Absence of Light Exposure Increases Pathogenicity of Pseudomonas aeruginosa Pneumonia-Associated Clinical Isolates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. P. aeruginosa Isolates
2.2. Phenotypic Profiling of P. aeruginosa
2.3. Preparation of the P. aeruginosa Fractions
2.4. Cell-Culture Maintenance
2.5. Characterization of the P. aeruginosa Fractions
2.5.1. DLS and Zeta Potential Analysis
2.5.2. Cell Morphology
2.5.3. Cytotoxicity Evaluation by the MTT Viability Assay
2.6. Statistical Analysis
3. Results
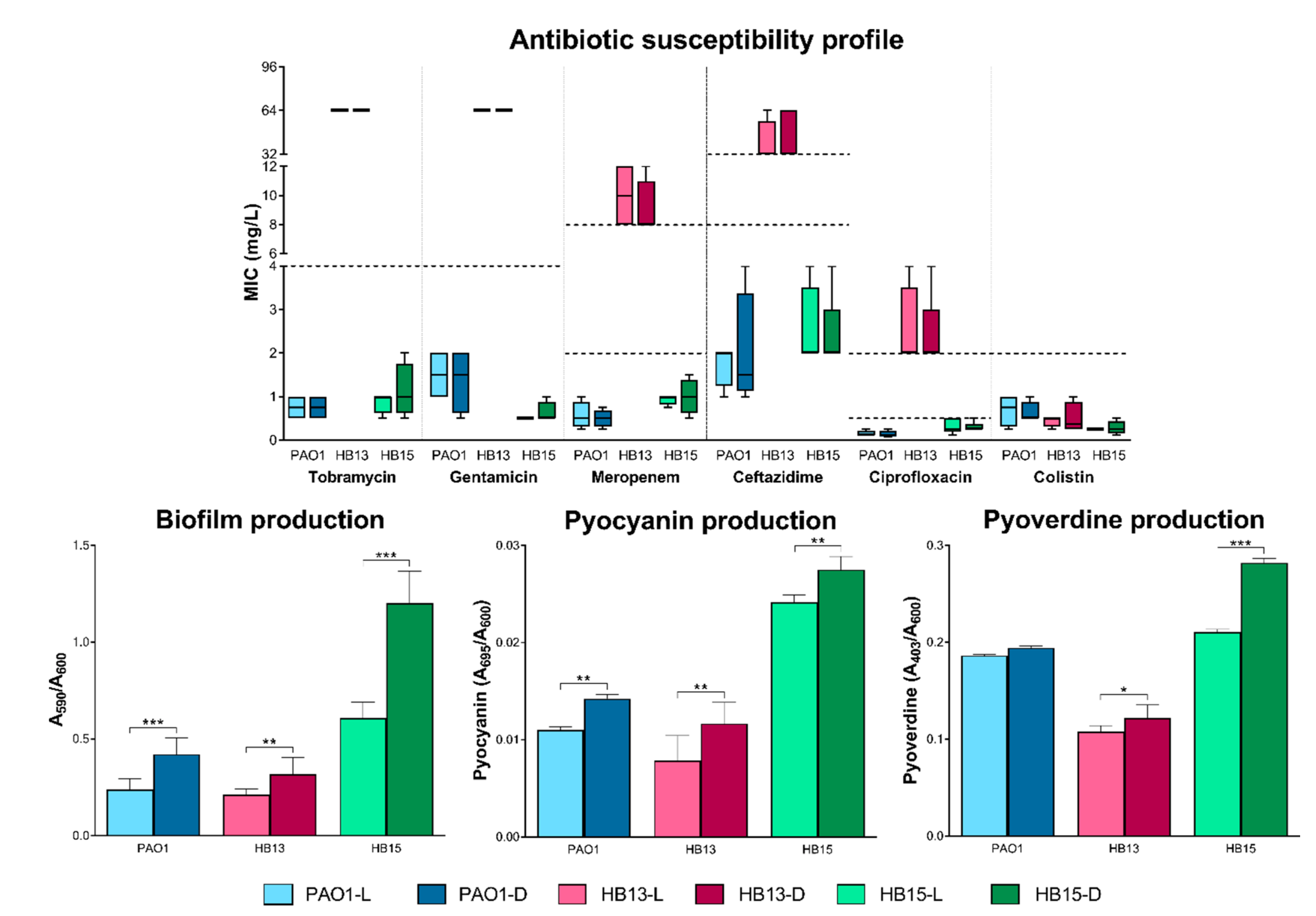
3.1. Phenotypic Plasticity Driven by Light Exposure
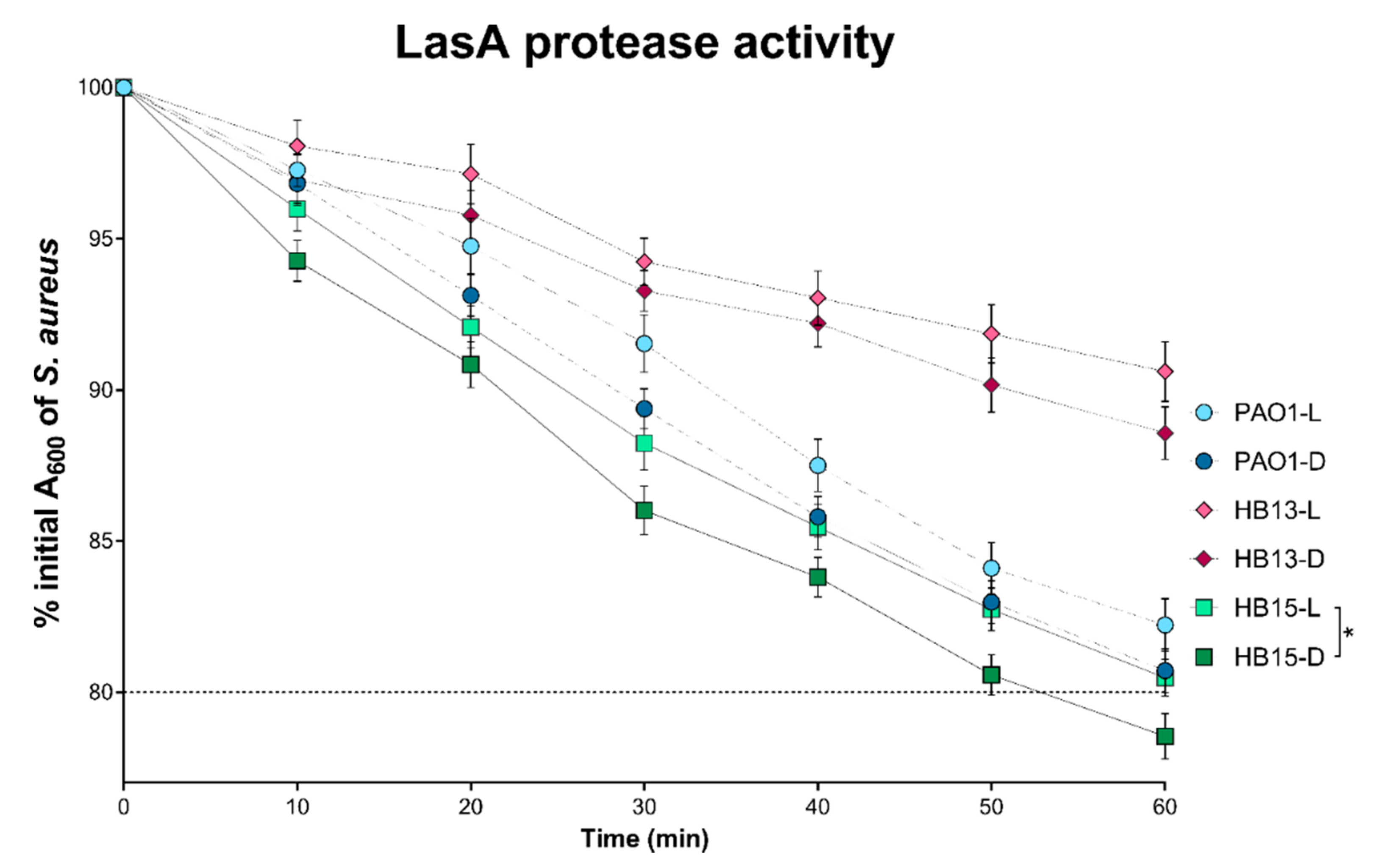
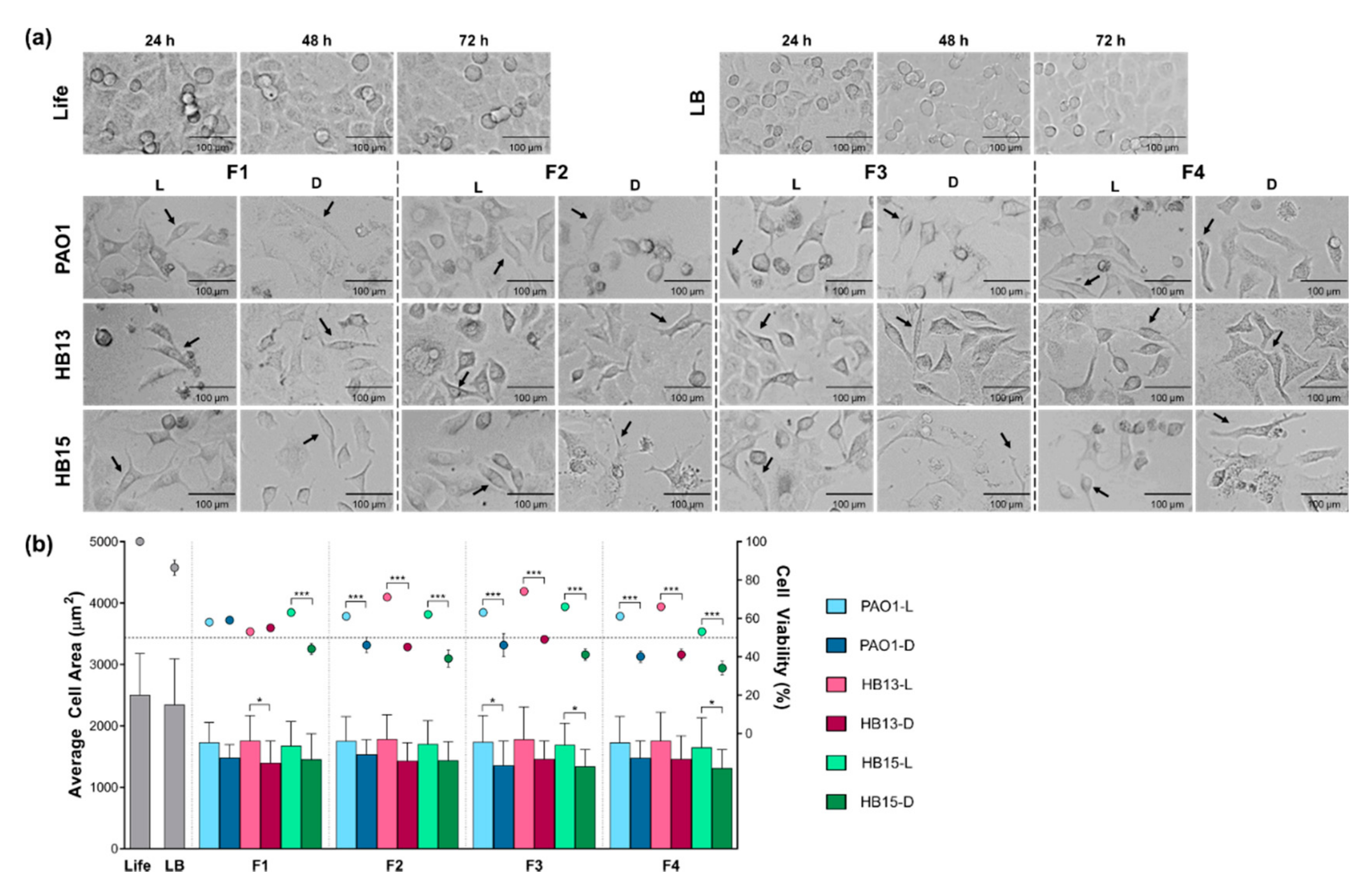
3.2. Assessment of the Pathogenic Potential of P. aeruginosa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Solh, A.A.; Alhajhusain, A. Update on the treatment of Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 2009, 64, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Sadikot, R.T.; Blackwell, T.S.; Christman, J.W.; Prince, A.S. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 1209–1223. [Google Scholar] [CrossRef] [Green Version]
- Gellatly, S.L.; Hancock, R.E.W. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.G.; Urbach, J.M.; Wu, G.; Liberati, N.T.; Feinbaum, R.L.; Miyata, S.; Diggins, L.T.; He, J.; Saucier, M.; Déziel, E.; et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006, 7, R90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef] [Green Version]
