Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals
Abstract
Simple Summary
Abstract
1. Introduction
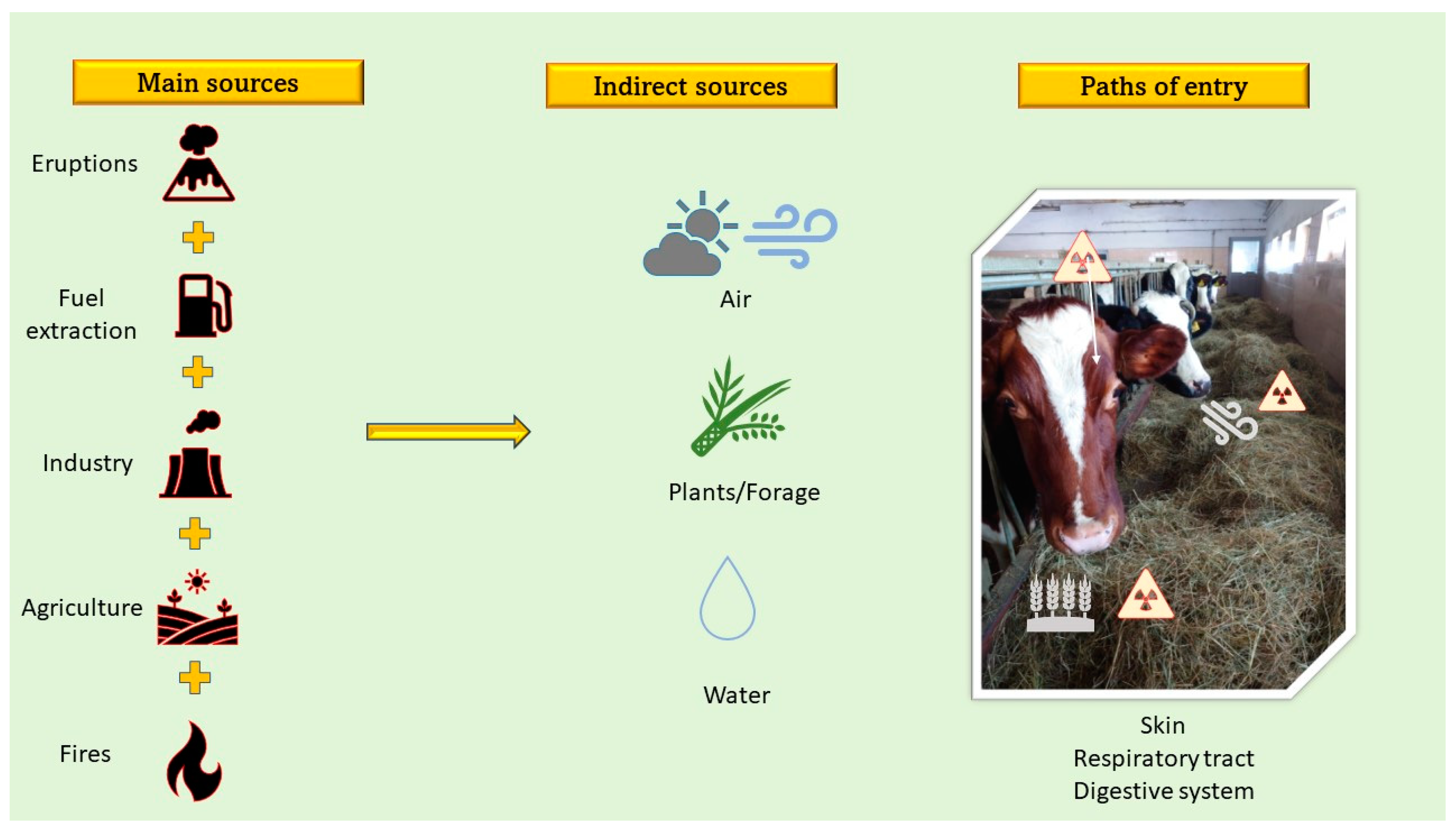
2. Toxic Metals and Sources
3. Fertility
3.1. Disorders of Gametogenesis
3.2. Gamete Dysfunction
3.3. Fetal Abnormalities/Stillbirths
3.4. Disturbances in the Synthesis of Reproductive Hormones
4. Toxic Effects of Metals
4.1. Cadmium
4.2. Lead
4.3. Arsenic
4.4. Mercury
5. Monitoring of Environmental Contamination with Toxic Metals
6. Preventing the Bioaccumulation of Toxic Metals
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volkov, R.; Ezhkova, A. Migration of heavy metals in the system “soil-plant-animal-livestock products”. BIO Web Conf. 2020, 27, 00068. [Google Scholar] [CrossRef]
- Bonnet, C.; Bouamra-Mechemache, Z.; Corre, T. An Environmental Tax Towards More Sustainable Food: Empirical Evidence of the Consumption of Animal Products in France. Ecol. Econ. 2018, 147, 48–61. [Google Scholar] [CrossRef]
- Marinova, D.; Bogueva, D. Planetary health and reduction in meat consumption. Sustain. Earth 2019, 2, 3. [Google Scholar] [CrossRef]
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2020–2029; OECD Publishing: Paris, France, 2020; ISBN 978-92-64-31767-3. [Google Scholar]
- Wang, X.; Zheng, G.; Chen, T.; Shi, X.; Wang, Y.; Nie, E.; Liu, J. Effect of phosphate amendments on improving the fertilizer efficiency and reducing the mobility of heavy metals during sewage sludge composting. J. Environ. Manag. 2019, 235, 124–132. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S. Impact of environmental contaminants on reproductive health of male domestic ruminants: A review. Environ. Sci. Pollut. Res. 2020, 27, 3819–3836. [Google Scholar] [CrossRef]
