A Recommendation for a Pre-Standardized Marine Microalgal Dry Weight Determination Protocol for Laboratory Scale Culture Using Ammonium Formate as a Washing Agent
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent, Microalgae, and Culture Medium
2.2. Total Dry Weight, Ash-Free Dry Weight Determination, and Assessment of Dry Weight
2.3. Statistical Analyses
3. Results
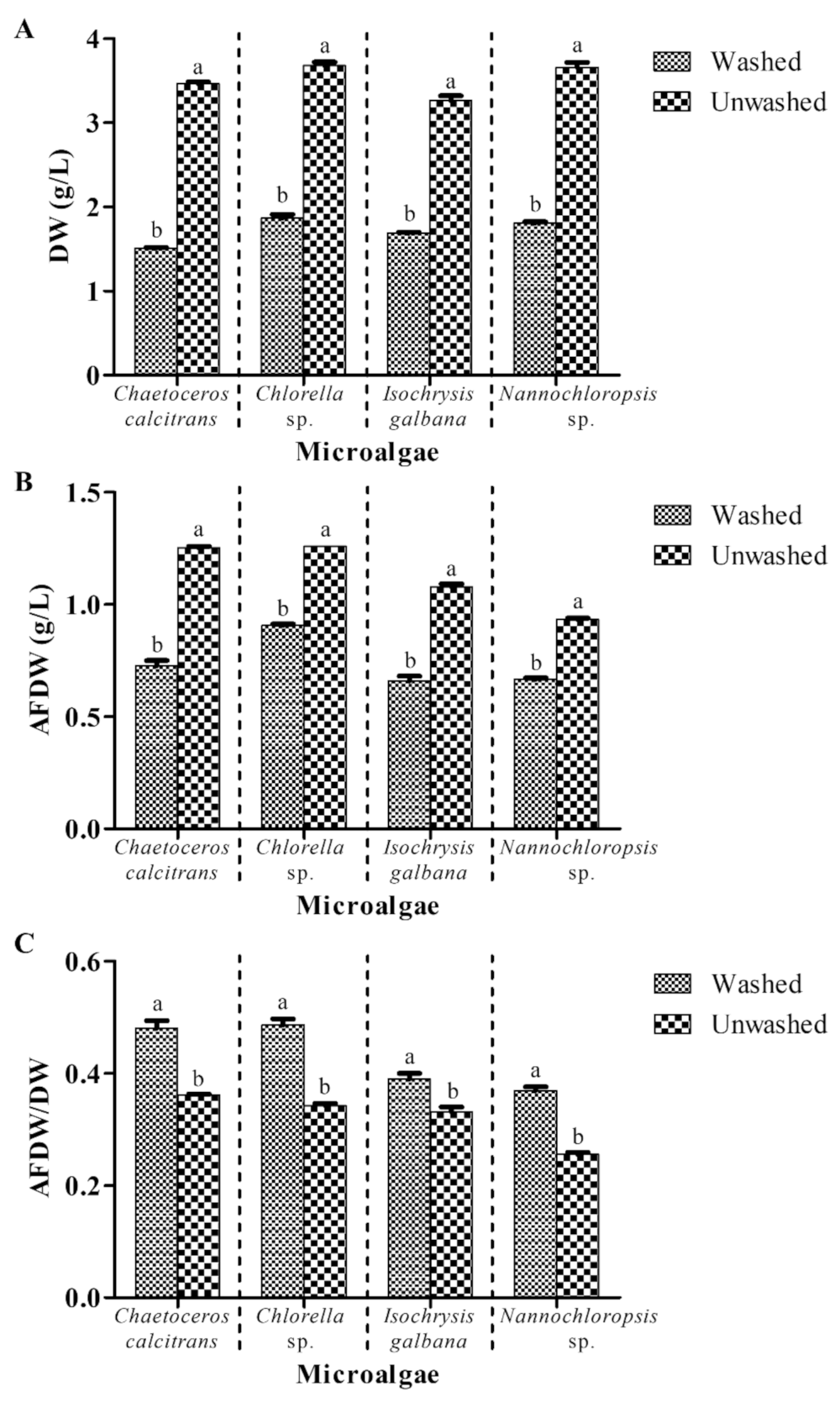
3.1. Washing Process Yielded Lower Microalgal Dry Weight and Higher AFDW/DW Ratio
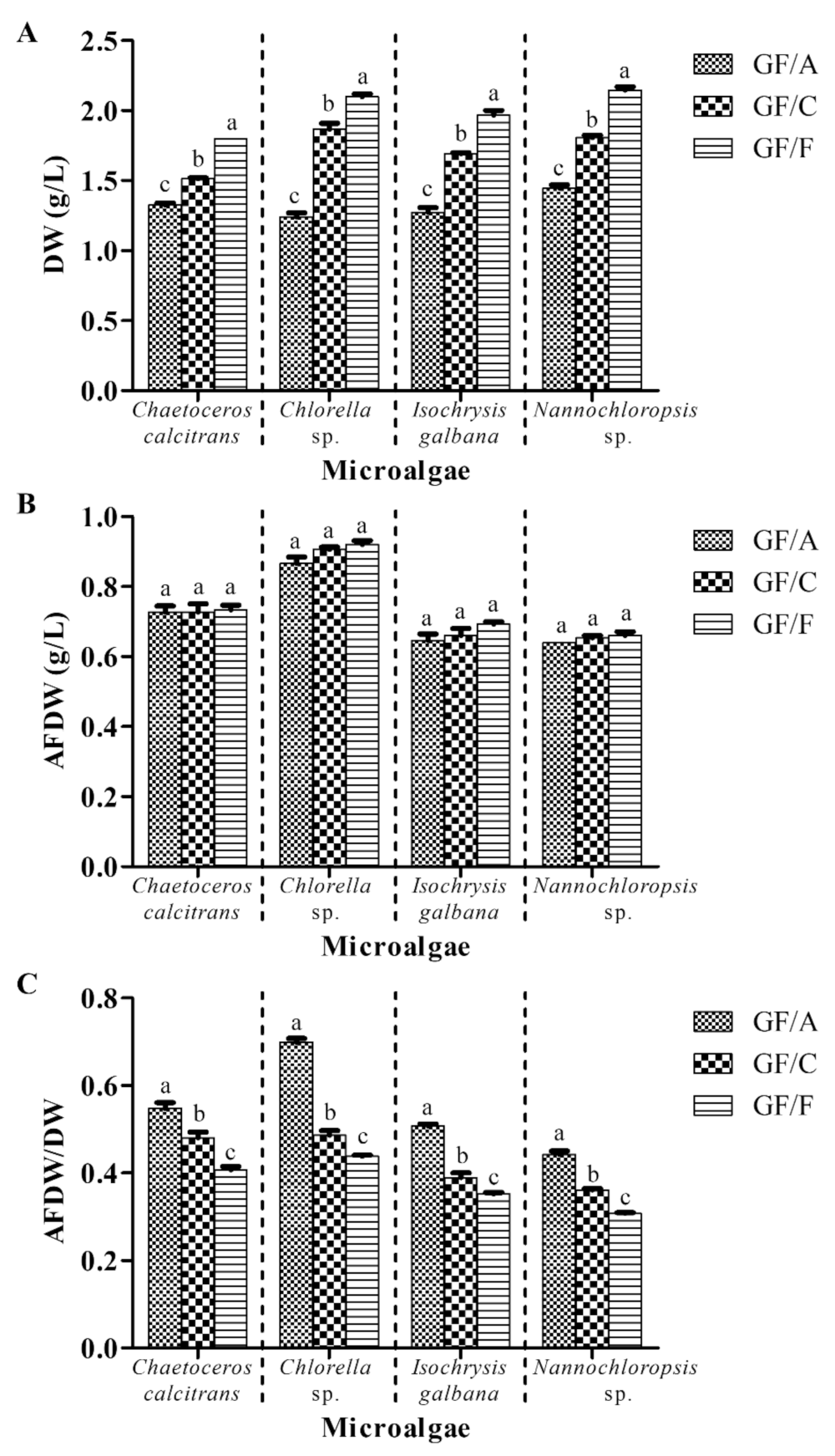
3.2. Glass Microfiber Filter Grade GF/A Is Recommended for Microalgal Biomass Dry Weight Determination
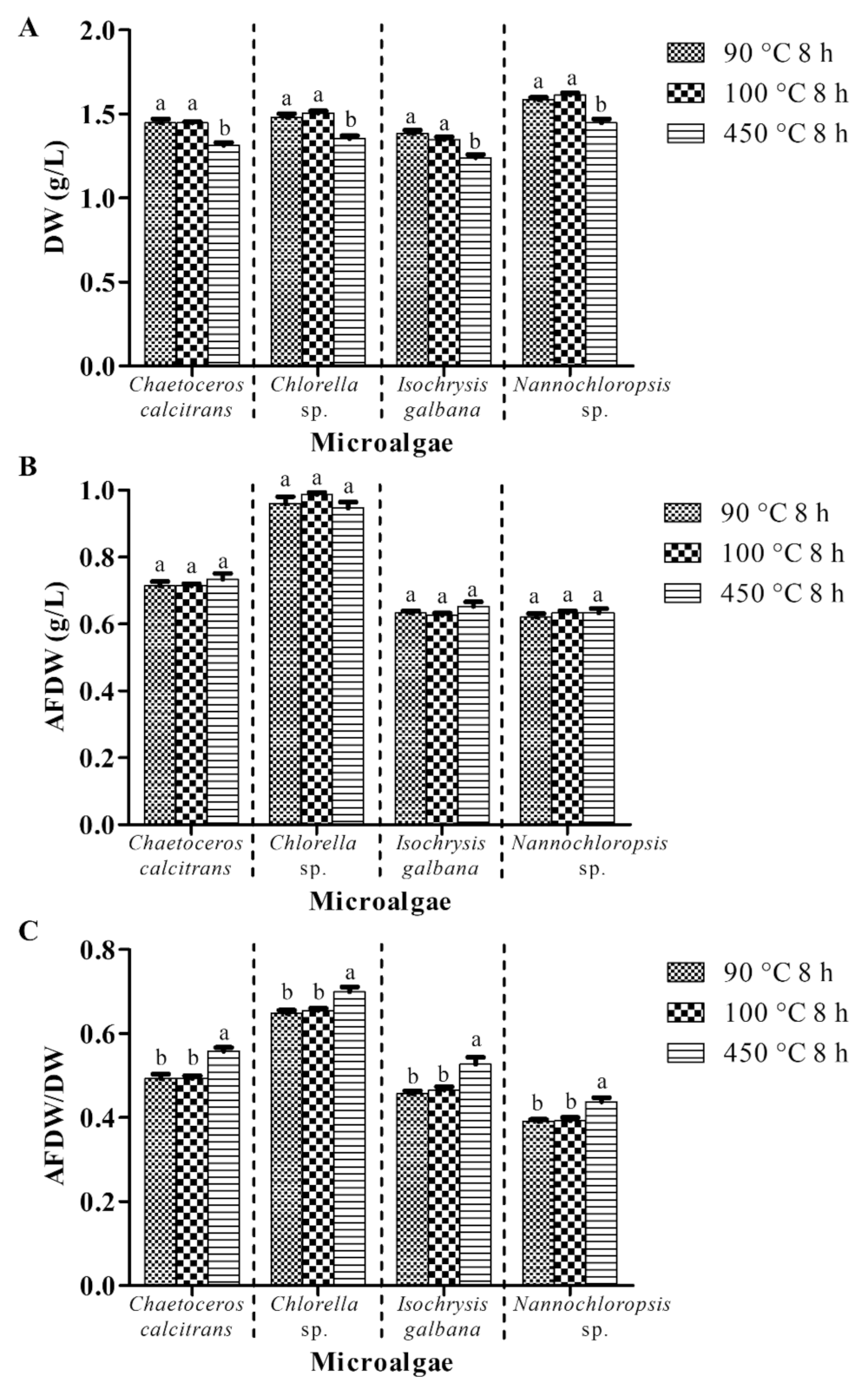
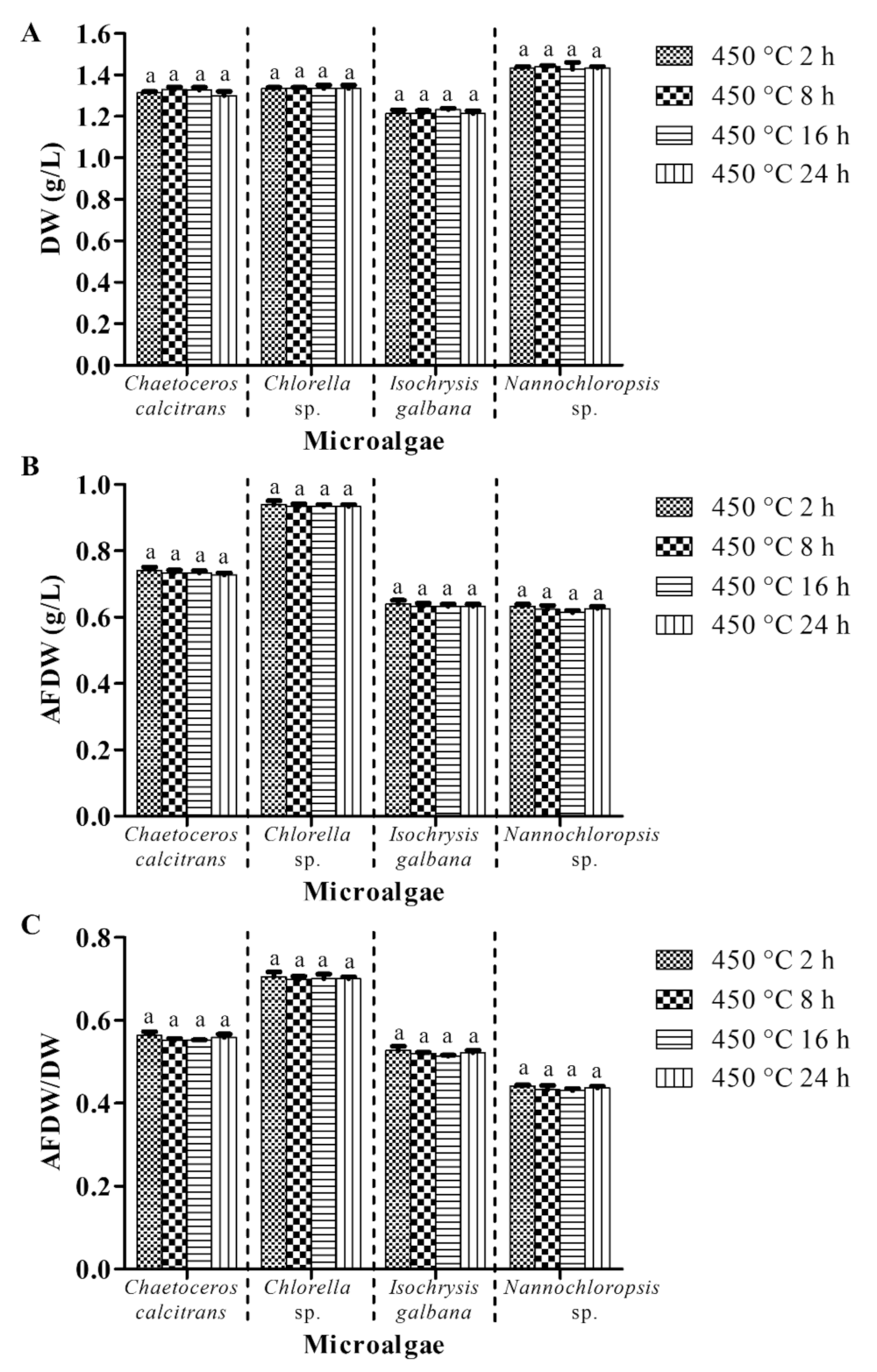
3.3. Combustion at 450 °C for Two Hours Is Suggested for the Pretreatment Purpose
3.3.1. Pretreatment of Glass Microfiber Filter at Different Temperatures
3.3.2. Pretreatment of Glass Microfiber Filter at 450 °C for Different Exposure Periods
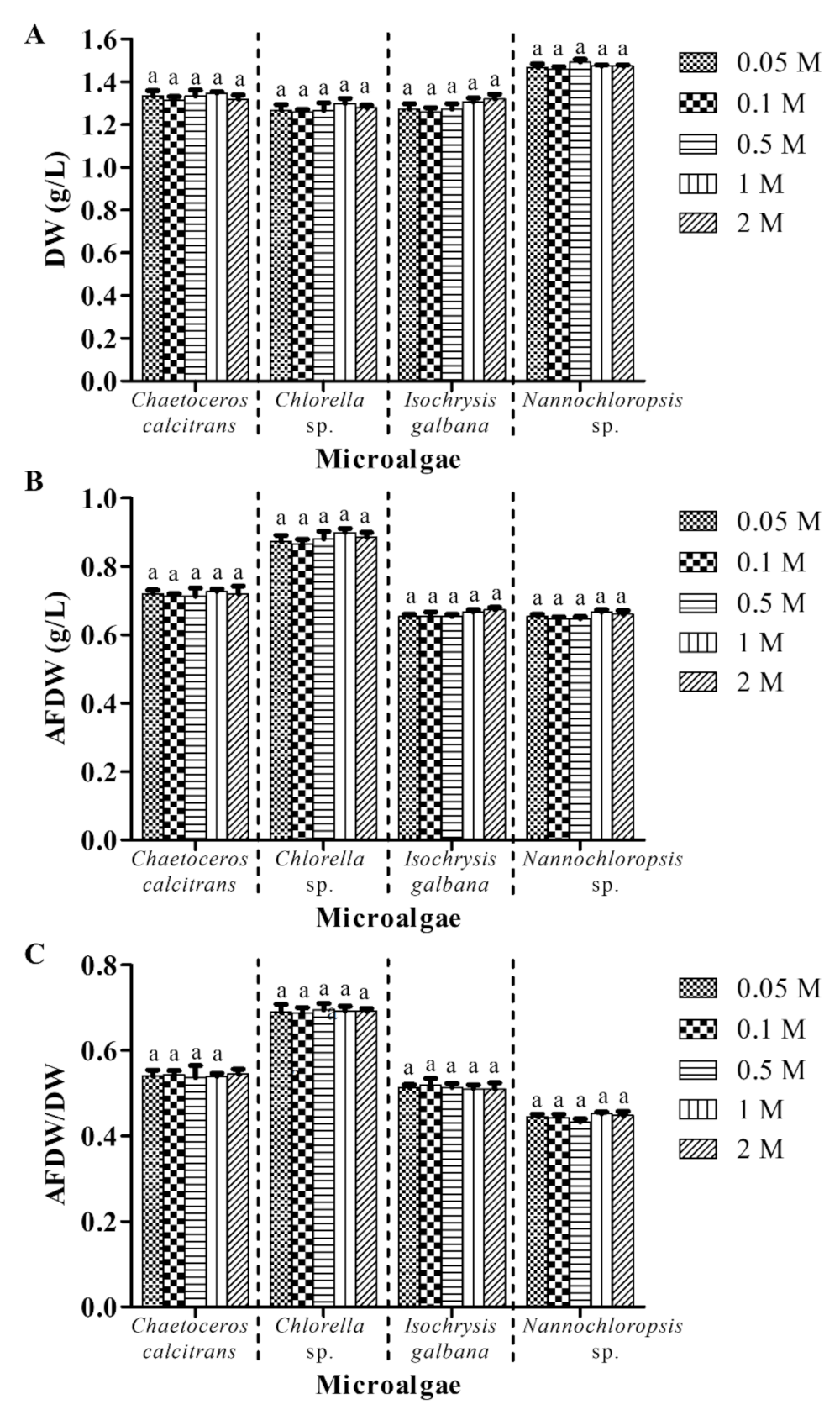
3.4. A Low Concentration Ammonium Formate (0.05 M) Is Sufficient to Remove Salts from Marine Microalgae
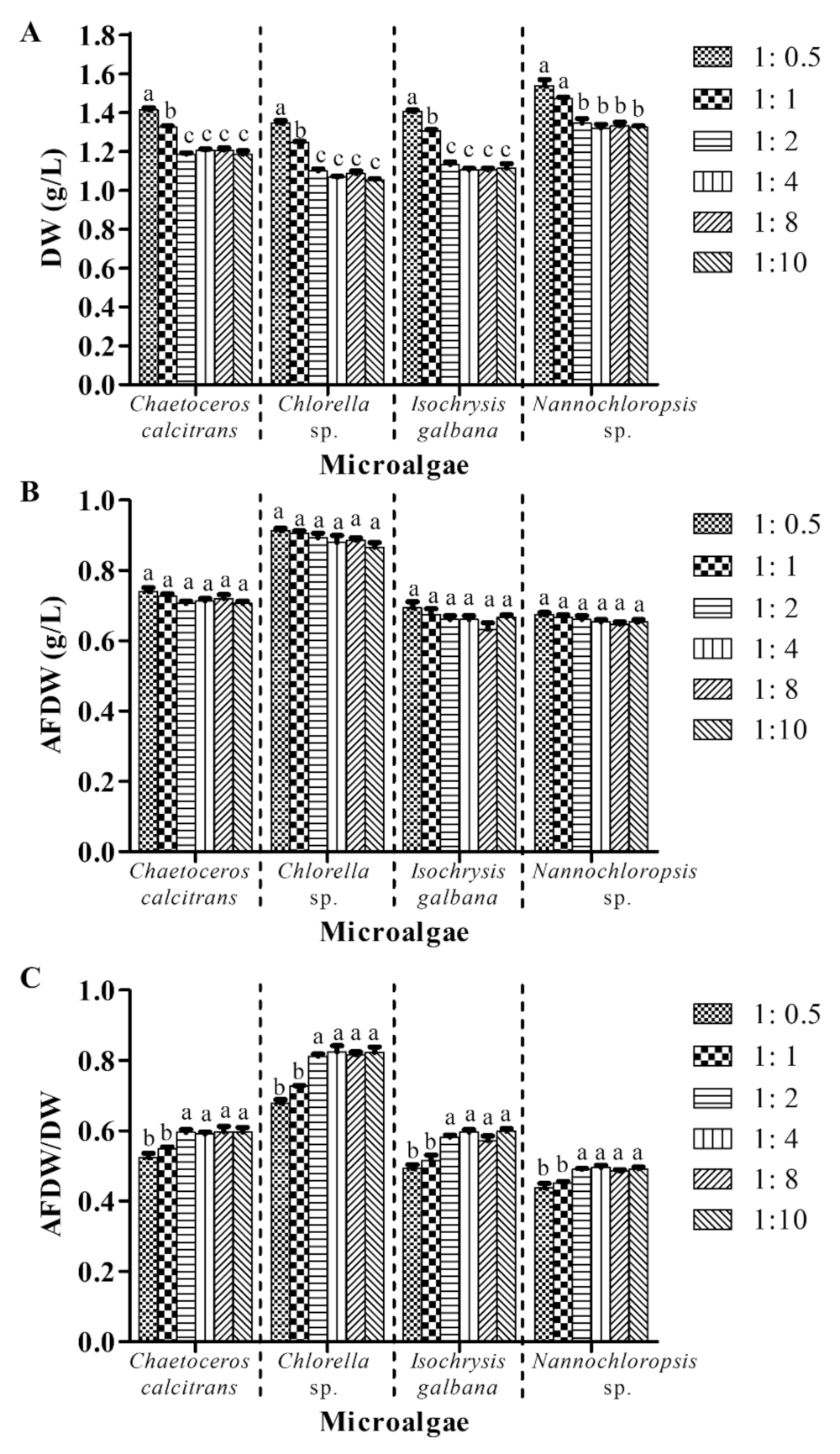
3.5. Utilization of 1:2 Culture: Washing Agent Ratio (v:v) Is Adequate to Provide Accurate Dry Weight for Marine Microalgae
