GARP: A Key Target to Evaluate Tumor Immunosuppressive Microenvironment
Abstract
Simple Summary
Abstract
1. Introduction
2. GARP Expression, Structure and Function
2.1. GARP Expression
2.2. GARP Structure
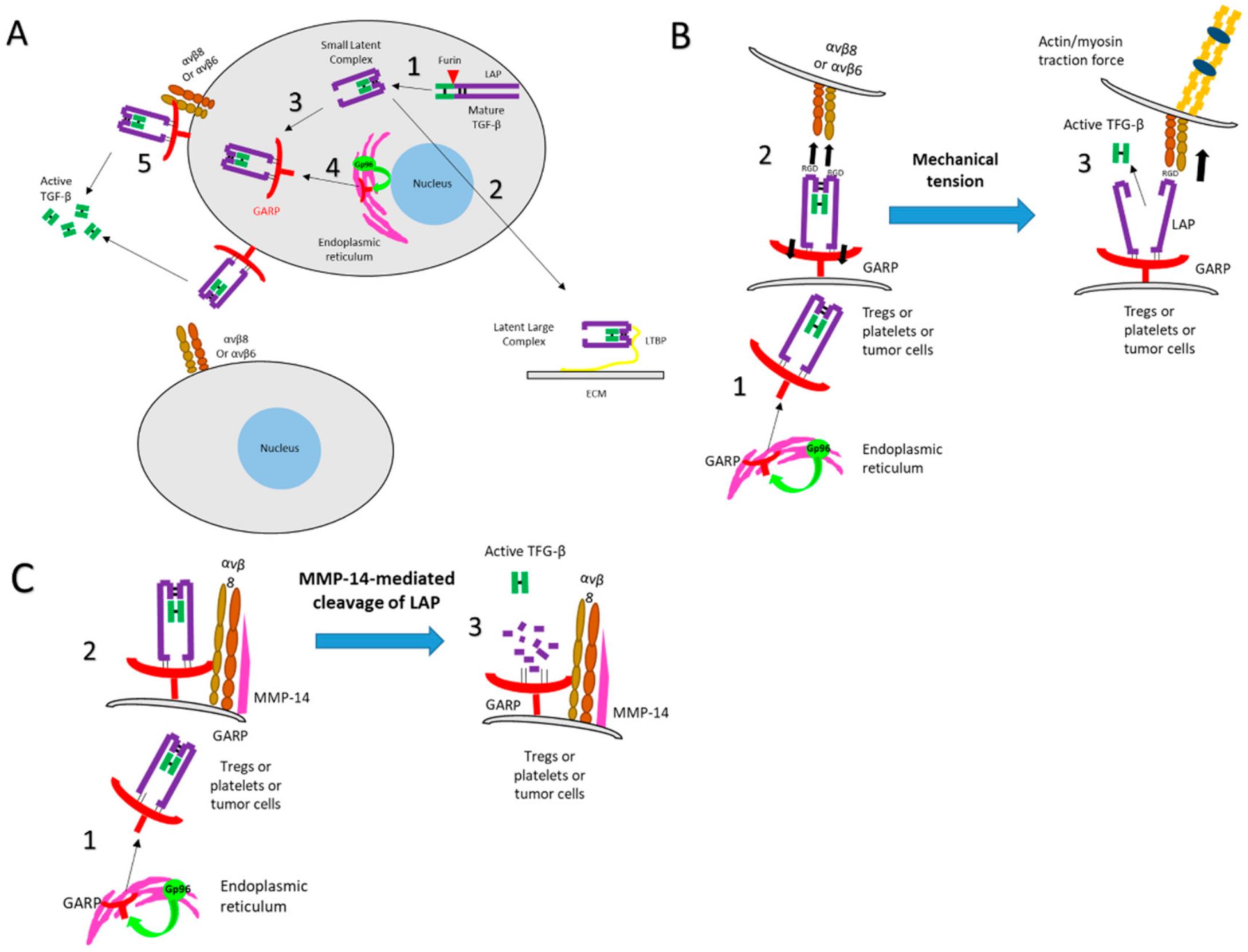
2.3. GARP Promotes the Activation of Biologically Active TGF-β
3. GARP in Cancer
3.1. GARP and Cancer Cells
3.2. GARP and Cells of the TME
3.2.1. Tregs
3.2.2. Platelets
3.2.3. Other Cells
3.3. Soluble GARP
3.3.1. Modulation of T-Cell Function by Soluble GARP
3.3.2. sGARP Influences the Polarization of Macrophages
4. GARP as a Therapeutic Target in Cancer
5. GARP as a Biomarker in Cancer: Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metelli, A.; Salem, M.; Wallace, C.H.; Wu, B.X.; Li, A.; Li, X.; Li, Z. Immunoregulatory Functions and the Therapeutic Implications of GARP-TGF-β in Inflammation and Cancer. J. Hematol. Oncol. 2018, 11, 24. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Grown Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J. Targeting TGF-β Signaling for Therapeutic Gain. Cold Spring Harb. Perspect. Biol. 2017, 9, a022301. [Google Scholar] [CrossRef]
- Lin, R.-L.; Zhao, L.-J. Mechanistic Basis and Clinical Relevance of the Role of Transforming Growth Factor-β in Cancer. Cancer Biol. Med. 2015, 12, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Brunen, D.; Willems, S.M.; Kellner, U.; Midgley, R.; Simon, I.; Bernards, R. TGF-β: An Emerging Player in Drug Resistance. Cell Cycle 2013, 12, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Derynck, R.; Budi, E.H. Specificity, Versatility, and Control of TGF-β Family Signaling. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Shevach, E.M. Garp as a Therapeutic Target for Modulation of T Regulatory Cell Function. Expert Opin. Ther. Targets 2017, 21, 191–200. [Google Scholar] [CrossRef]
- Metelli, A.; Wu, B.X.; Fugle, C.W.; Rachidi, S.; Sun, S.; Zhang, Y.; Wu, J.; Tomlinson, S.; Howe, P.H.; Yang, Y.; et al. Surface Expression of TGFβ Docking Receptor GARP Promotes Oncogenesis and Immune Tolerance in Breast Cancer. Cancer Res. 2016, 76, 7106–7117. [Google Scholar] [CrossRef]
- Liénart, S.; Merceron, R.; Vanderaa, C.; Lambert, F.; Colau, D.; Stockis, J.; van der Woning, B.; De Haard, H.; Saunders, M.; Coulie, P.G.; et al. Structural Basis of Latent TGF-Β1 Presentation and Activation by GARP on Human Regulatory T Cells. Science 2018, 362, 952–956. [Google Scholar] [CrossRef]
