Impact of the Epigenetically Regulated Hoxa-5 Gene in Neural Differentiation from Human Adipose-Derived Stem Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
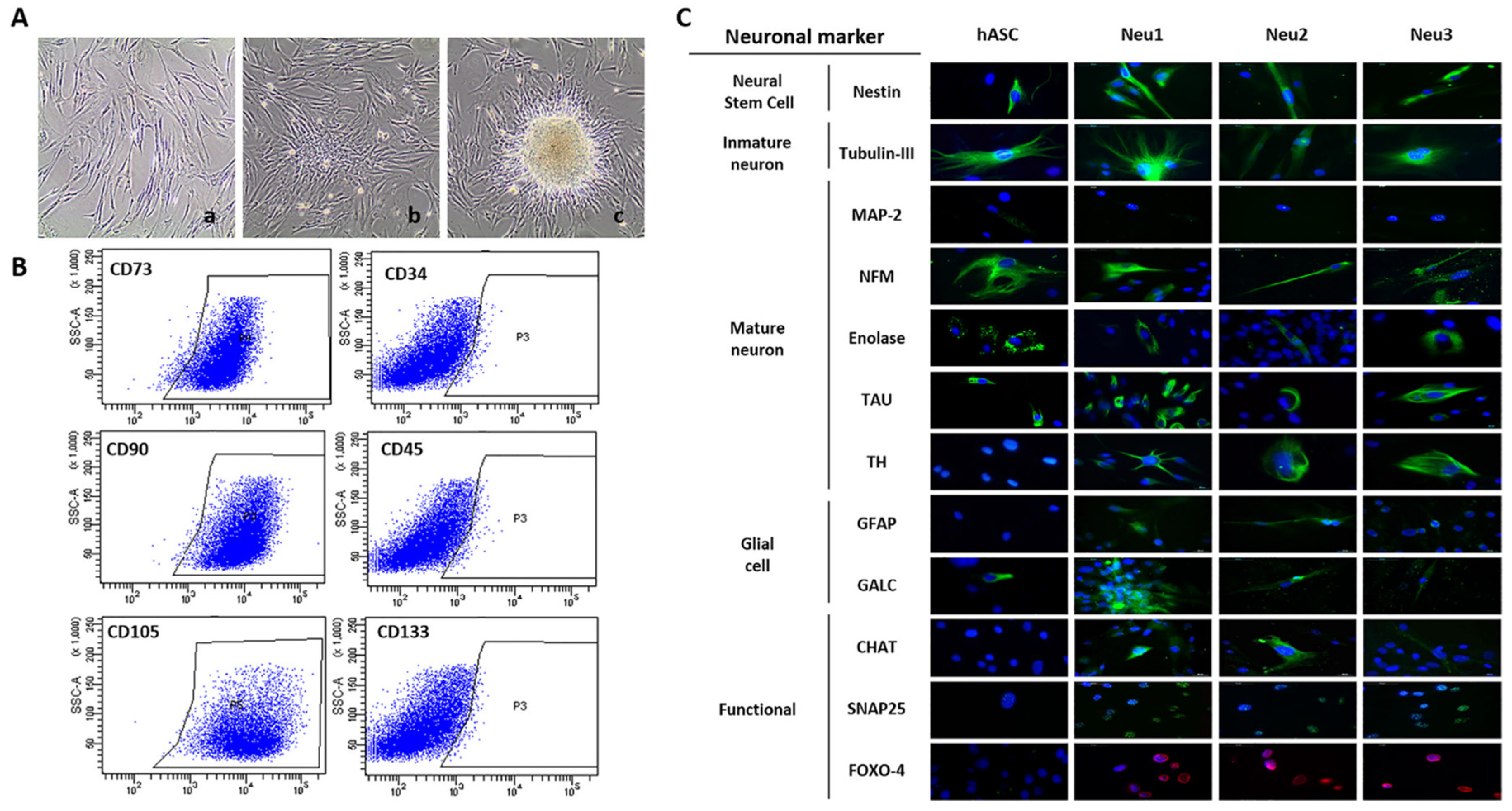
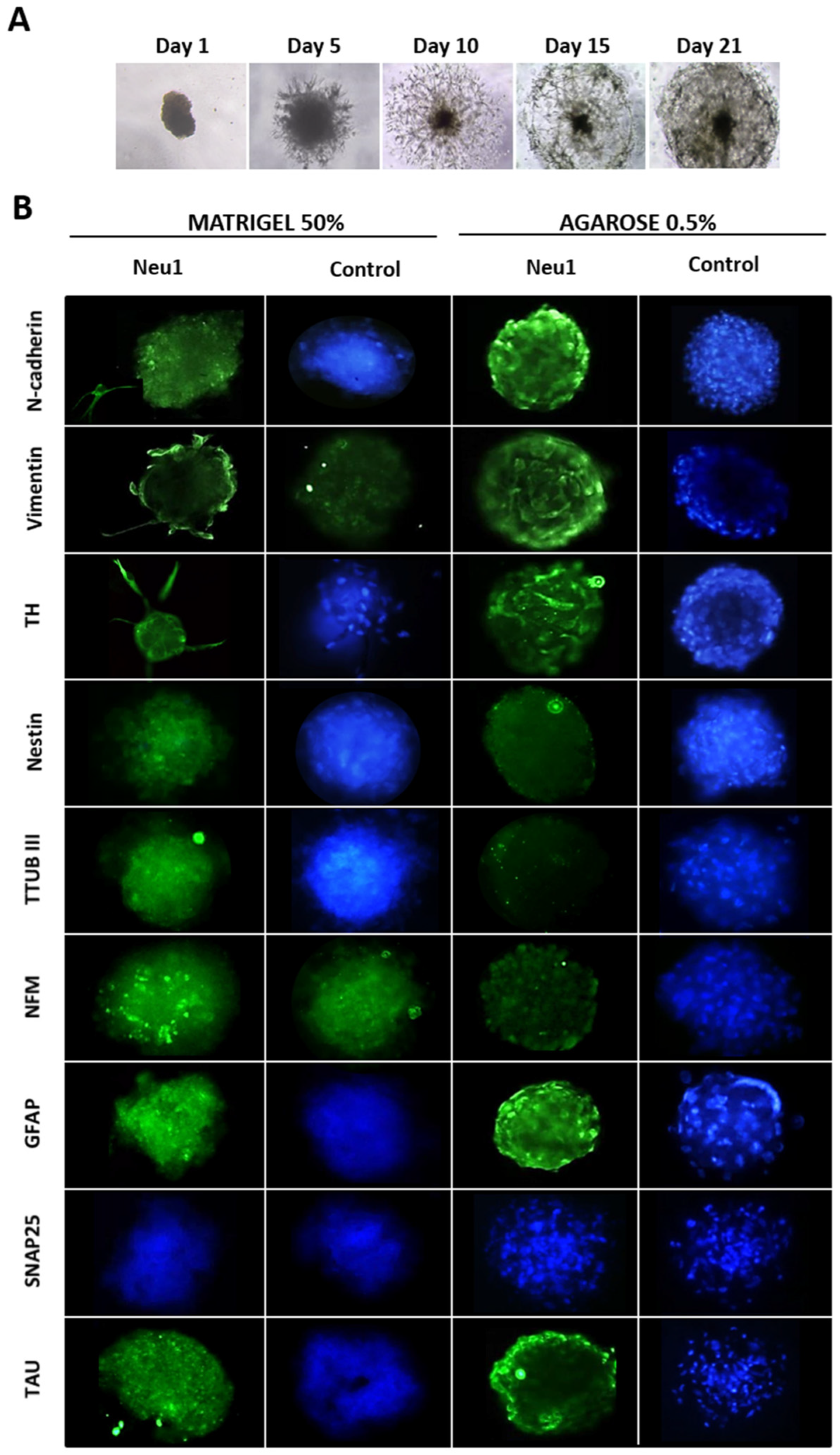
2.1. hASCs from Human Adipose Tissue, Neuronal Differentiation and Neurospheres Formation
2.2. Immunofluorescence Analysis of Neuronal Markers
2.3. Quantitative RT-PCR
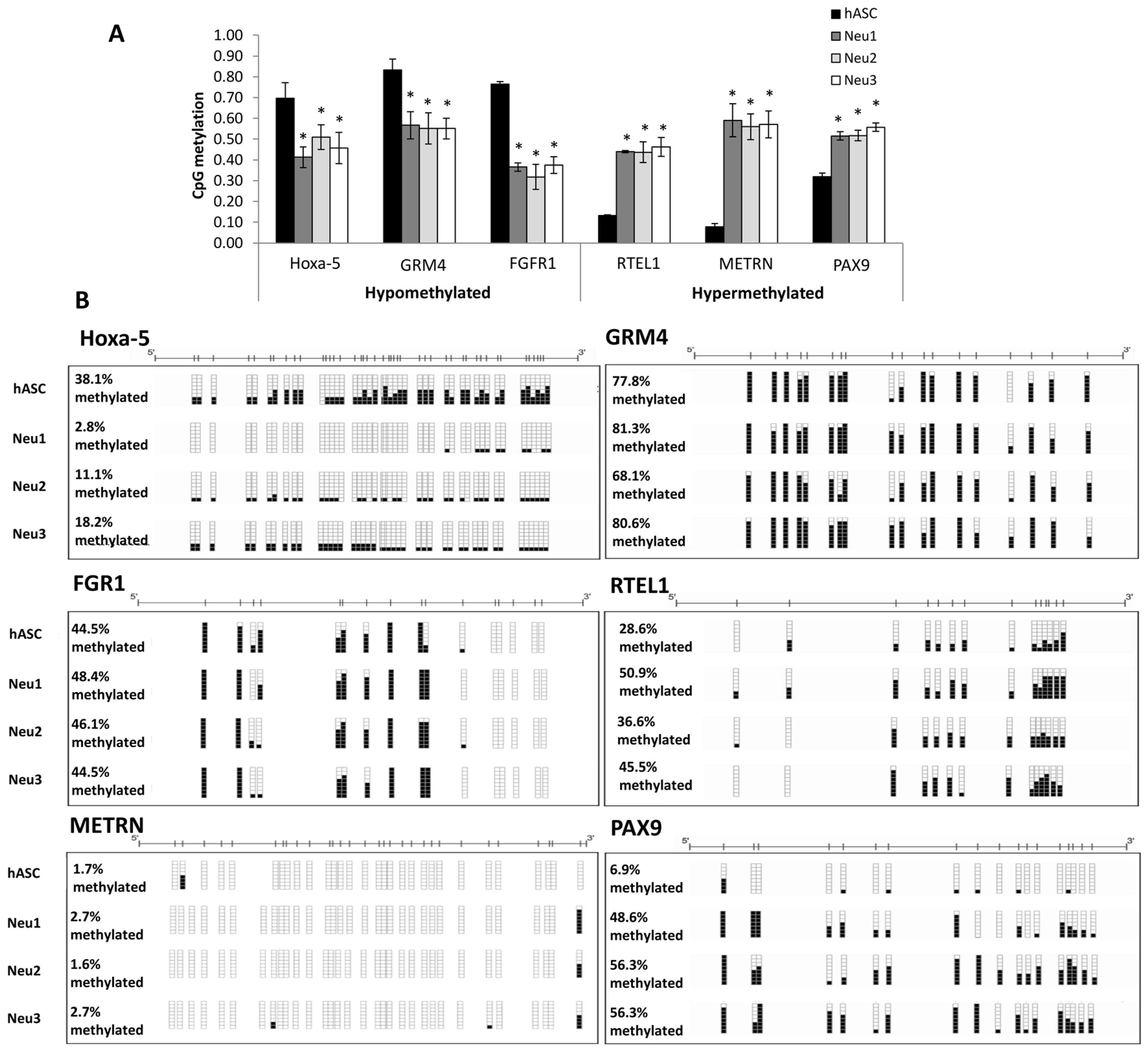
2.4. Genome-Wide CpG Methylation Profiling
2.5. Hierarchical Cluster Analysis and Definition of CpG Methylation Differences
2.6. Bisulfite Genomic Sequencing of Multiple Clones
2.7. Generation of a Stable hASCs Line Overexpressing Hoxa-5 Gene by Lentivirus
2.8. Generation of a Hoxa-5—Expressing hASCs Cell Line Using a CRISPR/dCas9 System
2.9. Statistical Analysis
3. Results
3.1. Neuronal Differentiation of hASCs
3.2. Neuronal Differentiation in hASCs Neurospheres
3.3. DNA Methylation Changes Associated with Neuronal Differentiation of hASCs
3.4. Modulation of Neuronal Markers in Stable hASC Line That Overexpresses the Hoxa-5 Gene
3.5. Neuronal Differentiation by Hoxa-5 Gene and Neu1 Medium in hASCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoudifar, N.; Doran, P.M. Mesenchymal stem cells derived from human adipose tissue. Methods Mol. Biol. 2015, 1340, 53–64. [Google Scholar]
- Perez-Campo, F.M.; Riancho, J.A. Epigenetic mechanisms regulating mesenchymal stem cell differentiation. Curr. Genom. 2015, 16, 368–383. [Google Scholar] [CrossRef][Green Version]
- Rajan, T.S.; Giacoppo, S.; Trubiani, O.; Diomede, F.; Piattelli, A.; Bramanti, P.; Mazzon, E. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp. Cell Res. 2016, 349, 152–161. [Google Scholar] [CrossRef]
