshahi, B. Separating gender composition effects from peer effects in education. Educ. Econ. 2017, 25, 112–126. [Google Scholar] [CrossRef]
- Oosterbeek, H.; Van Ewijk, R. Gender peer effects in university: Evidence from a randomized experiment. Econ. Educ. Rev. 2014, 38, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Castle, S.; Burchinal, M.; Horm, D.; Guss, S.; Bingham, G.E. Peer effects on low-income children’s learning and development. J. Sch. Psychol. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Justice, L.M.; Logan, J.A.R.; Lin, T.J.; Kaderavek, J.N. Peer Effects in Early Childhood Education: Testing the Assumptions of Special-Education Inclusion. Psychol. Sci. 2014, 25, 1722–1729. [Google Scholar] [CrossRef]
- Chiu, M.M.; Chow, B.W.Y.; Joh, S.W. Streaming, tracking and reading achievement: A multilevel analysis of students in 40 countries. J. Educ. Psychol. 2017, 109, 915–934. [Google Scholar] [CrossRef]
- Sacerdote, B. Experimental and Quasi-Experimental Analysis of Peer Effects: Two Steps Forward? Annu. Rev. Econom. 2014, 6, 253–272. [Google Scholar] [CrossRef]
- Zajonc, R. Social facilitation. Science 1965, 149, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, A.J.; Guedj, C.; Hadj-Bouziane, F.; Meunier, M.; Monfardini, E. Social Facilitation of Cognition in Rhesus Monkeys: Audience Vs. Coaction. Front. Behav. Neurosci. 2015, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- BBond, C.F.; Titus, L.J. Social facilitation: A meta-analysis of 241 studies. Psychol. Bull. 1983, 94, 265–292. [Google Scholar] [CrossRef]
- Tricoche, L.; Ferrand-Verdejo, J.; Pélisson, D.; Meunier, M. Peer Presence Effects on Eye Movements and Attentional Performance. Front. Behav. Neurosci. 2020, 13, 280. [Google Scholar] [CrossRef]
- Belletier, C.; Normand, A.; Huguet, P. Social-Facilitation-and-Impairment Effects: From Motivation to Cognition and the Social Brain. Curr. Dir. Psychol. Sci. 2019, 28, 260–265. [Google Scholar] [CrossRef]
- Guerin, B. Social Facilitation; Cambridge University Press: Cambridge, UK, 2010; ISBN 0521119790. [Google Scholar]
- Wolf, L.K.; Bazargani, N.; Kilford, E.J.; Dumontheil, I.; Blakemore, S.J. The audience effect in adolescence depends on who’s looking over your shoulder. J. Adolesc. 2015, 43, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Monfardini, E.; Reynaud, A.J.; Prado, J.; Meunier, M. Social modulation of cognition: Lessons from rhesus macaques relevant to education. Neurosci. Biobehav. Rev. 2017, 82, 45–57. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.M. Family and friends produce greater social facilitation of food intake than other companions. Physiol. Behav. 1994, 56, 445–455. [Google Scholar] [CrossRef]
- Lomi, A.; Snijders, T.A.B.; Steglich, C.E.G.; Torló, V.J. Why are some more peer than others? Evidence from a longitudinal study of social networks and individual academic performance. Soc. Sci. Res. 2011, 40, 1506–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addyman, C.; Fogelquist, C.; Levakova, L.; Rees, S. Social facilitation of laughter and smiles in preschool children. Front. Psychol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barkley, J.E.; Salvy, S.J.; Sanders, G.J.; Dey, S.; Von Carlowitz, K.P.; Williamson, M.L. Peer influence and physical activity behavior in young children: An experimental study. J. Phys. Act. Health 2014, 11, 404–409. [Google Scholar] [CrossRef]