- Verma, R.; Vijayalakshmy, K.; Chaudhiry, V. Detrimental impacts of heavy metals on animal reproduction: A review. J. Entomol. Zool. Stud. 2018, 6, 27–30. [Google Scholar]
- Das, A.; Joardar, M.; Chowdhury, N.R.; De, A.; Mridha, D.; Roychowdhury, T. Arsenic toxicity in livestock growing in arsenic endemic and control sites of West Bengal: Risk for human and environment. Environ. Geochem. Health 2021, 43, 3005–3025. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic dietary exposure to inorganic arsenic. EFSA J. 2021, 19, e06380. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- The Council of The European Union. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption, Official Journal of trhe European COmmunities, L 330/32, Brussels, 5 December 1998. Available online: https://www.consilium.europa.eu/en/council-eu/ (accessed on 1 September 2021).
- FAO. Who Joint Fao/Who Food Standards Programme Codex Committee On Contaminants. In Foods 13th Session; Codex Alimentarius Commission: Yogyakarta, Indonesia, 2019. [Google Scholar]
- FAO. OECD Dairy and dairy products. In Oecd-Fao Agricultural Outlook 2020–2029; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Shahbandeh, M. Consumption of Milk* Per Capita in the EU 2014–2020; Statista, 2021; Available online: https://www.statista.com/statistics/1192244/europe-per-capita-milk-consumption/ (accessed on 1 September 2021).
