Profile of Bacterial Infections in COVID-19 Patients: Antimicrobial Resistance in the Time of SARS-CoV-2
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Strains
2.2. Antimicrobial Testing
2.3. Statistical Analyses
3. Results
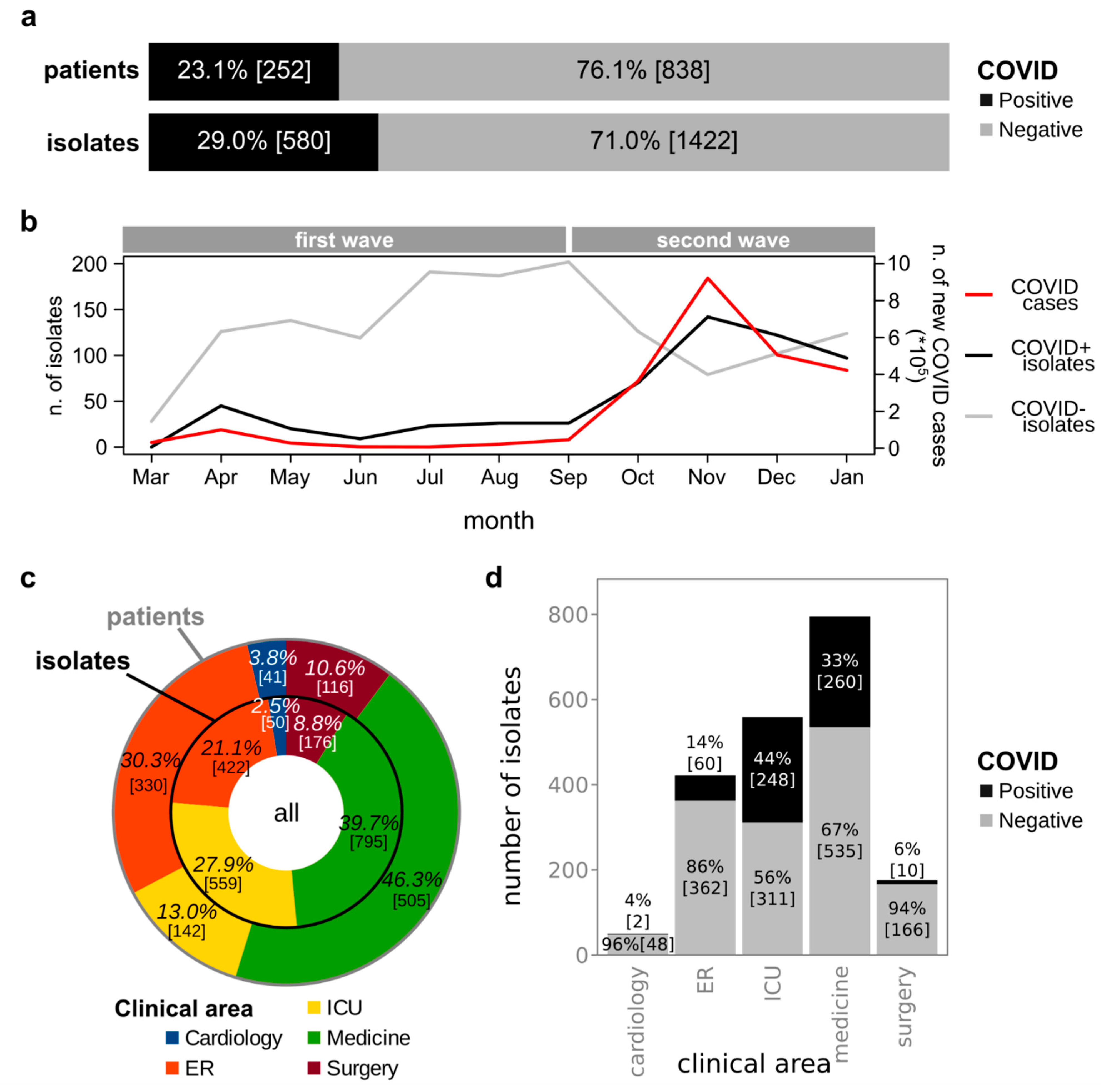
3.1. Isolation of Bacterial Strains from Clinical Specimens Collected from COVID+ and COVID− Patients
3.2. Microbial Species Isolated from COVID+ and COVID− Patients
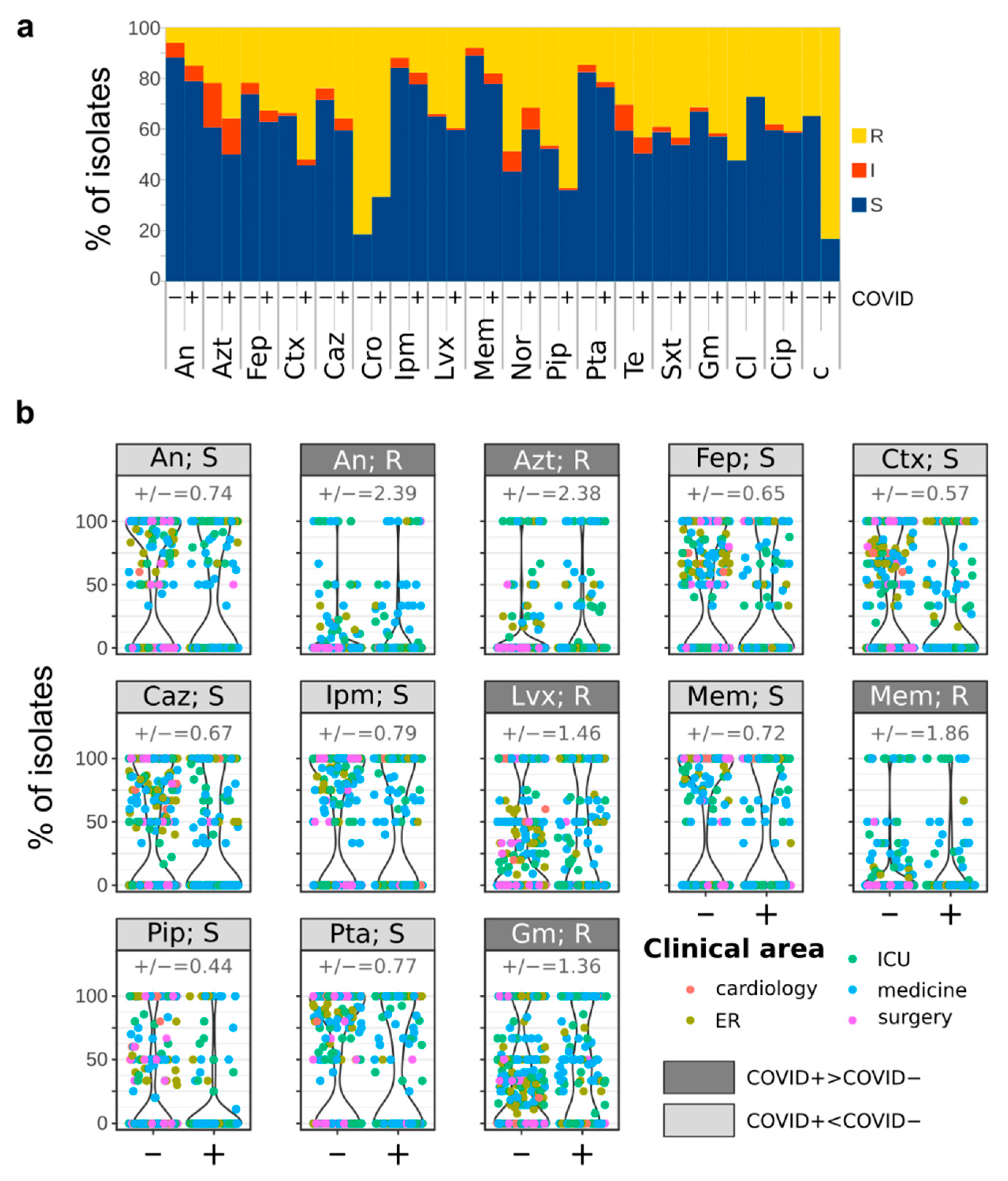
3.3. Antimicrobial Susceptibility of Bacterial Strains Isolated from COVID+ and COVID− Patients
3.4. Comparison of Bacterial Infections before and during the COVID-19 Pandemic
4. Discussion
5. Conclusions
- No significant differences were observed between COVID+ and COVID− in the distribution of isolates among clinical areas or isolation sources;
- Conversely, multiple strains from different species are more frequently isolated from COVID+ patients than from COVID− patients, and a significantly higher number of species was associated with COVID+ patients than with COVID− patients;
- The number of bacterial isolates from COVID+ patients was correlated with the number of new COVID-19 cases monitored at the national level;
- Several species identified only among COVID+ patients are commonly associated with infections in immuno-compromised patients or nosocomial infections. Klebsiella pneumoniae and Acinetobacter baumannii/haemolyticus were more abundant among COVID+ isolates;
- The fraction of microorganisms resistant to every tested antibiotic is higher in COVID+ than in COVID− isolates. Furthermore, COVID+ isolates show higher resistance to several antibiotics used as the first line therapeutic agents for the management of severe pneumonia;
- Resistance to trimethoprim/sulfamethoxazole was equally distributed between COVID+ and COVID− isolates;