- Wang, R.; Wan, Q.; Kozhaya, L.; Fujii, H.; Unutmaz, D. Identification of a Regulatory T Cell Specific Cell Surface Molecule That Mediates Suppressive Signals and Induces Foxp3 Expression. PLoS ONE 2008, 3, e2705. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.H.; Wu, B.X.; Salem, M.; Ansa-Addo, E.A.; Metelli, A.; Sun, S.; Gilkeson, G.; Shlomchik, M.J.; Liu, B.; Li, Z. B Lymphocytes Confer Immune Tolerance via Cell Surface GARP-TGF-β Complex. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Macaulay, I.C.; Tijssen, M.R.; Thijssen-Timmer, D.C.; Gusnanto, A.; Steward, M.; Burns, P.; Langford, C.F.; Ellis, P.D.; Dudbridge, F.; Zwaginga, J.-J.; et al. Comparative Gene Expression Profiling of in Vitro Differentiated Megakaryocytes and Erythroblasts Identifies Novel Activatory and Inhibitory Platelet Membrane Proteins. Blood 2007, 109, 3260–3269. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets Subvert T Cell Immunity against Cancer via GARP-TGFβ Axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef]
- Carrillo-Galvez, A.B.; Cobo, M.; Cuevas-Ocaña, S.; Gutiérrez-Guerrero, A.; Sánchez-Gilabert, A.; Bongarzone, P.; García-Pérez, A.; Muñoz, P.; Benabdellah, K.; Toscano, M.G.; et al. Mesenchymal Stromal Cells Express GARP/LRRC32 on Their Surface: Effects on Their Biology and Immunomodulatory Capacity. Stem Cells 2015, 33, 183–195. [Google Scholar] [CrossRef]
- Li, Y.; Kim, B.-G.; Qian, S.; Letterio, J.J.; Fung, J.J.; Lu, L.; Lin, F. Hepatic Stellate Cells Inhibit T Cells through Active TGF-Β1 from a Cell Surface-Bound Latent TGF-Β1/GARP Complex. J. Immunol. 2015, 195, 2648–2656. [Google Scholar] [CrossRef]
- Jin, H.; Sun, L.; Tang, L.; Yu, W.; Li, H. Expression of GARP Is Increased in Tumor-Infiltrating Regulatory T Cells and Is Correlated to Clinicopathology of Lung Cancer Patients. Front. Immunol 2017, 8, 138. [Google Scholar] [CrossRef]
- Hahn, S.A.; Neuhoff, A.; Landsberg, J.; Schupp, J.; Eberts, D.; Leukel, P.; Bros, M.; Weilbaecher, M.; Schuppan, D.; Grabbe, S.; et al. A Key Role of GARP in the Immune Suppressive Tumor Microenvironment. Oncotarget 2016, 7, 42996–43009. [Google Scholar] [CrossRef] [PubMed]
- Szepetowski, P.; Ollendorff, V.; Grosgeorge, J.; Courseaux, A.; Birnbaum, D.; Theillet, C.; Gaudray, P. DNA Amplification at 11q13.5-Q14 in Human Breast Cancer. Oncogene 1992, 7, 2513–2517. [Google Scholar]