- Ismail, A.; Riaz, M.; Akhtar, S.; Goodwill, J.E.; Sun, J. Heavy metals in milk: Global prevalence and health risk assessment. Toxin Rev. 2017, 38, 1–12. [Google Scholar] [CrossRef]
- Terver Ubwa, S.; Ejiga, R.; Chudi Okoye, P.-A.; Msughter Amua, Q. Assessment of Heavy metals in the Blood and Some Selected, Entrails of Cows, Goat and Pigs Slaughtered at Warukum SAbattoir, Makurdi-Nigeria. Adv. Anal. Chem. 2017, 7, 7–12. [Google Scholar] [CrossRef]
- Somasundaram, J.; Krishnasamy, R.; Svithri, P. Biotransfer of heave metals in Jersey cows. Indian J. Anim. Sci. 2005, 75, 1257–1260. [Google Scholar]
- Pšenková, M.; Toman, R.; Tančin, V. Concentrations of toxic metals and essential elements in raw cow milk from areas with potentially undisturbed and highly disturbed environment in Slovakia. Environ. Sci. Pollut. Res. 2020, 27, 26763–26772. [Google Scholar] [CrossRef]
- Akar, Y.; Ahmad, N.; Khalıd, M. The effect of cadmium on the bovine in vitro oocyte maturation and early embryo development. Int. J. Vet. Sci. Med. 2018, 6, S73–S77. [Google Scholar] [CrossRef]
- Kabala, C.; Galka, B.; Jezierski, P. Assessment and monitoring of soil and plant contamination with trace elements around Europe’s largest copper ore tailings impoundment. Sci. Total Environ. 2020, 738, 139918. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, K.A.; Movsesyan, H.S.; Ghazaryan, N.P.; Shalunts, S.V. Assessment of Heavy Metal Contamination of Soils Around Agarak (Ra) Copper-Molybdenum Mine Complex. Chem. Biol. 2016, 50, 34–42. [Google Scholar]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; de la Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of mining tailings in México for the possible recovery of strategic elements. J. S. Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Alam, M.S.; Silpa, M.V. Impacts of heavy metal feed contaminants in cattle farming. In Indo Australian Workshop Transfer of Mitigastion Technologies for Heat Stress in Farm Animals 5–7 February 2020; Bangalore, India, 2020; pp. 147–152. Available online: https://scholar.google.co.jp/scholar?q=Impacts+of+heavy+metal+feed+contaminants+in+cattle+farming&hl=zh-CN&as_sdt=0&as_vis=1&oi=scholart (accessed on 1 September 2021).
- Akarsu, S.A.; Yilmaz, M.; Niksarlioglu, S.; Kulahci, F.; Risvanli, A. Radioactivity, heavy metal and oxidative stress measurements in the follicular fluids of cattle bred near a coal-fired power plant. J. Anim. Plant Sci. 2017, 27, 373–378. [Google Scholar]
- Basri; Sakakibara, M.; Sera, K.; Kurniawan, I.A. Mercury Contamination of Cattle in Artisanal and Small-Scale Gold Mining in Bombana, Southeast Sulawesi, Indonesia. Geosciences 2017, 7, 133. [Google Scholar] [CrossRef]
- Keil, D.E.; Berger-Ritchie, J.; McMillin, G.A. Testing for Toxic Elements: A Focus on Arsenic, Cadmium, Lead, and Mercury. Lab. Med. 2011, 42, 735–742. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kňažická, Z.; Lukáčová, J.; Schneidgenová, M.; Goc, Z.; Greń, A.; Szabó, C.; Massányi, P.; Lukáč, N. The impact of lead and cadmium on selected motility, prooxidant and antioxidant parameters of bovine seminal plasma and spermatozoa. J. Environ. Sci. Health Part A 2013, 48, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Aglan, H.S.; Gebremedhn, S.; Salilew-Wondim, D.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell Tissue Res. 2020, 380, 643–655. [Google Scholar] [CrossRef]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Silva, E.F.D.S.J.D.; Missio, D.; Martinez, C.S.; Vassallo, D.V.; Peçanha, F.M.; Leivas, F.G.; Brum, D.D.S.; Wiggers, G.A. Mercury at environmental relevant levels affects spermatozoa function and fertility capacity in bovine sperm. J. Toxicol. Environ. Health A 2019, 82, 268–278. [Google Scholar] [CrossRef]