- Trubiani, O.; Guarnieri, S.; Diomede, F.; Mariggio, M.A.; Merciaro, I.; Morabito, C.; Cavalcanti, M.F.; Cocco, L.; Ramazzotti, G. Nuclear translocation of PKCalpha isoenzyme is involved in neurogenic commitment of human neural crest-derived periodontal ligament stem cells. Cell Signal. 2016, 28, 1631–1641. [Google Scholar] [CrossRef]
- Chun, S.Y.; Soker, S.; Jang, Y.J.; Kwon, T.G.; Yoo, E.S. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. J. Korean Med. Sci. 2016, 31, 171–177. [Google Scholar] [CrossRef]
- Ying, C.; Hu, W.; Cheng, B.; Zheng, X.; Li, S. Neural differentiation of rat adipose-derived stem cells in vitro. Cell. Mol. Neurobiol. 2012, 32, 1255–1263. [Google Scholar] [CrossRef]
- Marei, H.E.S.; El-Gamal, A.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Farag, A.; Cenciarelli, C.; Thomas, C.; Anwarul, H. Cholinergic and dopaminergic neuronal differentiation of human adipose tissue derived mesenchymal stem cells. J. Cell. Physiol. 2018, 233, 936–945. [Google Scholar] [CrossRef]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef]
- Dave, S.D.; Patel, C.N.; Vanikar, A.V.; Trivedi, H.L. In vitro differentiation of neural cells from human adipose tissue derived stromal cells. Neurol. India 2018, 66, 716–721. [Google Scholar] [CrossRef]
- Kang, Y.H.; Shivakumar, S.B.; Son, Y.B.; Bharti, D.; Jang, S.J.; Heo, K.S.; Park, W.U.; Byun, J.H.; Park, B.W.; Rho, G.J. Comparative analysis of three different protocols for cholinergic neuron differentiation in vitro using mesenchymal stem cells from human dental pulp. Anim. Cells Syst. 2019, 23, 275–287. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Zhao, S.; Jin, Y.; Zhou, F.; Yuan, P.; Cao, L.; Wang, J.; Qiu, Y.; Sun, C.; et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019, 10, 597. [Google Scholar] [CrossRef]
- Chudickova, M.; Bruza, P.; Zajicova, A.; Trosan, P.; Svobodova, L.; Javorkova, E.; Kubinova, S.; Holan, V. Targeted neural differentiation of murine mesenchymal stem cells by a protocol simulating the inflammatory site of neural injury. J. Tissue Eng. Regen. Med. 2017, 11, 1588–1597. [Google Scholar] [CrossRef]