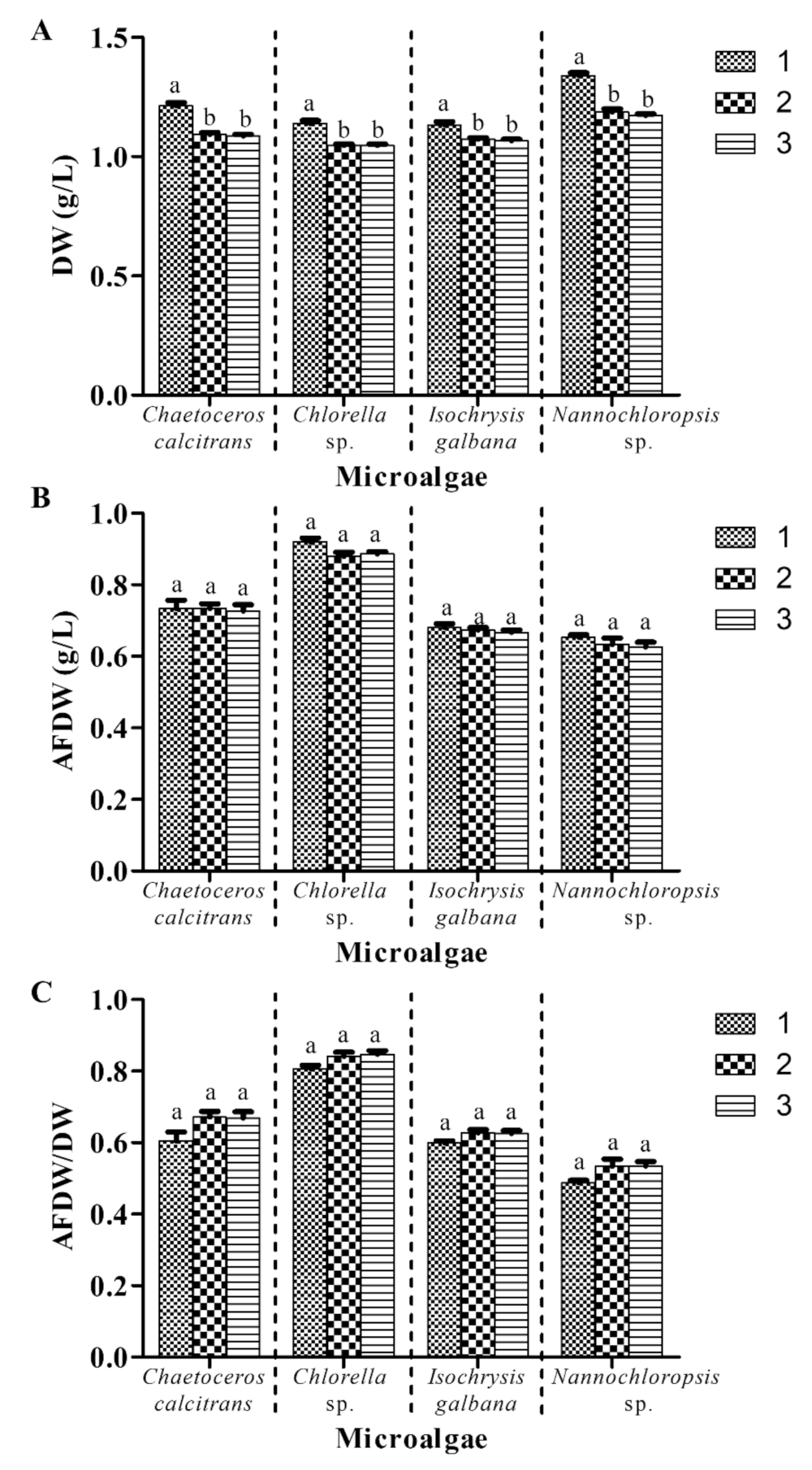
3.6. Two Cycles of Washing Process Are Proposed for Microalgal Biomass Dry Weight Determination
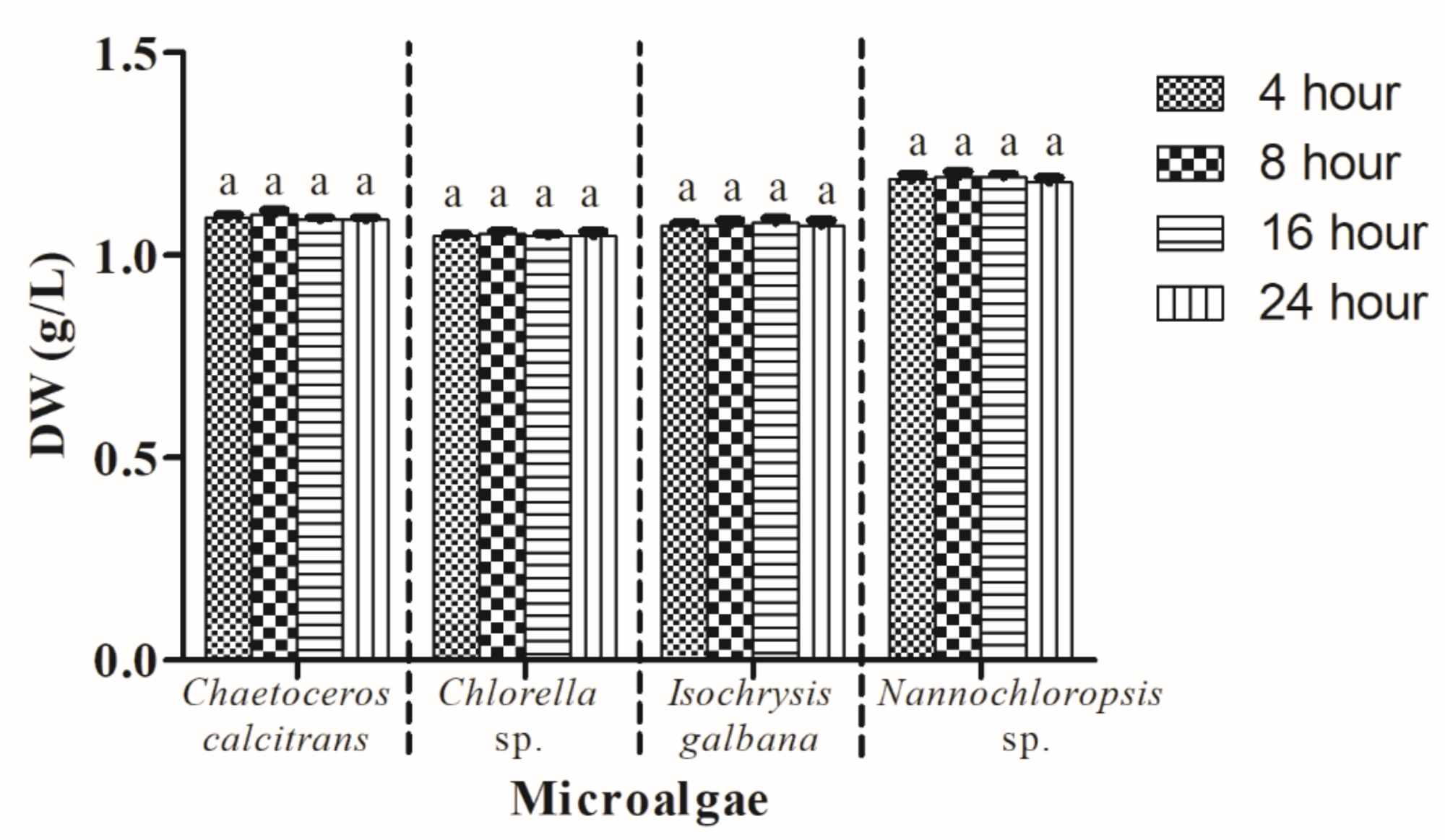
3.7. Drying Period of Microalgal Dry Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Stephens, E.; de Nys, R.; Ross, I.L.; Hankamer, B. Algae Fuels as an Alternative to Petroleum. J. Pet. Environ. Biotechnol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Chan, K.W.; Khong, N.M.H.; Yau, S.K. Production of Fucoxanthin-Rich Fraction (FxRF) from a Diatom, Chaetoceros Calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32. [Google Scholar] [CrossRef]
- Whyte, J.N.C. Biochemical Composition and Energy Content of Six Species of Phytoplankton Used in Mariculture of Bivalves. Aquaculture 1987, 60, 231–241. [Google Scholar] [CrossRef]
- Lim, Z.S.; Wong, R.R.; Wong, C.-Y.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Bibliometric Analysis of Research on Diesel Pollution in Antarctica and a Review on Remediation Techniques. Appl. Sci. 2021, 11, 1123. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K. Determination of Biomass Dry Weight of Marine Microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Borges, L.; Caldas, S.; D’Oca, M.G.M.; Abreu, P.C. Effect of