- Vessillier, S.; Delolme, F.; Bernillon, J.; Saulnier, J.; Wallach, J. Hydrolysis of glycine-containing elastin pentapeptides by LasA, a metalloelastase from Pseudomonas aeruginosa. Eur. J. Biochem. 2001, 268, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Rahme, L.G.; Ausubel, F.M.; Cao, H.; Drenkard, E.; Goumnerov, B.C.; Lau, G.W.; Mahajan-Miklos, S.; Plotnikova, J.; Tan, M.W.; Tsongalis, J.; et al. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 2000, 97, 8815–8821. [Google Scholar] [CrossRef] [Green Version]
- Kirienko, N.V.; Ausubel, F.M.; Ruvkun, G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 1821–1826. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomelsky, M.; Hoff, W.D. Light helps bacteria make important lifestyle decisions. Trends Microbiol. 2011, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kuk, J.; Moffat, K. Crystal structure of Pseudomonas aeruginosa bacteriophytochrome: Photoconversion and signal transduction. Proc. Natl. Acad. Sci. USA 2008, 105, 14715–14720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Horst, M.A.; Key, J.; Hellingwerf, K.J. Photosensing in chemotrophic, non-phototrophic bacteria: Let there be light sensing too. Trends Microbiol. 2007, 15, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Jemielita, M.; Stergioula, V.; Tikhonov, M.; Bassler, B.L. Photosensing and quorum sensing are integrated to control Pseudomonas aeruginosa collective behaviors. PLoS Biol. 2019, 17, e3000579. [Google Scholar] [CrossRef] [Green Version]