- Singer, W.; Dietz, A.B.; Zeller, A.D.; Gehrking, T.L.; Schmelzer, J.D.; Schmeichel, A.M.; Gehrking, J.A.; Suarez, M.D.; Sletten, D.M.; Minota Pacheco, K.V.; et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology 2019, 93, e77–e87. [Google Scholar] [CrossRef]
- Fernandez, O.; Izquierdo, G.; Fernandez, V.; Leyva, L.; Reyes, V.; Guerrero, M.; Leon, A.; Arnaiz, C.; Navarro, G.; Paramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, G.; Jiang, X.H. Fate determination in mesenchymal stem cells: A perspective from histone-modifying enzymes. Stem Cell Res. 2015, 6, 35. [Google Scholar] [CrossRef]
- Meissner, A.; Mikkelsen, T.S.; Gu, H.; Wernig, M.; Hanna, J.; Sivachenko, A.; Zhang, X.; Bernstein, B.E.; Nusbaum, C.; Jaffe, D.B.; et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 2008, 454, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Teven, C.M.; Liu, X.; Hu, N.; Tang, N.; Kim, S.H.; Huang, E.; Yang, K.; Li, M.; Gao, J.L.; Liu, H.; et al. Epigenetic regulation of mesenchymal stem cells: A focus on osteogenic and adipogenic differentiation. Stem Cells Int. 2011, 2011, 201371. [Google Scholar] [CrossRef] [PubMed]
- Mortada, I.; Mortada, R. Epigenetic changes in mesenchymal stem cells differentiation. Eur. J. Med. Genet. 2018, 61, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Van den Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Comprehensive DNA methylation and gene expression profiling in differentiating human adipocytes. J. Cell. Biochem. 2016, 117, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, A.R. Epigenetic modulators promote mesenchymal stem cell phenotype switches. Int. J. Biochem. Cell Biol. 2015, 64, 190–194. [Google Scholar] [CrossRef]
