Low SARS-CoV-2 Seroprevalence and No Active Infections among Dogs and Cats in Animal Shelters with Laboratory-Confirmed COVID-19 Human Cases among Employees
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
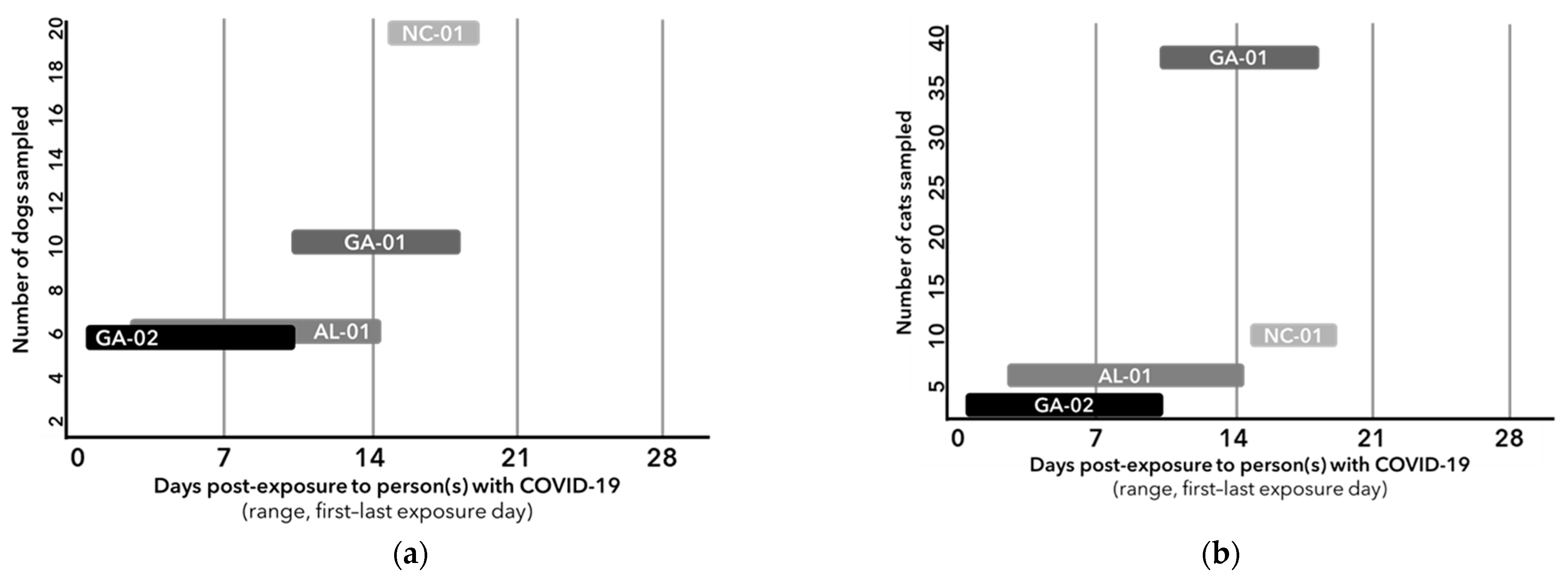
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimers
Appendix A
Appendix A.1. Animal Specimen Collection
Appendix A.2. Ribonucleic Acid Extraction and rRT-PCR
Appendix A.3. Virus Neutralization Assay
References
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, A.R. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission and response to reexposure of SARS-CoV-2 in domestic cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yuan, S.; Zhang, A.J.; Poon, V.K.; Chan, C.C.; Lee, A.C.; Fan, Z.; Li, C.; Liang, R.; Cao, J.; et al. Surgical mask partition reduces the risk of non-contact transmission in a golden Syrian hamster model for Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, S.-G.; Kim, S.-M.; Kim, E.-H.; Park, S.-J.; Yu, K.-M.; Chang, J.-H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; et al. Transmission of SARS-CoV-2 in Domestic Cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Smith, D.; Ghai, R.R.; Wallace, R.M.; Torchetti, M.K.; Loiacono, C.; Murrell, L.S.; Carpenter, A.; Moroff, S.; Rooney, J.A.; et al. First Reported Cases of SARS-CoV-2 Infection in Companion Animals—New York, March-April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Elia, G.; Grassi, A.; Giordano, A.; Desario, C.; Medardo, M.; Smith, S.L.; Anderson, E.R.; Prince, T.; Patterson, G.T.; et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 2020, 11, 6231. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Pauvolid-Corrêa, A.; Zecca, I.B.; Davila, E.; Auckland, L.D.; Roundy, C.M.; Tang, W.; Torchetti, M.; Killian, M.L.; Jenkins-Moore, M.; et al. SARS-CoV-2 Infections and Viral Isolations among Serially Tested Cats and Dogs in Households with Infected Owners in Texas, USA. Viruses 2021, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Goryoka, G.W.; Cossaboom, C.M.; Gharpure, R.; Dawson, P.; Tansey, C.; Rossow, J.; Mrotz, V.; Rooney, J.; Torchetti, M.; Loiacono, C.M.; et al. One Health Investigation of SARS-CoV-2 Infection and Seropositivity among Pets in Households with Confirmed Human COVID-19 Cases—Utah and Wisconsin, 2020. bioRxiv 2021. [Google Scholar] [CrossRef]
- Oreshkova, N.; jan Molenaar, R.; Vreman, S.; Harders, F.; Munnink, B.B.O.; der Honing, R.W.H.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.L.; Diel, D.G.; Wang, L.; Zec, S.; Laverack, M.; Martins, M.; Caserta, L.C.; Killian, M.L.; Terio, K.; Olmstead, C.; et al. SARS-COV-2 infection and longitudinal fecal screening in Malayan tigers (Panthera tigris jacksoni), Amur tigers (Panthera tigris altaica), and African lions (Panthera leo krugeri) at the Bronx Zoo, New York, USA. J. Zoo Wildl. Med. 2021, 51, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Coronavirus Resource Center. COVID-19 United States Cases by County. Johns Hopkins University & Medicine. Available online: https://coronavirus.jhu.edu/us-map (accessed on 7 September 2020).
- Centers for Disease Control and Prevention. Evaluation for SARS-CoV-2 Testing in Animals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/animals/animal-testing.html (accessed on 21 March 2021).