- Kahl, L.J.; Price-Whelan, A.; Dietrich, L.E.P. Light-mediated decreases in cyclic di-GMP levels inhibit structure formation in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2020, 202, e00117–e00120. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Soares-Castro, P.; Faustino, A.; Santos, H.M.; Capelo, J.L.; Santos, P. Identification of genomic loci associated with genotypic and phenotypic variation among Pseudomonas aeruginosa clinical isolates from pneumonia. Microb. Pathog. 2019, 136, 103702. [Google Scholar] [CrossRef] [PubMed]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing (M100–ED31); Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2021. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Zegans, M.E.; Wagner, J.C.; Cady, K.C.; Murphy, D.M.; Hammond, J.H.; O’Toole, G.A. Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J. Bacteriol. 2009, 191, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, P.; Smart, C.H.M.; Morgan, J.A.W.; Panagea, S.; Walshaw, M.J.; Hart, C.A.; Geffers, R.; Tümmler, B.; Winstanley, C. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J. Bacteriol. 2005, 187, 4908–4920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerley, D.F.; Lamont, I.L. Characterization and genetic manipulation of peptide synthetases in Pseudomonas aeruginosa PAO1 in order to generate novel pyoverdines. Chem. Biol. 2004, 11, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, G.L. Review of the folin phenol protein quantitation method of lowry, rosebrough, farr and randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef]
- Alves, N.J.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci. Rep. 2016, 6, 24866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baviskar, S.N. A quick & automated method for measuring cell area using ImageJ. Am. Biol. Teach. 2011, 73, 554–556. [Google Scholar]