- Fila-Danilow, A.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Kowalski, J. The influence of TSA and VPA on the in vitro differentiation of bone marrow mesenchymal stem cells into neuronal lineage cells: Gene expression studies. Postep. Hig. Med. Dosw. 2017, 71, 236–242. [Google Scholar] [CrossRef]
- Berdasco, M.; Esteller, M. Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 2019, 20, 109–127. [Google Scholar] [CrossRef]
- Du, P.; Kibbe, W.A.; Lin, S.M. lumi: A pipeline for processing Illumina microarray. Bioinformatics 2008, 24, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Lizen, B.; Hutlet, B.; Bissen, D.; Sauvegarde, D.; Hermant, M.; Ahn, M.T.; Gofflot, F. HOXA5 localization in postnatal and adult mouse brain is suggestive of regulatory roles in postmitotic neurons. J. Comp. Neurol. 2017, 525, 1155–1175. [Google Scholar] [CrossRef]
- Barsh, G.R.; Isabella, A.J.; Moens, C.B. Vagus motor neuron topographic map determined by parallel mechanisms of hox5 expression and time of axon initiation. Curr. Biol. 2017, 27, 3812–3825. [Google Scholar] [CrossRef]
- Rezsohazy, R.; Saurin, A.J.; Maurel-Zaffran, C.; Graba, Y. Cellular and molecular insights into Hox protein action. Development 2015, 142, 1212–1227. [Google Scholar] [CrossRef]
- Wang, M.Y.; Qiu, Y.H.; Cai, M.L.; Zhang, C.H.; Wang, X.W.; Liu, H.; Chen, Y.; Zhao, W.L.; Liu, J.B.; Shao, R.G. Role and molecular mechanism of stem cells in colorectal cancer initiation. J. Drug Target. 2020, 28, 1–10. [Google Scholar] [CrossRef]
- Berdasco, M.; Melguizo, C.; Prados, J.; Gomez, A.; Alaminos, M.; Pujana, M.A.; Lopez, M.; Setien, F.; Ortiz, R.; Zafra, I.; et al. DNA methylation plasticity of human adipose-derived stem cells in lineage commitment. Am. J. Pathol. 2012, 181, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Bossolasco, P.; Cova, L.; Calzarossa, C.; Rimoldi, S.G.; Borsotti, C.; Deliliers, G.L.; Silani, V.; Soligo, D.; Polli, E. Neuroglial differentiation of human bone marrow stem cells in vitro. Exp. Neurol. 2005, 193, 312–325. [Google Scholar] [CrossRef]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef]