- Carrillo-Gálvez, A.B.; Quintero, J.E.; Rodríguez, R.; Menéndez, S.T.; Victoria González, M.; Blanco-Lorenzo, V.; Allonca, E.; de Araújo Farias, V.; González-Correa, J.E.; Erill-Sagalés, N.; et al. GARP Promotes the Proliferation and Therapeutic Resistance of Bone Sarcoma Cancer Cells through the Activation of TGF-β. Cell Death Dis. 2020, 11, 985. [Google Scholar] [CrossRef]
- Li, K.; Chen, F.; Xie, H. Decreased FOXP3+ and GARP+ Tregs to Neoadjuvant Chemotherapy Associated with Favorable Prognosis in Advanced Gastric Cancer. OncoTargets 2016, 9, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Wallace, C.; Velegraki, M.; Li, A.; Ansa-Addo, E.; Metelli, A.; Kwon, H.; Riesenberg, B.; Wu, B.; Zhang, Y.; et al. GARP Dampens Cancer Immunity by Sustaining Function and Accumulation of Regulatory T Cells in the Colon. Cancer Res. 2019, 79, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. Higher Frequencies of GARP(+)CTLA-4(+)Foxp3(+) T Regulatory Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Are Associated with Impaired T-Cell Functionality. Cancer Res. 2013, 73, 2435–2444. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, M.; Yang, J.; Zheng, Y.; Xiao, Y.; Liu, W.; Ren, F. Increased Expression of GARP in Papillary Thyroid Carcinoma. Endocr. Pathol. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Zimmer, N.; Kim, E.; Schupp, J.; Sprang, B.; Leukel, P.; Khafaji, F.; Ringel, F.; Sommer, C.; Tuettenberg, J.; Tuettenberg, A. GARP as an Immune Regulatory Molecule in the Tumor Microenvironment of Glioblastoma Multiforme. Int. J. Mol. Sci. 2019, 20, 3676. [Google Scholar] [CrossRef]
- Hahn, S.A.; Stahl, H.F.; Becker, C.; Correll, A.; Schneider, F.-J.; Tuettenberg, A.; Jonuleit, H. Soluble GARP Has Potent Antiinflammatory and Immunomodulatory Impact on Human CD4+ T Cells. Blood 2013, 122, 1182–1191. [Google Scholar] [CrossRef]
- Fridrich, S.; Hahn, S.A.; Linzmaier, M.; Felten, M.; Zwarg, J.; Lennerz, V.; Tuettenberg, A.; Stöcker, W. How Soluble GARP Enhances TGFβ Activation. PLoS ONE 2016, 11, e0153290. [Google Scholar] [CrossRef]
- Roubin, R.; Pizette, S.; Ollendorff, V.; Planche, J.; Birnbaum, D.; Delapeyriere, O. Structure and Developmental Expression of Mouse Garp, a Gene Encoding a New Leucine-Rich Repeat-Containing Protein. Int. J. Dev. Biol. 1996, 40, 545–555. [Google Scholar] [PubMed]
- Ollendorff, V.; Noguchi, T.; deLapeyriere, O.; Birnbaum, D. The GARP Gene Encodes a New Member of the Family of Leucine-Rich Repeat-Containing Proteins. Cell Growth Differ. 1994, 5, 213–219. [Google Scholar]