- More COVID+ than COVID− isolates were resistant to every tested antibiotic.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nioi, M.; Napoli, P.E.; Lobina, J.; Fossarello, M.; d’Aloja, E. COVID-19 and Italian Healthcare Workers from the Initial Sacrifice to the mRNA Vaccine: Pandemic Chrono-History, Epidemiological Data, Ethical Dilemmas, and Future Challenges. Front. Public Health 2021, 8, 1037. [Google Scholar] [CrossRef]
- Chirico, F.; Nucera, G.; Szarpak, L. COVID-19 mortality in Italy: The first wave was more severe and deadly, but only in Lombardy region. J. Infect. 2021, 83, e16. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Hydroxychloroquine for COVID-19: The end of the line? BMJ 2020, 369, m2378. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Yacouba, A.; Olowo-Okere, A.; Yunusa, I. Repurposing of Antibiotics for Clinical Management of COVID-19: A Narrative Review. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 37. [Google Scholar] [CrossRef]
- Chalichem, N.S.S.; Bethapudi, B.; Mundkinajeddu, D. Aminoglycosides Can Be a Better Choice over Macrolides in COVID-19 Regimen: Plausible Mechanism for Repurposing Strategy. Med. Hypotheses 2020, 144, 109984. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Maurya, A.K.; Mishra, N.; Siddique, H.R. Virtual Screening, ADME/T, and Binding Free Energy Analysis of Anti-Viral, Anti-Protease, and Anti-Infectious Compounds against NSP10/NSP16 Methyltransferase and Main Protease of SARS CoV-2. J. Recept. Signal Transduct. Res. 2020, 40, 605–612. [Google Scholar] [CrossRef]
- Scroggs, S.L.P.; Offerdahl, D.K.; Flather, D.P.; Morris, C.N.; Kendall, B.L.; Broeckel, R.M.; Beare, P.A.; Bloom, M.E. Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses 2020, 13, 8. [Google Scholar] [CrossRef]