- U.S. Department of Agriculture. SARS-CoV-2 Case Definition as of 18 June 2020; U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services: Ames, IA, USA, 2020. Available online: https://www.aphis.usda.gov/animal_health/one_health/downloads/SARS-CoV-2-case-definition.pdf (accessed on 7 September 2021).
- Centers for Disease Control and Prevention. Real-Time RT-PCR Panel for Detection of 2019-nCoV; US Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. Available online: https://www.fda.gov/media/134922/download (accessed on 7 July 2020).
- Wang, X.; Guo, X.; Xin, Q.; Pan, Y.; Li, J.; Chu, Y.; Feng, Y.; Wang, Q. Neutralizing Antibodies Responses to SARS-CoV-2 in COVID-19 Inpatients and Convalescent Patients. Clin. Infect. Dis. 2020, 152, 82–87. [Google Scholar]
- Shelter Animals Count: The National Database. 2020 Animal Sheltering Statistics. Available online: https://www.shelteranimalscount.org/data-dashboards (accessed on 7 September 2020).
- Zhang, Q.; Zhang, H.; Gao, J.; Huang, K.; Yang, Y.; Hui, X.; He, X.; Li, C.; Gong, W.; Zhang, Y.; et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg. Microbes Infect. 2020, 9, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Temmam, S.; Barbarino, A.; Maso, D.; Behillil, S.; Enouf, V.; Huon, C.; Jaraud, A.; Chevallier, L.; Backovic, M.; Pérot, P.; et al. Absence of SARS-CoV-2 infection in cats and dogs in close contact with a cluster of COVID-19 patients in a veterinary campus. One Health 2020, 10, 100164. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.; Peiris, M.; Tam, K.W.S.; Law, P.Y.T.; Brackman, C.J.; To, E.M.W.; Yu, V.Y.T.; Chu, D.K.W.; Perera, R.A.P.M.; Sit, T.H.C. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerg. Infect. Dis. 2020, 26, 3071–3074. [Google Scholar] [CrossRef] [PubMed]
| Shelter ID | Total Animals n = 96 | Animal Species | Total Specimens n = 702 | Specimen Type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dog n = 42 | Cat N = 54 | Nasal Swabs * n = 120 | OP Swabs * n = 92 | Rectal Swabs * n = 95 | Fecal Specimens * n = 65 | Blood Specimens * n = 91 | Fur Swabs n = 148 | |||
| GA-01 | 47 | 10 | 37 | 292 | 47 | 45 † | 47 | 30 † | 38 † | 47 |
| GA-02 | 7 | 6 | 1 | 41 | 7 | 7 | 6† | 0 † | 7 | 7 |
| AL-01 | 12 | 6 | 6 | 85 | 12 | 12 | 12 | 7 † | 12 | 12 |
| NC-01 | 30 | 20 | 10 | 284 | 54 †,‡ | 28 | 30 | 28 | 31 § | 82 ‡ |
| Overall (n = 96) | |
|---|---|
| Reason for intake of the animal | |
| Owner surrender unrelated to COVID-19 | 21 (21.9%) |
| Stray/loose animal | 55 (57.3%) |
| Bite hold | 3 (3.1%) |
| Returned by adopter or foster | 1 (1.0%) |
| Transferred from another facility (e.g., animal shelter) | 16 (16.7%) |
| Source of exposure of the animal to SARS-CoV-2 | |
| Facility employee (paid or unpaid) | 92 (95.8%) |
| No known exposure * | 4 (4.0%) |
| Daily duration of the interaction between the animal and the person(s) with confirmed COVID-19 | |
| <1 h | 51 (53.1%) |
| 1–3 h | 5 (5.2%) |
| Unknown | 6 (6.3%) |
| No known exposure * | 4 (4.0%) |
| Data not collected (North Carolina shelter) | 34 (35.4%) |
| Types of interaction the person(s) with confirmed COVID-19 had with the animal | |