- Tondreau, T.; Dejeneffe, M.; Meuleman, N.; Stamatopoulos, B.; Delforge, A.; Martiat, P.; Bron, D.; Lagneaux, L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genom. 2008, 9, 166. [Google Scholar] [CrossRef]
- Yang, E.; Liu, N.; Tang, Y.; Hu, Y.; Zhang, P.; Pan, C.; Dong, S.; Zhang, Y.; Tang, Z. Generation of neurospheres from human adipose-derived stem cells. BioMed Res. Int. 2015, 2015, 743714. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef]
- Fortin, J.P.; Labbe, A.; Lemire, M.; Zanke, B.W.; Hudson, T.J.; Fertig, E.J.; Greenwood, C.M.; Hansen, K.D. Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol. 2014, 15, 503. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Cano-Rodriguez, D.; Gjaltema, R.A.; Jilderda, L.J.; Jellema, P.; Dokter-Fokkens, J.; Ruiters, M.H.; Rots, M.G. Writing of H3K4Me3 overcomes epigenetic silencing in a sustained but context-dependent manner. Nat. Commun. 2016, 7, 12284. [Google Scholar] [CrossRef]
- Maeder, M.L.; Linder, S.J.; Cascio, V.M.; Fu, Y.; Ho, Q.H.; Joung, J.K. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 2013, 10, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cao, L.; Shen, Z.; Shao, L.; Gao, S.; Zhang, C.; Lu, J.; Li, W. Materials for neural differentiation, trans-differentiation, and modeling of neurological disease. Adv. Mater. 2018, 30, e1705684. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Amirpour, N.; Niapour, A.; Razavi, S. An overview of neural differentiation potential of human adipose derived stem cells. Stem Cell Rev. Rep. 2016, 12, 26–41. [Google Scholar] [CrossRef]
- Garcez, R.C.; Teixeira, B.L.; Schmitt Sdos, S.; Alvarez-Silva, M.; Trentin, A.G. Epidermal growth factor (EGF) promotes the in vitro differentiation of neural crest cells to neurons and melanocytes. Cell. Mol. Neurobiol. 2009, 29, 1087–1091. [Google Scholar] [CrossRef]