- Gonzales, T.; Jackson, M.L.; Nicolson, A. The Effects of Peer Presence on Variables Maintaining Moderate-to-Vigorous Physical Activity in Children. Behav. Modif. 2019. [Google Scholar] [CrossRef]
- Dumontheil, I.; Wolf, L.K.; Blakemore, S.-J. Audience effects on the neural correlates of relational reasoning in adolescence. Neuropsychologia 2016, 87, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiner, K.; Li, A.; Cohen, A.O.; Steinberg, L.; Bonnie, R.J.; Scott, E.S.; Taylor-Thompson, K.; Rudolph, M.D.; Chein, J.; Richeson, J.A.; et al. Combined effects of peer presence, social cues, and rewards on cognitive control in adolescents. Dev. Psychobiol. 2018, 60, 292–302. [Google Scholar] [CrossRef]
- Chein, J.; Albert, D.; Brien, L.O.; Uckert, K.; Steinberg, L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Van Hoorn, J.; Van Dijk, E.; Güroğlu, B.; Crone, E.A. Neural correlates of prosocial peer influence on public goods game donations during adolescence. Soc. Cogn. Affect. Neurosci. 2016, 11, 923–933. [Google Scholar] [CrossRef]
- Somerville, L.H.; Haddara, N.; Sasse, S.F.; Skwara, A.C.; Moran, J.M.; Figner, B. Dissecting “Peer Presence” and “Decisions” to Deepen Understanding of Peer Influence on Adolescent Risky Choice. Child Dev. 2019, 90, 2086–2103. [Google Scholar] [CrossRef]
- Phillips, B.M.; Clancy-Menchetti, J.; Lonigan, C.J. Successful Phonological Awareness Instruction With Preschool Children: Lessons From the Classroom. Top. Early Child Spec. Educ. 2008, 28, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Starr, A.; Libertus, M.E.; Brannon, E.M. Number sense in infancy predicts mathematical abilities in childhood. Proc. Natl. Acad. Sci. USA 2013, 110, 18116–18120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, A.M.F.; Suzuki, S.; O’doherty, J.P.; Li, S.-C.; Eppinger, B. Supplemental Material for Risk Contagion by Peers Affects Learning and Decision-Making in Adolescents. J. Exp. Psychol. Gen. 2019. [Google Scholar] [CrossRef] [PubMed]
- Van Hoorn, J.; Crone, E.A.; Van Leijenhorst, L. Hanging Out With the Right Crowd: Peer Influence on Risk-Taking Behavior in Adolescence. J. Res. Adolesc. 2017, 27, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telzer, E.H.; Rogers, C.R.; Van Hoorn, J. Neural Correlates of Social Influence on Risk Taking and Substance Use in Adolescents. Curr. Addict. Reports 2017, 4, 333–341. [Google Scholar] [CrossRef]
- Prado, J.; Mutreja, R.; Zhang, H.; Mehta, R.; Desroches, A.S.; Minas, J.E.; Booth, J.R. Distinct representations of subtraction and multiplication in the neural systems for numerosity and language. Hum. Brain Mapp. 2011, 32, 1932–1947. [Google Scholar] [CrossRef] [Green Version]
- Prado, J.; Mutreja, R.; Booth, J.R. Developmental dissociation in the neural responses to simple multiplication and subtraction problems. Dev. Sci. 2014, 17, 537–552. [Google Scholar] [CrossRef] [Green Version]
- Schuch, S.; Konrad, K. Investigating task inhibition in children versus adults: A diffusion model analysis. J. Exp. Child Psychol. 2017, 156, 143–167. [Google Scholar] [CrossRef]
- RRatcliff, R.; Love, J.; Thompson, C.A.; Opfer, J.E. Children are not like older adults: A diffusion model analysis of developmental changes in speeded responses. Child Dev. 2012, 83, 367–381. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S. Précis of the number sense. Mind Language 2001, 16, 16–36. [Google Scholar] [CrossRef]
- Hyde, D.C.; Khanum, S.; Spelke, E.S. Brief non-symbolic, approximate number practice enhances subsequent exact symbolic arithmetic in children. Cognition 2014, 131, 92–107. [Google Scholar] [CrossRef] [Green Version]