- Zhao, L.; Ru, Y.; Liu, M.; Tang, J.; Zheng, J.; Wu, B.; Gu, Y.; Shi, H. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoubb, A.; Agarwald, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Llamas Luceño, N.; de Souza Ramos Angrimani, D.; de Cássia Bicudo, L.; Szymańska, K.J.; Van Poucke, M.; Demeyere, K.; Meyer, E.; Peelman, L.; Mullaart, E.; Broekhuijse, M.L.W.J.; et al. Exposing dairy bulls to high temperature-humidity index during spermatogenesis compromises subsequent embryo development in vitro. Theriogenology 2020, 141, 16–25. [Google Scholar] [CrossRef]
- Rao, T.K.S.; Mohanty, T.K.; Bhakat, M. Assessment of antioxidants for preservation of crossbred bull semen in Tris based extender. Indian J. Anim. Res. 2017, 1–5. Available online: https://scholar.google.co.jp/scholar?q=Assessment+of+antioxidants+for+preservation+of+crossbred+bull+semen+in+Tris+based+extender&hl=zh-CN&as_sdt=0&as_vis=1&oi=scholart (accessed on 1 September 2021). [CrossRef][Green Version]
- Chand, N.; Tyagi, S.; Prasad, R.; Dutta, D.; Sirohi, A.S.; Sharma, A.; Tyagi, R. Effect of heavy metals on oxidative markers and semen quality parameters in HF crossbred bulls. Indian J. Anim. Sci. 2019, 89, 5. [Google Scholar]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.J.; Chaube, R. Differential Effects of Heavy Metals (Cadmium, Cobalt, Lead and Mercury) on Oocyte Maturation and Ovulation of the Catfish Heteropneustes fossilis: An In Vitro Study. Turk. J. Fish. Aquat. Sci. 2018, 18, 1205–1214. [Google Scholar] [CrossRef]
- Mahey, S. A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl. Sci. 2020, 2, 12. [Google Scholar] [CrossRef]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Nikoloff, N.; Pascua, A.M.; Furnus, C.C. High copper concentrations produce genotoxicity and cytotoxicity in bovine cumulus cells. Environ. Sci. Pollut. Res. 2017, 24, 20041–20049. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Yamashiro, H.; Abe, Y.; Fukuda, T.; Kino, Y.; Kawaguchi, I.; Kuwahara, Y.; Fukumoto, M.; Takahashi, S.; Suzuki, M.; Kobayashi, J.; et al. Effects of radioactive caesium on bull testes after the Fukushima nuclear plant accident. Sci. Rep. 2013, 3, 2850. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Yousafzai, A.M. Chromium toxicity in fish: A review article. J. Entomol. Zool. Stud. 2017, 5, 1483–1488. [Google Scholar]
- Sevim, Ç.; Doğan, E.; Comakli, S. Cardiovascular disease and toxic metals. Curr. Opin. Toxicol. 2020, 19, 88–92. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-M.; Lo, K.; Zheng, T.-Z.; Yang, J.-L.; Bai, Y.-N.; Feng, Y.-Q.; Cheng, N.; Liu, S.-M. Environmental heavy metals and cardiovascular diseases: Status and future direction. Chronic Dis. Transl. Med. 2020, 6, 251–259. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Liu, X.; Zhang, C.; Wang, P.; Zhao, X. Circulatory Levels of Toxic Metals (Aluminum, Cadmium, Mercury, Lead) in Patients with Alzheimer’s Disease: A Quantitative Meta-Analysis and Systematic Review. J. Alzheimers Dis. 2018, 62, 361–372. [Google Scholar] [CrossRef]
- Arslan, H.H.; Saripinar Aksu, D.; Ozdemir, S.; Yavuz, O.; Or, M.E.; Barutcu, U.B. Anayol Yakınında Yaşayan Sığırlarda Kan Ağır Metal, İz Element Seviyeleri ve Antioksidan Metabolizma Arasındaki İlişkinin Değerlendirilmesi. Kafkas Univ. Vet. Fak. Derg. 2009. [Google Scholar] [CrossRef]
- Suhartono, E.; Thalib, I.; Aflanie, I.; Noor, Z.; Idroes, R. Study of Interaction between Cadmium and Bovine Serum Albumin with UV-Vis Spectrocopy Approach. IOP Conf. Ser. Mater. Sci. Eng. 2018, 350, 012008. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mandalunis, P.M. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public. Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ugulu, I.; Umar, S.; Ahmad, K.; Mehmood, N.; Ashfaq, A.; Bashir, H.; Sohail, M. Potential Toxic Metal Accumulation in Soil, Forage and Blood Plasma of Buffaloes Sampled from Jhang, Pakistan. Bull. Environ. Contam. Toxicol. 2018, 101, 235–242. [Google Scholar] [CrossRef]
- López-Alonso, M.; Rey-Crespo, F.; Herrero-Latorre, C.; Miranda, M. Identifying sources of metal exposure in organic and conventional dairy farming. Chemosphere 2017, 185, 1048–1055. [Google Scholar] [CrossRef]