- Tran, D.Q.; Andersson, J.; Wang, R.; Ramsey, H.; Unutmaz, D.; Shevach, E.M. GARP (LRRC32) Is Essential for the Surface Expression of Latent TGF-Beta on Platelets and Activated FOXP3+ Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 13445–13450. [Google Scholar] [CrossRef]
- Zhou, Q.; Haupt, S.; Prots, I.; Thümmler, K.; Kremmer, E.; Lipsky, P.E.; Schulze-Koops, H.; Skapenko, A. MiR-142-3p Is Involved in CD25+ CD4 T Cell Proliferation by Targeting the Expression of Glycoprotein A Repetitions Predominant. J. Immunol. 2013, 190, 6579–6588. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.X.; Metelli, A.; Thaxton, J.E.; Hong, F.; Rachidi, S.; Ansa-Addo, E.; Sun, S.; Vasu, C.; Yang, Y.; et al. GP96 Is a GARP Chaperone and Controls Regulatory T Cell Functions. J. Clin. Investig. 2015, 125, 859–869. [Google Scholar] [CrossRef]
- Liu, C.-J.; Lin, S.-C.; Chen, Y.-J.; Chang, K.-M.; Chang, K.-W. Array-Comparative Genomic Hybridization to Detect Genomewide Changes in Microdissected Primary and Metastatic Oral Squamous Cell Carcinomas. Mol. Carcinog. 2006, 45, 721–731. [Google Scholar] [CrossRef]
- Martinez-Cardús, A.; Martinez-Balibrea, E.; Bandrés, E.; Malumbres, R.; Ginés, A.; Manzano, J.L.; Taron, M.; Garcia-Foncillas, J.; Abad, A. Pharmacogenomic Approach for the Identification of Novel Determinants of Acquired Resistance to Oxaliplatin in Colorectal Cancer. Mol. Cancer Ther. 2009, 8, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Hughes-Davies, L.; Vallès, H.; Orsetti, B.; Cuny, M.; Ursule, L.; Kouzarides, T.; Theillet, C. Amplification of the BRCA2 Pathway Gene EMSY in Sporadic Breast Cancer Is Related to Negative Outcome. Clin. Cancer Res. 2004, 10, 5785–5791. [Google Scholar] [CrossRef]
- DeRycke, M.S.; Charbonneau, B.; Preston, C.C.; Kalli, K.R.; Knutson, K.L.; Rider, D.N.; Goode, E.L. Toward Understanding the Genetics of Regulatory T Cells in Ovarian Cancer. Oncoimmunology 2013, 2, e24535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stockis, J.; Dedobbeleer, O.; Lucas, S. Role of GARP in the Activation of Latent TGF-Β1. Mol. Biosyst. 2017, 13, 1925–1935. [Google Scholar] [CrossRef]
- Bank, R.P.D. RCSB PDB—6GFF: Structure of GARP (LRRC32) in Complex with Latent TGF-Beta1 and MHG-8 Fab. Available online: https://www.rcsb.org/structure/6GFF (accessed on 1 July 2021).