Harvesting Processes on the Lipid Yield and Fatty Acid Profile of the Marine Microalga Nannochloropsis oculata. Aquac. Reports 2016, 4, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Knoshaug, E.P.; Dong, T.; Spiller, R.; Nagle, N.; Pienkos, P.T. Pretreatment and Fermentation of Salt-Water Grown Algal Biomass as a Feedstock for Biofuels and High-Value Biochemicals. Algal Res. 2018, 36, 239–248. [Google Scholar] [CrossRef]
- Dong, T.; Van Wychen, S.; Nagle, N.; Pienkos, P.T.; Laurens, L.M.L. Impact of Biochemical Composition on Susceptibility of Algal Biomass to Acid-Catalyzed Pretreatment for Sugar and Lipid Recovery. Algal Res. 2016, 18, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Khong, N.M.H.; Chan, K.W.; Yau, S.K. Efficient Solvent Extraction of Antioxidant-Rich Extract from a Tropical Diatom, Chaetoceros Calcitrans (Paulsen) Takano 1968. Asian Pac. J. Trop. Biomed. 2015, 5, 796–801. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis Strains for Simultaneous Production of Docosahexaenoic Acid and Fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Eklöf, J.; Austin, Å.; Bergström, U.; Donadi, S.; Eriksson, B.D.H.K.; Hansen, J.; Sundblad, G. Size Matters: Relationships between Body Size, Dry Weight and Ash-Free Dry Weight of Common Coastal, Aquatic Invertebrates in the Baltic Sea. PeerJ 2016, 5, e2906. [Google Scholar] [CrossRef] [Green Version]