- Adamse, P.; Van der Fels-Klerx, H.J.; de Jong, J. Cadmium, lead, mercury and arsenic in animal feed and feed materials–trend analysis of monitoring results. Food Addit. Contam. Part A 2017, 34, 1298–1311. [Google Scholar] [CrossRef]
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy metal and pesticide levels in dairy products: Evaluation of human health risk. Food Chem. Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef] [PubMed]
- European Union European Union. Commission regulation (EC) no. 1881/ 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006, 364, 5–24. [Google Scholar]
- Kołacz, R.; Dobrzański, Z.; Kupczyński, R.; Cwynar, P.; Opaliński, S.; Pogoda-Sewerniak, K. Impact of the copper industry on the content of selected heavy metals and biochemical indicators in the blood of dairy cows. Med. Weter. 2017, 73, 171–175. [Google Scholar] [CrossRef][Green Version]
- Kołacz, R.; Spiak, Z.; Dobrzański, Z.; Opaliński, S.; Kowalska, N.; Cwynar, P.; Kupczyński, R. Copper and zinc concentrations in plant and animal raw materials collected in the vicinity of the Żelazny Most waste treatment tailings pond. J. Elem. 2020, 25, 1423–1434. [Google Scholar] [CrossRef]
- Kupczyński, R.; Bednarski, M.; Śpitalniak, K.; Pogoda-Sewerniak, K. Effects of protein-iron complex concentrate supplementation on iron metabolism, oxidative and immune status in preweaning calves. Int. J. Mol. Sci. 2017, 18, 1501. [Google Scholar] [CrossRef]
- Xing, T.; Gao, F.; Tume, R.K.; Zhou, G.; Xu, X. Stress Effects on Meat Quality: A Mechanistic Perspective. Compr. Rev. Food Sci. Food Saf. 2019, 18, 380–401. [Google Scholar] [CrossRef]
- Harada, M.; Honma, Y.; Yoshizumi, T.; Kumamoto, K.; Oe, S.; Harada, N.; Tanimoto, A.; Yabuki, K.; Karasuyama, T.; Yoneda, A.; et al. Idiopathic copper toxicosis: Is abnormal copper metabolism a primary cause of this disease? Med. Mol. Morphol. 2020, 53, 50–55. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Wang, S.-L.; Fadrowski, J.J.; Navas-Acien, A.; Kuo, C.-C. Urinary Concentration Correction Methods for Arsenic, Cadmium, and Mercury: A Systematic Review of Practice-Based Evidence. Curr. Environ. Health Rep. 2019, 6, 188–199. [Google Scholar] [CrossRef] [PubMed]
- McGeehan, S.; Baszler, T.; Gaskill, C.; Johnson, J.; Smith, L.; Raisbeck, M.; Schrier, N.; Harris, H.; Talcott, P. Interlaboratory comparison of heavy metal testing in animal diagnostic specimens and feed using inductively coupled plasma–mass spectrometry. J. Vet. Diagn. Investig. 2020, 32, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M. Heavy metal concentrations in bovine tissues (muscle, liver and kidney) and their relationship with heavy metal contents in consumed feed. Ecotoxicol. Environ. Saf. 2018, 154, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wei, Z.; Wei, D.; Ahmed Mohamed, T.; Yu, H.; Xie, X.; Zhu, L.; Zhao, Y. Roles of adding biochar and montmorillonite alone on reducing the bioavailability of heavy metals during chicken manure composting. Bioresour. Technol. 2019, 294, 122199. [Google Scholar] [CrossRef]
- Li, S.; Zou, D.; Li, L.; Wu, L.; Liu, F.; Zeng, X.; Wang, H.; Zhu, Y.; Xiao, Z. Evolution of heavy metals during thermal treatment of manure: A critical review and outlooks. Chemosphere 2020, 247, 125962. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, F.; Elbasiouny, H.; Ali, R.; Brevik, E.C. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water. Air. Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]

| Toxic Metal | Source | Penetration into Body | Bioaccumulation | Toxic Effect | References |
|---|---|---|---|---|---|