- Wang, R.; Zhu, J.; Dong, X.; Shi, M.; Lu, C.; Springer, T.A. GARP Regulates the Bioavailability and Activation of TGFβ. Mol. Biol. Cell 2012, 23, 1129–1139. [Google Scholar] [CrossRef]
- Stockis, J.; Colau, D.; Coulie, P.G.; Lucas, S. Membrane Protein GARP Is a Receptor for Latent TGF-Beta on the Surface of Activated Human Treg. Eur. J. Immunol. 2009, 39, 3315–3322. [Google Scholar] [CrossRef]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and Extracellular TGF-β Signaling in Cancer: Some Recent Topics. Front. Med. 2018, 12, 387–411. [Google Scholar] [CrossRef]
- Edwards, J.P.; Thornton, A.M.; Shevach, E.M. Release of Active TGF-Β1 from the Latent TGF-Β1/GARP Complex on T Regulatory Cells Is Mediated by Integrin Β8. J. Immunol. 2014, 193, 2843–2849. [Google Scholar] [CrossRef]
- Howley, B.V.; Hussey, G.S.; Link, L.A.; Howe, P.H. Translational Regulation of Inhibin ΒA by TGFβ via the RNA-Binding Protein HnRNP E1 Enhances the Invasiveness of Epithelial-to-Mesenchymal Transitioned Cells. Oncogene 2016, 35, 1725–1735. [Google Scholar] [CrossRef]
- Sun, L.; Jin, H.; Li, H. GARP: A Surface Molecule of Regulatory T Cells That Is Involved in the Regulatory Function and TGF-β Releasing. Oncotarget 2016, 7, 42826–42836. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Li, J.; Kulkarni, A.B.; Perruche, S.; Chen, W. A Critical Function for TGF-Beta Signaling in the Development of Natural CD4+CD25+Foxp3+ Regulatory T Cells. Nat. Immunol. 2008, 9, 632–640. [Google Scholar] [CrossRef]
- Zheng, Y.; Josefowicz, S.; Chaudhry, A.; Peng, X.P.; Forbush, K.; Rudensky, A.Y. Role of Conserved Non-Coding DNA Elements in the Foxp3 Gene in Regulatory T-Cell Fate. Nature 2010, 463, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kozhaya, L.; Mercer, F.; Khaitan, A.; Fujii, H.; Unutmaz, D. Expression of GARP Selectively Identifies Activated Human FOXP3 Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 13439–13444. [Google Scholar] [CrossRef]
- Probst-Kepper, M.; Geffers, R.; Kröger, A.; Viegas, N.; Erck, C.; Hecht, H.-J.; Lünsdorf, H.; Roubin, R.; Moharregh-Khiabani, D.; Wagner, K.; et al. GARP: A Key Receptor Controlling FOXP3 in Human Regulatory T Cells. J. Cell Mol. Med. 2009, 13, 3343–3357. [Google Scholar] [CrossRef]
- Reinwald, S.; Wiethe, C.; Westendorf, A.M.; Breloer, M.; Probst-Kepper, M.; Fleischer, B.; Steinkasserer, A.; Buer, J.; Hansen, W. CD83 Expression in CD4+ T Cells Modulates Inflammation and Autoimmunity. J. Immunol. 2008, 180, 5890–5897. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Novak, A.J.; Ziesmer, S.C.; Witzig, T.E.; Ansell, S.M. CD70+ Non-Hodgkin Lymphoma B Cells Induce Foxp3 Expression and Regulatory Function in Intratumoral CD4+CD25 T Cells. Blood 2007, 110, 2537–2544. [Google Scholar] [CrossRef]
- Probst-Kepper, M.; Kröger, A.; Garritsen, H.S.P.; Buer, J. Perspectives on Regulatory T Cell Therapies. Transfus Med. Hemother. 2009, 36, 302–308. [Google Scholar] [CrossRef]
- Jurk, K.; Kehrel, B.E. Platelets: Physiology and Biochemistry. Semin. Thromb. Hemost. 2005, 31, 381–392. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Zimmerman, G.A. Platelets: Signaling Cells in the Immune Continuum. Trends Immunol. 2004, 25, 489–495. [Google Scholar] [CrossRef]