- Hiroyuki, S.; Susumu, K. New Restriction Endonucleases from Flavobacterium Okeanokoites (FokI) and Micrococcus Luteus (MluI). Gene 1981, 16, 73–78. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae Cultivation in a Wastewater Dominated by Carpet Mill Effluents for Biofuel Applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Santos, A.M.; Janssen, M.; Lamers, P.P.; Evers, W.A.C.; Wijffels, R.H. Growth of Oil Accumulating Microalga Neochloris Oleoabundans under Alkaline-Saline Conditions. Bioresour. Technol. 2012, 104, 593–599. [Google Scholar] [CrossRef]
- Khatoon, H.; Banerjee, S.; Syahiran, M.S.; Bt, N.; Noordin, M.; Munafi, A.; Bolong, A.; Endut, A. Re-Use of Aquaculture Wastewater in Cultivating Microalgae as Live Feed for Aquaculture Organisms. Desalin. Water Treat. 2016, 57, 29295–29302. [Google Scholar] [CrossRef]
- Ferreira, M.; Coutinho, P.; Seixas, P.; Fábregas, J.; Otero, A. Enriching Rotifers with “Premium” Microalgae. Nannochloropsis Gaditana. Mar. Biotechnol. 2009, 11, 585–595. [Google Scholar] [CrossRef]