| Petting/cuddling | 54 (56.3%) |
| Cleaning the animal’s housing | 51 (53.1%) |
| Going for walks or play yards | 49 (51.0%) |
| Feeding | 50 (52.1%) |
| Play groups with other animals | 35 (36.5%) |
| Veterinary care | 7 (7.3%) |
| Indirect—within 6 feet but no direct contact | 6 (6.3%) |
| Behavioral assessments or training | 3 (3.1%) |
| In staff member’s office full time | 2 (2.1%) |
| Grooming | 1 (1.0%) |
| Picked up | 1 (1.0%) |
| Transport, bringing into intake | 1 (1.0%) |
| Quarantine setting for the animal(s) exposed to SARS-CoV-2 | |
| Yes, quarantine area for animals exposed to SARS-CoV-2 or with other medical conditions or exposures | 3 (3.1%) |
| No | 93 (96.9%) |
| Type of animal interaction with other animals (e.g., dogs participating in play group) | |
| Play group | 4 (4.2%) |
| Goes out to the yard | 1 (1.0%) |
| Interacts with other outdoor cats on shelter property | 1 (1.0%) |
| No interaction with other animals | 1 (1.0%) |
| Unknown | 89 (92.7%) |
| Animal’s current housing | |
| Housed individually, with other animals in the same room (e.g., a kennel with multiple, single-animal enclosures in one room) | 61 (63.5%) |
| Co-housed with other animals (e.g., communal cat room, multiple dogs in one run) | 30 (31.3%) |
| Housed individually, no other animals in the room (e.g., in a staff member’s office) | 3 (3.1%) |
| Free-roaming outdoors (community cats) | 1 (1.0%) |
| Other, unspecified | 1 (1.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossaboom, C.M.; Medley, A.M.; Spengler, J.R.; Kukielka, E.A.; Goryoka, G.W.; Baird, T.; Bhavsar, S.; Campbell, S.; Campbell, T.S.; Christensen, D.; et al. Low SARS-CoV-2 Seroprevalence and No Active Infections among Dogs and Cats in Animal Shelters with Laboratory-Confirmed COVID-19 Human Cases among Employees. Biology 2021, 10, 898. https://doi.org/10.3390/biology10090898
Cossaboom CM, Medley AM, Spengler JR, Kukielka EA, Goryoka GW, Baird T, Bhavsar S, Campbell S, Campbell TS, Christensen D, et al. Low SARS-CoV-2 Seroprevalence and No Active Infections among Dogs and Cats in Animal Shelters with Laboratory-Confirmed COVID-19 Human Cases among Employees. Biology. 2021; 10(9):898. https://doi.org/10.3390/biology10090898
Chicago/Turabian StyleCossaboom, Caitlin M., Alexandra M. Medley, Jessica R. Spengler, Esther A. Kukielka, Grace W. Goryoka, Tiffany Baird, Swity Bhavsar, Stefanie Campbell, Thomas S. Campbell, Daniel Christensen, and et al. 2021. "Low SARS-CoV-2 Seroprevalence and No Active Infections among Dogs and Cats in Animal Shelters with Laboratory-Confirmed COVID-19 Human Cases among Employees" Biology 10, no. 9: 898. https://doi.org/10.3390/biology10090898
APA StyleCossaboom, C. M., Medley, A. M., Spengler, J. R., Kukielka, E. A., Goryoka, G. W., Baird, T., Bhavsar, S., Campbell, S., Campbell, T. S., Christensen, D., Condrey, J. A., Dawson, P., Doty, J. B., Feldpausch, A., Gabel, J., Jones, D., Lim, A., Loiacono, C. M., Jenkins-Moore, M., ... Barton Behravesh, C. (2021). Low SARS-CoV-2 Seroprevalence and No Active Infections among Dogs and Cats in Animal Shelters with Laboratory-Confirmed COVID-19 Human Cases among Employees. Biology, 10(9), 898. https://doi.org/10.3390/biology10090898