- Möhle, R.; Green, D.; Moore, M.A.; Nachman, R.L.; Rafii, S. Constitutive Production and Thrombin-Induced Release of Vascular Endothelial Growth Factor by Human Megakaryocytes and Platelets. Proc. Natl. Acad. Sci. USA 1997, 94, 663–668. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of Platelets to Tumour Metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gabazza, E.C.; Takeya, H.; Deguchi, H.; Urano, H.; Adachi, Y.; Suzuki, K. Prothrombin and Its Derivatives Stimulate Motility of Melanoma Cells. Thromb. Haemost. 1998, 80, 407–412. [Google Scholar] [CrossRef]
- Erpenbeck, L.; Schön, M.P. Deadly Allies: The Fatal Interplay between Platelets and Metastasizing Cancer Cells. Blood 2010, 115, 3427–3436. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-like Transition and Promotes Metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Pinedo, H.M.; Verheul, H.M.; D’Amato, R.J.; Folkman, J. Involvement of Platelets in Tumour Angiogenesis? Lancet 1998, 352, 1775–1777. [Google Scholar] [CrossRef]
- Sierko, E.; Wojtukiewicz, M.Z. Platelets and Angiogenesis in Malignancy. Semin. Thromb. Hemost. 2004, 30, 95–108. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Zimmer, N.; Krebs, F.K.; Zimmer, S.; Mitzel-Rink, H.; Kumm, E.J.; Jurk, K.; Grabbe, S.; Loquai, C.; Tuettenberg, A. Platelet-Derived GARP Induces Peripheral Regulatory T Cells—Potential Impact on T Cell Suppression in Patients with Melanoma-Associated Thrombocytosis. Cancers 2020, 12, 3653. [Google Scholar] [CrossRef]
- Hu, Q.; Hisamatsu, T.; Haemmerle, M.; Cho, M.S.; Pradeep, S.; Rupaimoole, R.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Wong, S.T.C.; Sood, A.K.; et al. Role of Platelet-Derived Tgfβ1 in the Progression of Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5611–5621. [Google Scholar] [CrossRef]
- Kopp, H.-G.; Placke, T.; Salih, H.R. Platelet-Derived Transforming Growth Factor-Beta down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef]
- Metelli, A.; Wu, B.X.; Riesenberg, B.; Guglietta, S.; Huck, J.D.; Mills, C.; Li, A.; Rachidi, S.; Krieg, C.; Rubinstein, M.P.; et al. Thrombin Contributes to Cancer Immune Evasion via Proteolysis of Platelet-Bound GARP to Activate LTGF-β. Sci. Transl. Med. 2020, 12, eaay4860. [Google Scholar] [CrossRef]
- Philippe, C.; Philippe, B.; Fouqueray, B.; Perez, J.; Lebret, M.; Baud, L. Protection from Tumor Necrosis Factor-Mediated Cytolysis by Platelets. Am. J. Pathol. 1993, 143, 1713–1723. [Google Scholar]
- Haselmayer, P.; Grosse-Hovest, L.; von Landenberg, P.; Schild, H.; Radsak, M.P. TREM-1 Ligand Expression on Platelets Enhances Neutrophil Activation. Blood 2007, 110, 1029–1035. [Google Scholar] [CrossRef]
- Stockis, J.; Liénart, S.; Colau, D.; Collignon, A.; Nishimura, S.L.; Sheppard, D.; Coulie, P.G.; Lucas, S. Blocking Immunosuppression by Human Tregs in Vivo with Antibodies Targeting Integrin AVβ8. Proc. Natl. Acad. Sci. USA 2017, 114, E10161–E10168. [Google Scholar] [CrossRef]