- Tinoco, A.; Ribeiro, A.; Oliveira, C.; Parpot, P.; Gomes, A.; Cavaco-Paulo, A. Albumin/asparaginase capsules prepared by ultrasound to retain ammonia. Appl. Microbiol. Biotechnol. 2016, 100, 9499–9508. [Google Scholar] [CrossRef] [Green Version]
- Toska, J.; Sun, Y.; Carbonell, D.A.; Foster, A.N.S.; Jacobs, M.R.; Pearlman, E.; Rietsch, A. Diversity of virulence phenotypes among type III secretion negative Pseudomonas aeruginosa clinical isolates. PLoS ONE 2014, 9, e86829. [Google Scholar] [CrossRef]
- Barbieri, J.T.; Riese, M.J.; Aktories, K. Bacterial toxins that modify the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 2002, 18, 315–344. [Google Scholar] [CrossRef]
- Broquet, A.; Asehnoune, K. Apoptosis induced by Pseudomonas aeruginosa: A lonely killer? Microb. Biotechnol. 2015, 8, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Sunny, S.S.; Oliphant, V.; Perry, J.; Brodlie, M.; Johnson, G.E.; Ward, C.; Gould, K.; Corris, P.A.; De Soyza, A.; et al. Pseudomonas aeruginosa accentuates epithelial-to-mesenchymal transition in the airway. Eur. Respir. J. 2011, 37, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Visible light-induced killing of bacteria as a function of wavelength: Implication for wound healing. Lasers Surg. Med. 2010, 42, 467–472. [Google Scholar] [CrossRef] [PubMed]
| MTT Endpoint | IC50 (μg/mL) ± SD | ||
|---|---|---|---|
| PAO1 | HB13 | HB15 | |
| 24 h | 4.91 ± 0.14 | 3.73 ± 0.11 | 3.08 ± 0.08 |
| 48 h | 4.66 ± 0.21 | 3.33 ± 0.07 | 2.79 ± 0.05 |
| 72 h | 3.61 ± 0.05 | 2.91 ± 0.05 | 1.59 ± 0.04 |
| P. aeruginosa | Fraction | Cell Viability (%) ± SD | ΔL→D | ΔL→D/IC50 | |