- Khademizadeh, M.; Messripour, M.; Ghasemi, N.; Momen Beik, F.; Movahedian Attar, A. Differentiation of adult human mesenchymal stem cells into dopaminergic neurons. Res. Pharm. Sci. 2019, 14, 209–215. [Google Scholar] [CrossRef]
- Ii, M.; Nishimura, H.; Sekiguchi, H.; Kamei, N.; Yokoyama, A.; Horii, M.; Asahara, T. Concurrent vasculogenesis and neurogenesis from adult neural stem cells. Circ. Res. 2009, 105, 860–868. [Google Scholar] [CrossRef]
- Honda, S.; Kagoshima, M.; Wanaka, A.; Tohyama, M.; Matsumoto, K.; Nakamura, T. Localization and functional coupling of HGF and c-Met/HGF receptor in rat brain: Implication as neurotrophic factor. Brain Res. Mol. Brain Res. 1995, 32, 197–210. [Google Scholar] [CrossRef]
- Deng, J.; Petersen, B.E.; Steindler, D.A.; Jorgensen, M.L.; Laywell, E.D. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 2006, 24, 1054–1064. [Google Scholar] [CrossRef]
- Wang, T.T.; Tio, M.; Lee, W.; Beerheide, W.; Udolph, G. Neural differentiation of mesenchymal-like stem cells from cord blood is mediated by PKA. Biochem. Biophys. Res. Commun. 2007, 357, 1021–1027. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, S.; Pandey, A.; Kumar, D.; Rajpurohit, C.S.; Khanna, V.K.; Pant, A.B. Secretome of differentiated PC12 cells enhances neuronal differentiation in human mesenchymal stem cells via NGF-like mechanism. Mol. Neurobiol. 2018, 55, 8293–8305. [Google Scholar] [CrossRef] [PubMed]
- Turac, G.; Duruksu, G.; Karaoz, E. The effect of recombinant tyrosine hydroxylase expression on the neurogenic differentiation potency of mesenchymal stem cells. Neurospine 2018, 15, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, D.; Boyer, L.; Lutz, M.; Merkwirth, C.; Morantte, I.; Tse, C.; Spencer, B.; Page, L.; Masliah, E.; Berggren, W.T.; et al. FOXO4 is