| Pb | Mining Pb-acid batteries, gasoline in petrol, pigments in paints, electronic wastes, pesticides, fires, volcanic eruptions | Ingestion of contaminate feed and water | Liver, kidney, brain, bones, testes, epididymis, seminal vesicle, ejaculate, follicle fluid | Male: Damage to sperm reduces spermatozoa count and motility, azoospermia, asthenozoospermia, morphological abnormalities of sperm and disorders in prostatic function Female: Abortion, infertility, pregnancy, hypertension, premature calving, follicular artesia | [7,28,35,36] |
| Hg | Coal combustion, gold mining, pesticides, volcanic eruptions, wildfire | Ingestion of contaminated feed and water, inhalation | Brain, kidney, boses, blood, hair, liver | Damage of testicular cells reduces semen quality and spermatogenesis, increases oxidative stress, and damages sperm membrane Fetotoxic, Ataxia, neuromuscular incoordination, convulsions | [6,25,30,36] |
| As | Mining, smelting, combustion of fossil fuel, production of chemotherapeutic drugs of cancer, production of glass, volcano eruptions | Ingestion of contaminated feed and water | Liver, heart, lungs, kidney, blood, keratin tissues | Decrease of testosterone, LH, FSH, increase of cortisol, Leydig cells, atrophy Ataxia, anorexia, diarrhea, hepatoxic | [6,8,26,36] |
| Cd | Combustion of fossil fuels, Ni-Cd batteries, mining and smelting operations, volcano eruptions, forest fires, dust storm, erosion | Ingestion of contaminate feed and water | Kidney, lungs, bones, testes, epididymis, seminal vesicle, ejaculate | Male: Abnormalities of sperm caused separated tail of sperm, a decrease in the mass of the testes, changes in Sertoli cells and seminal tubules, damage sperm DNA, decreases antioxidant status in semen, Female: reduces ovarian function, suppresses oocyte maturation, cytotoxic to oocytes, reduces oocytogenesis | [6,19,35,37] |
| Co | Mining, incinerators, leaching, hard metal production, batteries | Through the digestive system | Bones | Reduces oocyte maturation, ovulation, gametogenesis, | [37,38] |
| Cu | Mining, cement production, coatings and painting, transport, metal mining | Ingestion with contaminated feed | Lung, spleen, liver, kidney, intestine | Reduces mitochondrial activity, induces apoptosis of cumulus cells, damages ovaries, causes abnormalities of sperm, causes the separated tail of sperm, reduces sperm motility | [6,36,39] |
| Mn | Transport, soil fertilizers, waste management, industry | Through the digestive system or respiratory system | Follicular fluid, | Damage acrosome and plasma membranes reduce fertility | [6,39] |
| Ni | Coal combustion | Through the digestive system | Kidneys | Causes separated flagellum of spermatozoa and reduces sperm concentration and motility | [6,40] |
| Cs | Radiography, gamma radiation | Through ionizing radiation | Testicles, vas deferens | Reduces fertility causes abnormal sperm and azoospermia; damages of spermatogonia impairs male and female germinal cells, induces testicular cancer, the radioactivity of cesium is transferred from cow to the fetus via the placenta | [6,41] |
| Cr | Leather tanneries, textile industry, steel industry, coal combustion, wood burning, production of dyes and wood preservatives | Ingestion with contaminated feed | Liver, kidney, | tumors in stomach | [40,42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrzecińska, M.; Kowalczyk, A.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals. Biology 2021, 10, 882. https://doi.org/10.3390/biology10090882
Wrzecińska M, Kowalczyk A, Cwynar P, Czerniawska-Piątkowska E. Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals. Biology. 2021; 10(9):882. https://doi.org/10.3390/biology10090882
Chicago/Turabian StyleWrzecińska, Marcjanna, Alicja Kowalczyk, Przemysław Cwynar, and Ewa Czerniawska-Piątkowska. 2021. "Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals" Biology 10, no. 9: 882. https://doi.org/10.3390/biology10090882
APA StyleWrzecińska, M., Kowalczyk, A., Cwynar, P., & Czerniawska-Piątkowska, E. (2021). Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals. Biology, 10(9), 882. https://doi.org/10.3390/biology10090882