- Brown, M.R.; Farmer, C.L. Riboflavin Content of Six Species of Microalgae Used in Mariculture. J. Appl. Phycol. 1994, 6, 61–65. [Google Scholar] [CrossRef]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The Vitamin Content of Microalgae Used in Aquaculture. J. Appl. Phycol. 1999, 247–255. [Google Scholar] [CrossRef]
- Neto, W.A.F.; Mendes, C.R.B.; Abreu, P.C. Carotenoid Production by the Marine Microalgae Nannochloropsis Oculata in Different Low-cost Culture Media. Aquac. Res. 2018, 49, 2527–2535. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Khong, N.M.H.; Shaharuddin, N.A.; Yusoff, F.M.D. A Simple 18S RDNA Approach for the Identification of Cultured Eukaryotic Microalgae with an Emphasis on Primers. J. Microbiol. Methods 2020, 172, 105890. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.; Eaton, A.; Clesceri, L. 5310 Total Organic Carbon (TOC). In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; pp. 19–24. [Google Scholar]
- Bombar, D.; Paerl, R.W.; Anderson, R.; Riemann, L. Filtration via Conventional Glass Fiber Filters in 15N2 Tracer Assays Fails to Capture All Nitrogen-Fixing Prokaryotes. Front. Mar. Sci. 2018, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Breuer, G.; Evers, W.A.C.; de Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of Fatty Acid Content and Composition in Microalgae. J. Vis. Exp. JoVE 2013. [Google Scholar] [CrossRef] [Green Version]