|---|---|---|---|---|---|
| L | D | ||||
| PAO1 | F1 | 58 ± 2 | 59 ± 3 | +1 | +0.2 |
| F2 | 61 ± 2 | 46 ± 4 | −15 | −3.2 | |
| F3 | 63 ± 3 | 46 ± 7 | −17 | −3.6 | |
| F4 | 61 ± 3 | 40 ± 5 | −21 | −4.5 | |
| HB13 | F1 | 53 ± 3 | 55 ± 1 | +2 | +0.6 |
| F2 | 71 ± 6 | 45 ± 1 | −26 | −7.8 | |
| F3 | 74 ± 4 | 49 ± 2 | −25 | −7.5 | |
| F4 | 66 ± 2 | 41 ± 3 | −25 | −7.5 | |
| HB15 | F1 | 63 ± 5 | 44 ± 4 | −19 | −6.8 |
| F2 | 62 ± 4 | 39 ± 5 | −23 | −8.2 | |
| F3 | 66 ± 3 | 41 ± 3 | −25 | −9.0 | |
| F4 | 53 ± 3 | 34 ± 4 | −19 | −6.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, C.S.; Ribeiro, A.; Gomes, A.C.; Santos, P.M. Absence of Light Exposure Increases Pathogenicity of Pseudomonas aeruginosa Pneumonia-Associated Clinical Isolates. Biology 2021, 10, 837. https://doi.org/10.3390/biology10090837
Mesquita CS, Ribeiro A, Gomes AC, Santos PM. Absence of Light Exposure Increases Pathogenicity of Pseudomonas aeruginosa Pneumonia-Associated Clinical Isolates. Biology. 2021; 10(9):837. https://doi.org/10.3390/biology10090837
Chicago/Turabian StyleMesquita, Cristina S., Artur Ribeiro, Andreia C. Gomes, and Pedro M. Santos. 2021. "Absence of Light Exposure Increases Pathogenicity of Pseudomonas aeruginosa Pneumonia-Associated Clinical Isolates" Biology 10, no. 9: 837. https://doi.org/10.3390/biology10090837
APA StyleMesquita, C. S., Ribeiro, A., Gomes, A. C., & Santos, P. M. (2021). Absence of Light Exposure Increases Pathogenicity of Pseudomonas aeruginosa Pneumonia-Associated Clinical Isolates. Biology, 10(9), 837. https://doi.org/10.3390/biology10090837