- Lombardy Section Italian Society Infectious and Tropical Diseases. Vademecum for the Treatment of People with COVID-19. Edition 2.0, 13 March 2020. Infez. Med. 2020, 28, 143–152. [Google Scholar] [PubMed]
- Società Italiana di Malattie Infettive e Tropicali. Vademecum per la cura delle persone con infezione da SARS-CoV-2. Version 3.0. 3 November 2020. Available online: https://www.simit.org/images/Vademecum%203.0%20del%2003.11.2020.pdf (accessed on 18 August 2021).
- Società Italiana di Medicina Generale e delle Cure Primarie. LA TERAPIA DOMICILIARE DEL COVID-19. Version 1.2. 27 April 2021. Available online: https://www.simg.it/Coronavirus/La%20terapia%20domiciliare%20del%20COVID-19_27042021.pdf (accessed on 18 August 2021).
- Zhang, A.; Hobman, E.V.; De Barro, P.; Young, A.; Carter, D.J.; Byrne, M. Self-Medication with Antibiotics for Protection against COVID-19: The Role of Psychological Distress, Knowledge Of, and Experiences with Antibiotics. Antibiotics 2021, 10, 232. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t Neglect Antimicrobial Stewardship Principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Yam, E.L.Y. COVID-19 Will Further Exacerbate Global Antimicrobial Resistance. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Clinical Management of COVID-19. Interim Guidance; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- ESCMID-European Society of Clinical Microbiology; Infectious Diseases. EUCAST: Clinical Breakpoints and Dosing of Antibiotics; ESCMID-European Society of Clinical Microbiology, Infectious Diseases: Basel, Switzerland, 2021. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanini, I.; Cavalieri, D. Metagenomic approaches to investigate the contribution of the vineyard environment to the quality of wine fermentation: Potentials and difficulties. Front. Microbiol. 2018, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 18 August 2021).