- Volkman, J.K.; Brown, M.R.; Dunstan, G.A.; Jeffrey, S.W. The Biochemical Composition of Marine Microalgae from the Class Eustigmatophyceae. J. Phycol. 1993, 29, 69–78. [Google Scholar] [CrossRef]
- Guimarães, B.O.; Gremmen, P.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Effect of Ammonium Formate Washing on the Elemental Composition Determination in Nannochloropsis oceanica. Aquaculture 2021, 538, 736526. [Google Scholar] [CrossRef]
- Piwowar, A.M.; Lockyer, N.P.; Vickerman, J.C. Salt Effects on Ion Formation in Desorption Mass Spectrometry: An Investigation into the Role of Alkali Chlorides on Peak Suppression in Time-of-Flight-Secondary Ion Mass Spectrometry. Anal. Chem. 2009, 81, 1040–1048. [Google Scholar] [CrossRef]
- Berman, E.S.F.; Fortson, S.L.; Checchi, K.D.; Wu, L.; Felton, J.S.; Wu, K.J.J.; Kulp, K.S. Preparation of Single Cells for Imaging/Profiling Mass Spectrometry. Am. Soc. Mass Spectrom. 2008, 19, 1230–1236. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Qiu, R.; Zhang, W.; Dong, H.; Zhao, Z.; Zhang, T.; Wei, X.; Cai, X. The Study of Operating Variables in Soil Washing with EDTA. Environ. Pollut. 2009, 157, 229–236. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Pan, L.-Y.; Hong, M.-J.; Lee, A.-C. The Effects of Temperature on the Growth of and Ammonia Uptake by Marine Microalgae. Bot. Stud. 2012, 53, 125–133. [Google Scholar]