necessary for neural differentiation of human embryonic stem cells. Aging Cell 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Catela, C.; Shin, M.M.; Lee, D.H.; Liu, J.P.; Dasen, J.S. Hox proteins coordinate motor neuron differentiation and connectivity programs through Ret/Gfralpha genes. Cell Rep. 2016, 14, 1901–1915. [Google Scholar] [CrossRef] [PubMed]
- Philippidou, P.; Walsh, C.M.; Aubin, J.; Jeannotte, L.; Dasen, J.S. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat. Neurosci. 2012, 15, 1636–1644. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, R.; Jiménez-Luna, C.; Ortiz, R.; Setién, F.; López, M.; Perazzoli, G.; Esteller, M.; Berdasco, M.; Prados, J.; Melguizo, C. Impact of the Epigenetically Regulated Hoxa-5 Gene in Neural Differentiation from Human Adipose-Derived Stem Cells. Biology 2021, 10, 802. https://doi.org/10.3390/biology10080802
Hernández R, Jiménez-Luna C, Ortiz R, Setién F, López M, Perazzoli G, Esteller M, Berdasco M, Prados J, Melguizo C. Impact of the Epigenetically Regulated Hoxa-5 Gene in Neural Differentiation from Human Adipose-Derived Stem Cells. Biology. 2021; 10(8):802. https://doi.org/10.3390/biology10080802
Chicago/Turabian StyleHernández, Rosa, Cristina Jiménez-Luna, Raúl Ortiz, Fernando Setién, Miguel López, Gloria Perazzoli, Manel Esteller, María Berdasco, Jose Prados, and Consolación Melguizo. 2021. "Impact of the Epigenetically Regulated Hoxa-5 Gene in Neural Differentiation from Human Adipose-Derived Stem Cells" Biology 10, no. 8: 802. https://doi.org/10.3390/biology10080802
APA StyleHernández, R., Jiménez-Luna, C., Ortiz, R., Setién, F., López, M., Perazzoli, G., Esteller, M., Berdasco, M., Prados, J., & Melguizo, C. (2021). Impact of the Epigenetically Regulated Hoxa-5 Gene in Neural Differentiation from Human Adipose-Derived Stem Cells. Biology, 10(8), 802. https://doi.org/10.3390/biology10080802





