Simple Summary
As an important enzyme system in organisms, P450 multi-functional oxidase not only participates in the metabolism and synthesis of substances in organisms but can also maintain the normal physiological functions of organisms under stress. As one of the important rice pests, the harm caused by white-backed planthoppers has been increasing in recent years. Although the application of chemical pesticides as one of the prevention and control measures can slow down the harm of white-backed planthoppers, its resistance is also rising rapidly. Among them, the generation of metabolic resistance dominated by the P450 enzyme is more common. In this study, we measured the expression of ten P450 gene in vivo situations with a background of chlorpyrifos resistance in white-backed planthoppers. After selecting the two genes with the highest expression, the function of these two genes in the pesticide resistance process was verified by RNA interference and provided a theoretical basis for a follow-up study of the molecular mechanism of the P450 gene mediated by pesticide resistance formation.
Abstract
The white-back planthopper (WBPH), Sogatella furcifera, mainly harms rice and occurs in most rice regions in China and Asia. With the use of chemical pesticides, S. furcifera has developed varying degrees of resistance to a variety of pesticides. In our study, a chlorpyrifos-resistant population (44.25-fold) was built through six generations of screening with a sublethal dose of chlorpyrifos (LD50) from a field population. The expression levels of ten selected resistance-related P450 genes were analyzed by RT-qPCR and found that CYP408A3 and CYP6CS3 were significantly more expressed in the third instar nymphs of the XY17-G5 and XY17-G6 populations, about 25-fold more than the Sus-Lab strain, respectively (p < 0.01). To elucidate their molecular function in the development of resistance towards chlorpyrifos, we cloned two P450 full lengths and predicted their tertiary protein structures. CYP408A3 and CYP6CS3 were also downregulated after injecting dsCYP408A3, dsCYP6CS3, or their mixture compared to the control group. Moreover, the mortality rates of the dsCYP6CS3 (91.7%) and the mixture injection treatment (93.3%) treated by the LC50 concentration of chlorpyrifos were significantly higher than the blank control group (51.7%) and dsCYP408A3 injection treatment (69.3%) at 72 h (p < 0.01). Meanwhile, the P450 enzyme activities in the dsRNA treatments were lower than that in the control (XY17-G6) (p < 0.01). Therefore, the P450 gene CYP6CS3 may be one of the main genes in the development of chlorpyrifos resistance in S. furcifera.
1. Introduction
The white-backed planthopper (WBPH), Sogatella furcifera (Horváth) (Hemiptera: Delphacidae), is among the most notorious insect pests of the rice crop in Asia. It usually feeds on rice stalks with its stinging mouthparts and can carry and spread southern Rice Black Streaked dwarf virus (RBSDV), which can harm the normal physiological growth of rice plants [1,2,3,4,5,6,7]. Over the past few decades, the most effective measure against S. furcifera is the use of pesticides. Consequently, one of the major crises is drug resistance caused by the inappropriate use and abuse of pesticides, as S. furcifera appears to have an extraordinary ability to develop resistance to a variety of insecticides, including chlorpyrifos [8,9,10,11,12]. A field WBPH resistance for chlorpyrifos was reported in Guizhou, Sichuan Province and many other rice growing areas of China in the past 15 years [11,12,13]. As a traditional and highly effective organophosphorus insecticide, Chlorpyrifos continues to be used in the control of pests such as Lepidoptera, Diptera, and Hemiptera [14]. However, due to the complex environment of pesticide use and the improvement of insect adaptability, organophosphate pesticides such as chlorpyrifos are suffering a new risk crisis of being banned. The pesticide industry is ambivalent about such a move, and chlorpyrifos is still a commonly used insecticide to control agricultural pests [15,16]. However, due to its stable nature and low utilization efficiency after its application (in the effective period of time to the full effect), many countries and regions are restricting the use of chlorpyrifos in order to reduce the excessive use of chlorpyriphos and reduce the generation of resistance in target species [17,18]. In addition, rice has now reached 363.3 million hectares since it was planted on a large scale in China. There is a buffering period for the replacement of insecticides, and chlorpyrifos is still used in many rice-growing areas. Therefore, it is still necessary to explore the mechanism of chlorpyrifos resistance through basic research in order to provide a theoretical basis for the subsequent research and development of biological pesticides and the promotion of new technologies [19,20].