- Schipper, K.; Al Muraikhi, M.; Alghasal, G.S.H.S.; Saadaoui, I.; Bounnit, T.; Rasheed, R.; Dalgamouni, T.; Al Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Potential of Novel Desert Microalgae and Cyanobacteria for Commercial Applications and CO2 Sequestration. J. Appl. Phycol. 2019, 31, 2231–2243. [Google Scholar] [CrossRef] [Green Version]
| Glass Microfiber Filter Grade | Pretreatment (Combustion) | Concentration of Ammonium Formate (M) | Culture: Ammonium Formate Ratio (v:v) | Cycle of Washing Process | Drying Period (hour) | Drying Temperature (°C) | Reference |
|---|---|---|---|---|---|---|---|
| GF/F | 450 °C, 2 h | 0.5 | 1:2 | 1 | - | 95 | [6] |
| GF/C | 90 °C, 4 h | 0.65 | 1:1 | 1 | 4 | 90 | [15] |
| GF/F | 450 °C, 2 h | 0.5 | 1:2 | 2 | 24 | 95 | [16] |
| - | 100 °C, 4 h | 0.5 | 1:1 | 1 | 4 | 100 | [17] |
| GF/C | - | 0.5 | 1:1 | 3 | Overnight | 80 | [18] |
| GF/C | 450 °C, 16 h | 0.5 | 1:0.75 | 1 | 16 | 100 | [19] |
| GF/C | 450 °C, 16 h | 0.5 | - | 1 | 16 | 100 | [20] |