- Ehri, L.C.; Nunes, S.R.; Willows, D.M.; Schuster, B.V.; Yaghoub-Zadeh, Z.; Shanahan, T. Phonemic Awareness Instruction Helps Children Learn to Read: Evidence From the National Reading Panel’s Meta-Analysis. Read. Res. Q. 2001, 36, 250–287. [Google Scholar] [CrossRef]
- Reimers, S.; Maylor, E.A. Task switching across the life Span: Effects of age on general and specific switch costs. Dev. Psychol. 2005, 41, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Gebuis, T.; Reynvoet, B. Generating nonsymbolic number stimuli. Behav. Res. Methods 2011, 43, 981–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Hondt, M.; Leybaert, J. Lateralization effects during semantic and rhyme judgement tasks in deaf and hearing subjects. Brain Lang. 2003, 87, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, M.; Demont, E.; Metz-Lutz, M.N.; Leybaert, J.; Alegria, J. Why do deaf participants have a lower performance than hearing participants in a visual rhyming task: A phonological hypothesis. Read. Writ. 2014, 27, 31–54. [Google Scholar] [CrossRef] [Green Version]
- New, B.; Pallier, C.; Ferrand, L.; Matos, R. Une base de données lexicales du français contemporain sur internet: LEXIQUE. Annee. Psychol. 2001, 101, 447–462. [Google Scholar] [CrossRef]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 2012, 42, 2031–2035. [Google Scholar] [CrossRef] [Green Version]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ding, M.; Kluger, B.M. Change in intraindividual variability over time as a key metric for defining performance-based cognitive fatigability. Brain Cogn. 2014, 85, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmon, D.C.; Kazakov, D.; Santos, O.; Kassel, M.T. Non-Gaussian Distributional Analyses of Reaction Times (RT): Improvements that Increase Efficacy of RT Tasks for Describing Cognitive Processes. Neuropsychol. Rev. 2018, 28, 359–376. [Google Scholar] [CrossRef]
- Heathcote, A.; Popiel, S.J.; Mewhort, D.J.K. Analysis of response time distributions: An example using the stroop task. Psychol. Bull. 1991, 109, 340–347. [Google Scholar] [CrossRef]
- Onagawa, R.; Shinya, M.; Ota, K.; Kudo, K. Risk aversion in the adjustment of speed-accuracy tradeoff depending on time constraints. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Bays, P.M.; Husain, M. Rapid decision-making under risk. Cogn. Neurosci. 2012, 3, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, R.; Thompson, C.A.; McKoon, G. Modeling individual differences in response time and accuracy in numeracy. Cognition 2015, 137, 115–136. [Google Scholar] [CrossRef] [Green Version]
- Ratcliff, R.; McKoon, G. The Diffusion Decision Model: Theory and Data for Two-Choice Decision Tasks. Neural Comput. 2008, 20, 873–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, A.; Voss, J.; Lerche, V. Assessing cognitive processes with diffusion model analyses: A tutorial based on fast-dm-30. Front. Psychol. 2015, 6, 336. [Google Scholar] [CrossRef] [Green Version]
- Voss, A.; Voss, J. A fast numerical algorithm for the estimation of diffusion model parameters. J. Math. Psychol. 2008, 52, 1–9. [Google Scholar] [CrossRef]
- Aron, A.; Aron, E.N.; Smollan, D. Inclusion of Other in the Self Scale and the Structure of Interpersonal Closeness. J. Pers. Soc. Psychol. 1992, 63, 596–612. [Google Scholar] [CrossRef]