- Westfall, P.H.; Young, S.S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment; John Wiley & Sons: Hoboken, NJ, USA, 1993; ISBN 9780471557616. [Google Scholar]
- R core team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat. Soc. Series B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hasell, J.; Mathieu, E.; Beltekian, D.; Macdonald, B.; Giattino, C.; Ortiz-Ospina, E.; Roser, M.; Ritchie, H. A Cross-Country Database of COVID-19 Testing. Sci. Data 2020, 7, 345. [Google Scholar] [CrossRef]
- Stefanini, I.; Boni, M.; Silvaplana, P.; Lovera, P.; Pelassa, S.; De Renzi, G.; Mognetti, B. Antimicrobial Resistance, an Update from the Ward: Increased Incidence of New Potential Pathogens and Site of Infection-Specific Antibacterial Resistances. Antibiotics 2020, 9, 631. [Google Scholar] [CrossRef]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the Treatment of Nosocomial Pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, N.K.D.; Veeraraghavan, B. Accurate Identification and Epidemiological Characterization of Burkholderia Cepacia Complex: An Update. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, M.P.; Adley, C.C. Sphingomonas Paucimobilis: A Persistent Gram-Negative Nosocomial Infectious Organism. J. Hosp. Infect. 2010, 75, 153–157. [Google Scholar] [CrossRef]
- Oyeka, M.; Antony, S. Citrobacter braakii Bacteremia: Case Report and Review of the Literature. Infect. Disord. Drug Targets 2017, 17, 59–63. [Google Scholar] [CrossRef]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the Mechanisms of Acinetobacter Baumannii Virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Quan, J.; Dai, H.; Liao, W.; Zhao, D.; Shi, Q.; Zhang, L.; Shi, K.; Akova, M.; Yu, Y. Etiology and Prevalence of ESBLs in Adult Community-Onset Urinary Tract Infections in East China: A Prospective Multicenter Study. J. Infect. 2021, 83. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Finco, G.; Demontis, R.; Fossarello, M.; d’Aloja, E. Fear of the COVID-19 and medical liability. Insights from a series of 130 consecutives medico-legal claims evaluated in a single institution during SARS-CoV-2-related pandemic. Signa Vitae 2021, 17, 79–85. [Google Scholar]
- Böcher, S.; Tønning, B.; Skov, R.L.; Prag, J. Staphylococcus lugdunensis, a Common Cause of Skin and Soft Tissue Infections in the Community. J. Clin. Microbiol. 2009, 47, 946–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanini, I.; De Renzi, G.; Foddai, E.; Cordani, E.; Mognetti, B. Profile of Bacterial Infections in COVID-19 Patients: Antimicrobial Resistance in the Time of SARS-CoV-2. Biology 2021, 10, 822. https://doi.org/10.3390/biology10090822
Stefanini I, De Renzi G, Foddai E, Cordani E, Mognetti B. Profile of Bacterial Infections in COVID-19 Patients: Antimicrobial Resistance in the Time of SARS-CoV-2. Biology. 2021; 10(9):822. https://doi.org/10.3390/biology10090822
Chicago/Turabian StyleStefanini, Irene, Giuseppe De Renzi, Elisa Foddai, Elisa Cordani, and Barbara Mognetti. 2021. "Profile of Bacterial Infections in COVID-19 Patients: Antimicrobial Resistance in the Time of SARS-CoV-2" Biology 10, no. 9: 822. https://doi.org/10.3390/biology10090822
APA StyleStefanini, I., De Renzi, G., Foddai, E., Cordani, E., & Mognetti, B. (2021). Profile of Bacterial Infections in COVID-19 Patients: Antimicrobial Resistance in the Time of SARS-CoV-2. Biology, 10(9), 822. https://doi.org/10.3390/biology10090822