| GF/A | - | 0.05 | - | 1 | 48 | 60 | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaw, Y.S.; Tan, H.T.; Sopawong, A.; Shaharuddin, N.A.; Omar, A.R.; Yusoff, F.M. A Recommendation for a Pre-Standardized Marine Microalgal Dry Weight Determination Protocol for Laboratory Scale Culture Using Ammonium Formate as a Washing Agent. Biology 2021, 10, 799. https://doi.org/10.3390/biology10080799
Khaw YS, Tan HT, Sopawong A, Shaharuddin NA, Omar AR, Yusoff FM. A Recommendation for a Pre-Standardized Marine Microalgal Dry Weight Determination Protocol for Laboratory Scale Culture Using Ammonium Formate as a Washing Agent. Biology. 2021; 10(8):799. https://doi.org/10.3390/biology10080799
Chicago/Turabian StyleKhaw, Yam Sim, Hui Teng Tan, Arissara Sopawong, Noor Azmi Shaharuddin, Abdul Rahman Omar, and Fatimah Md. Yusoff. 2021. "A Recommendation for a Pre-Standardized Marine Microalgal Dry Weight Determination Protocol for Laboratory Scale Culture Using Ammonium Formate as a Washing Agent" Biology 10, no. 8: 799. https://doi.org/10.3390/biology10080799
APA StyleKhaw, Y. S., Tan, H. T., Sopawong, A., Shaharuddin, N. A., Omar, A. R., & Yusoff, F. M. (2021). A Recommendation for a Pre-Standardized Marine Microalgal Dry Weight Determination Protocol for Laboratory Scale Culture Using Ammonium Formate as a Washing Agent. Biology, 10(8), 799. https://doi.org/10.3390/biology10080799