- Gächter, S.; Starmer, C.; Tufano, F. Measuring the closeness of relationships: A comprehensive evaluation of the “inclusion of the other in the self” scale. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Cameron, L.; Rutland, A.; Brown, R.; Douch, R. Changing children’s intergroup attitudes toward refugees: Testing different models of extended contact. Child Dev. 2006, 77, 1208–1219. [Google Scholar] [CrossRef]
- Uziel, L. Individual differences in the social facilitation effect: A review and meta-analysis. J. Res. Pers. 2007, 41, 579–601. [Google Scholar] [CrossRef]
- Losier, G.F.; Vallerand, R.J.; Blais, M.R. Construction et validation de l’Échelle des Perceptions de Compétence dans les Domaines de Vie (EPCDV). Sci. Comport. 1993, 23, 1–16. [Google Scholar]
- Bouffard, T.; Seidah, A.; McIntyre, M.; Boivin, M.; Vezeau, C.; Cantin, S. Mesure de l’estime de soi à l’adolescence: Version canadienne-française du Self-Perception Profile for Adolescents de Harter. Can. J. Behav. Sci. 2002, 34, 158–162. [Google Scholar] [CrossRef]
- Plaisant, O.; Courtois, R.; Réveillère, C.; Mendelsohn, G.A.; John, O.P. Validation par analyse factorielle du Big Five Inventory français (BFI-Fr). Analyse convergente avec le NEO-PI-R. In Annales Médico-Psychologiques, Revue Psychiatrique; Elsevier Masson: Paris, France, 2010; Volume 168, pp. 97–106. [Google Scholar] [CrossRef] [Green Version]
- Olivier, M.; Herve, M. The Big Five Questionnaire for Children (BFQ-C): A French validation on 8- to 14-year-old children. Pers. Individ. Dif. 2015, 87, 55–58. [Google Scholar] [CrossRef]
- Steinberg, L.; Monahan, K.C. Age Differences in Resistance to Peer Influence. Dev Psychol. 2007, 43, 1531–1543. [Google Scholar] [CrossRef] [Green Version]
- Herman, C.P. The social facilitation of eating. A review. Appetite 2015, 86, 61–73. [Google Scholar] [CrossRef]
- Van Belle, J.; Van Raalten, T.; Bos, D.J.; Zandbelt, B.B.; Oranje, B.; Durston, S. Capturing the dynamics of response variability in the brain in ADHD. NeuroImage Clin. 2015, 7, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Tamnes, C.K.; Fjell, A.M.; Westlye, L.T.; Østby, Y.; Walhovd, K.B. Becoming consistent: Developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J. Neurosci. 2012, 32, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Bond, C. Social facilitation: A self-presentational view. J. Pers. Soc. Psychol. 1982, 42, 1042–1050. [Google Scholar] [CrossRef]
- Gerardin, P.; Miquée, A.; Urquizar, C.; Pélisson, D. Functional activation of the cerebral cortex related to sensorimotor adaptation of reactive and voluntary saccades. Neuroimage 2012, 61, 1100–1112. [Google Scholar] [CrossRef]
- Telzer, E.H. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Dev. Cogn. Neurosci. 2016, 17, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Van Duijvenvoorde, A.C.K.; Peters, S.; Braams, B.R.; Crone, E.A. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neurosci. Biobehav. Rev. 2016, 70, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Dickstein, R.; Gargan, M. Developmental effects in social facilitation and in being a model. J. Psychol. Interdiscip. Appl. 1978, 99, 143–150. [Google Scholar] [CrossRef]
- Bevington, J.; Wishart, J.G. The influence of classroom peers on cognitive performance in children with behavionral problems. Br. J. Educ. Psychol. 1999, 69, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, L.; Blakemore, S.J. Is there heightened sensitivity to social reward in adolescence? Curr. Opin. Neurobiol. 2016, 40, 81–85. [Google Scholar] [CrossRef]