- Qian, W.-J.; Monroe, M.E.; Liu, T.; Jacobs, J.M.; Anderson, G.A.; Shen, Y.; Moore, R.J.; Anderson, D.J.; Zhang, R.; Calvano, S.E.; et al. Quantitative Proteome Analysis of Human Plasma Following in Vivo Lipopolysaccharide Administration Using 16O/18O Labeling and the Accurate Mass and Time Tag Approach. Mol. Cell Proteom. 2005, 4, 700–709. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Gao, L.; Zhao, Y.; Lai, J.; Lu, D.; Bao, R.; Jia, B.; Zhong, L.; Wang, F.; et al. Noninvasive Imaging of CD206-Positive M2 Macrophages as an Early Biomarker for Post-Chemotherapy Tumor Relapse and Lymph Node Metastasis. Theranostics 2017, 7, 4276–4288. [Google Scholar] [CrossRef]
- Erreni, M.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011, 4, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Chaumonnot, K.; Masson, S.; Sikner, H.; Bouchard, A.; Baverel, V.; Bellaye, P.-S.; Collin, B.; Garrido, C.; Kohli, E. The HSP GRP94 Interacts with Macrophage Intracellular Complement C3 and Impacts M2 Profile during ER Stress. Cell Death Dis. 2021, 12, 114. [Google Scholar] [CrossRef]
- Cuende, J.; Liénart, S.; Dedobbeleer, O.; van der Woning, B.; De Boeck, G.; Stockis, J.; Huygens, C.; Colau, D.; Somja, J.; Delvenne, P.; et al. Monoclonal Antibodies against GARP/TGF-Β1 Complexes Inhibit the Immunosuppressive Activity of Human Regulatory T Cells in Vivo. Sci. Transl. Med. 2015, 7, 284ra56. [Google Scholar] [CrossRef]
- de Streel, G.; Bertrand, C.; Chalon, N.; Liénart, S.; Bricard, O.; Lecomte, S.; Devreux, J.; Gaignage, M.; De Boeck, G.; Mariën, L.; et al. Selective Inhibition of TGF-Β1 Produced by GARP-Expressing Tregs Overcomes Resistance to PD-1/PD-L1 Blockade in Cancer. Nat. Commun. 2020, 11, 4545. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-Dependent Depletion of Tumor-Infiltrating Regulatory T Cells Co-Defines the Efficacy of Anti-CTLA-4 Therapy against Melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Xing, H.; Liang, C.; Xu, X.; Sun, H.; Ma, X.; Jiang, Z. Mesenchymal Stroma/Stem-like Cells of GARP Knockdown Inhibits Cell Proliferation and Invasion of Mouse Colon Cancer Cells (MC38) through Exosomes. J. Cell Mol. Med. 2020, 24, 13984–13990. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchard, A.; Collin, B.; Garrido, C.; Bellaye, P.-S.; Kohli, E. GARP: A Key Target to Evaluate Tumor Immunosuppressive Microenvironment. Biology 2021, 10, 836. https://doi.org/10.3390/biology10090836
Bouchard A, Collin B, Garrido C, Bellaye P-S, Kohli E. GARP: A Key Target to Evaluate Tumor Immunosuppressive Microenvironment. Biology. 2021; 10(9):836. https://doi.org/10.3390/biology10090836
Chicago/Turabian StyleBouchard, Alexanne, Bertrand Collin, Carmen Garrido, Pierre-Simon Bellaye, and Evelyne Kohli. 2021. "GARP: A Key Target to Evaluate Tumor Immunosuppressive Microenvironment" Biology 10, no. 9: 836. https://doi.org/10.3390/biology10090836
APA StyleBouchard, A., Collin, B., Garrido, C., Bellaye, P.-S., & Kohli, E. (2021). GARP: A Key Target to Evaluate Tumor Immunosuppressive Microenvironment. Biology, 10(9), 836. https://doi.org/10.3390/biology10090836