- Knoll, L.J.; Magis-Weinberg, L.; Speekenbrink, M.; Blakemore, S.J. Social Influence on Risk Perception During Adolescence. Psychol. Sci. 2015, 26, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerville, L.H. Sensitivity to social evaluation. Curr. Dir. Psychol. Sci. 2013, 22, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, D.; De Smedt, B.; Grabner, R.H. Neuroeducation—A critical overview of an emerging field. Neuroethics 2012, 5, 105–117. [Google Scholar] [CrossRef]
- Horvath, J.C.; Donoghue, G.M. A bridge too far-revisited: Reframing Bruer’s Neuroeducation Argument for Modern Science of Learning Practitioners. Front. Psychol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotellar, C.; Cain, J. Research, Perspectives, and Recommendations on Implementing the Flipped Classroom. Am. J. Pharm. Educ. 2016, 80, 34. [Google Scholar] [CrossRef] [Green Version]
- Sanna, L.J. Self-efficacy theory: Implications for social facilitation and social loafing. J. Pers. Soc. Psychol. 1992, 62, 774–786. [Google Scholar] [CrossRef]
- Bhatia, P.; Delem, M.; Léone, J.; Boisin, E.; Cheylus, A.; Gardes, M.L.; Prado, J. The ratio processing system and its role in fraction understanding: Evidence from a match-to-sample task in children and adults with and without dyscalculia. Q. J. Exp. Psychol. 2020, 73, 2158–2176. [Google Scholar] [CrossRef]
- Huguet, P.; Galvaing, M.P.; Monteil, J.; Dumas, F. Social presence effects in the Stroop task: Further evidence for an attentional view of social facilitation. J. Pers. Soc. Psychol. 1999, 77, 1011–1025. [Google Scholar] [CrossRef]
- Huguet, P.; Barbet, I.; Belletier, C.; Monteil, J.; Fagot, J. Cognitive control under social influence in baboons. J. Exp. Psychol. Gen. 2014, 143, 2067–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normand, A.; Croizet, J.-C. Upward Social Comparison Generates Attentional Focusing When the Dimension of Comparison is Self-Threatening. Soc. Cogn. 2013, 31, 336–348. [Google Scholar] [CrossRef]
- Tesser, A.; Millar, M.; Moore, J. Some Affective Consequences of Social Comparison and Reflection Processes: The Pain and Pleasure of Being Close. J. Personal. Soc. Psychol. 1988, 54, 49–61. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tricoche, L.; Monfardini, E.; Reynaud, A.J.; Epinat-Duclos, J.; Pélisson, D.; Prado, J.; Meunier, M. Peer Presence Effect on Numerosity and Phonological Comparisons in 4th Graders: When Working with a SchoolMate Makes Children More Adult-like. Biology 2021, 10, 902. https://doi.org/10.3390/biology10090902
Tricoche L, Monfardini E, Reynaud AJ, Epinat-Duclos J, Pélisson D, Prado J, Meunier M. Peer Presence Effect on Numerosity and Phonological Comparisons in 4th Graders: When Working with a SchoolMate Makes Children More Adult-like. Biology. 2021; 10(9):902. https://doi.org/10.3390/biology10090902
Chicago/Turabian StyleTricoche, Leslie, Elisabetta Monfardini, Amélie J. Reynaud, Justine Epinat-Duclos, Denis Pélisson, Jérôme Prado, and Martine Meunier. 2021. "Peer Presence Effect on Numerosity and Phonological Comparisons in 4th Graders: When Working with a SchoolMate Makes Children More Adult-like" Biology 10, no. 9: 902. https://doi.org/10.3390/biology10090902
APA StyleTricoche, L., Monfardini, E., Reynaud, A. J., Epinat-Duclos, J., Pélisson, D., Prado, J., & Meunier, M. (2021). Peer Presence Effect on Numerosity and Phonological Comparisons in 4th Graders: When Working with a SchoolMate Makes Children More Adult-like. Biology, 10(9), 902. https://doi.org/10.3390/biology10090902