Under natural conditions, when pest are subjected to long-term chemical stress, the development of resistance is a natural phenomenon in which the most significant factor is the enhanced metabolic activity of the enzymes related to insecticide resistance, such as mixed-function oxidases (MFO), carboxylesterases (CarEs), glutathione S-transferases (GSTs) [21], and particularly, the cytochrome P450 monooxygenases (P450s), which plays an important role in mediating the stress resistance of pests, such as insecticide metabolism [22,23]. Many studies have been performed relating an elevated P450 activity with an observed pesticide resistance in rice planthoppers. Zhang et al. [24] found that the four P450 genes played a major role in the metabolic resistance of Nilaparvata lugens (Stal) (Homoptera: Delphacidae) for about 25 generations, screened with the sublethal dosage of imidacloprid. Another study suggested that the overexpression of P450s could contribute to insecticide resistance in the field Laodelphax striatellus (Fallén) populations of Jiangsu Province, especially for the multiple types of resistance [25].
It is widely accepted that the increased controlled transcriptional expression of detoxing-related metabolic genes leads to an increase in the insect enzyme levels. For example, Bao et al. [26] detected the expression levels of two P450 cytopigments (CYP6ER1 and CYP6AY1) related to imidacloprid resistance in several laboratory and field populations of brown planthopper and found that the P450 gene CYP6ER1 in all the field populations was significantly overexpressed 7- to 24-fold, as compared to the laboratory susceptible strain. The study of Yang et al. [27] also found that the CYP3, CYP4, and CYP6 families of S. furcifera were more closely associated with detoxification processes, and finally, resulted in a tolerance against imidacloprid. Although it is well-known that the dysregulation of the P450 genes played a key role in the process of insecticidal resistance, its molecular mechanism is still uncertain and needs to further explain its functions through some molecular technologies, such as RNA interference (RNAi). Liao et al. [28] specifically detected a significant overexpression of the P450 gene CYP6ER1 in N. lugens (36.87-fold change) and then subsequently significantly increased the sensitivity of the drug-resistant populations towards sulfoxaflor through RNAi. Xu et al. [29] reported that multiple P450 genes could be associated with the resistance to chlorpyrifos and imidacloprid via feeding dsRNA in N. lugens. The study by Mao et al. [30] showed that the twelve P450 genes were disorders in the resistant strain of N. lugens, but when this resistance strain was injected with dsCYP6ER1, it returned to a susceptibility against nitenpyram.
In this study, two highly expressed P450 genes, CYP408A3 and CYP6CS3, were selected from the XY17-G6 population (resistance ratio of 44.25-fold), which was obtained from a field resistant population and successively screened for six generations with the LD50 dose of chlorpyrifos. In addition, the full lengths of the two genes were cloned, and we conducted two gene interferences on XY17-6G through RNAi technology and then determined the susceptibility towards chlorpyrifos, and the activity of select P450 enzymes also was determined. Our preliminary results provided a preliminary insight into the expression changes of some P450 genes in WBPH during chlorpyrifos resistance and their relationship with regulation. The findings of this study are expected to be helpful in awareness of the WBPH resistance against chlorpyrifos, especially for the mechanism of resistance in the wild population after the mixed use of various pesticides.
2. Materials and Methods
2.1. Insects
The susceptible strains of S. furcifera (Lab-HN strain) (LC50: 3.262 μg/mL) were provided by Hunan Agricultural University. They were normally raised and domesticated in the laboratory for 15 years and have not been exposed to any pesticides and were uninterrupted when reared on rice seedlings (TN1) in our laboratory at a temperature of 26 ± 1 °C, relative humidity of 85% ± 10%, and photoperiod of 14 L:10 D. The field population (XY17) was collected in 2017 from Xuyong County, Sichuan Province, China.
The resistant population was collected from the field and screened with a chlorpyrifos LD50 dose (144.345 μg/mL) for 6 successive generations and proceeded as the resistant-chlorpyrifos population with 44.25-fold resistance.
2.2. Insecticides and Chemicals
Chlorpyrifos, a 98% technical product, was provided by Hubei Sharonda Co., Ltd. (Jingzhou, China). Triton X-100 was purchased from Chengdu Haobo Technology Co., Ltd. (Chengdu, China). DTT, PMSF, NADPH, and bovine serum albumin (BSA) were purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China), as were EDTA-Na2 and Sodium Dodecyl Sulfate (SDS).
2.3. Bioassay
The indoor resistant population was screened by the rice seedling impregnation method, with little modification [16,31]. Third instar nymphs were used in the study. We soaked the rice seedlings and let them dry and wrapped the roots with cotton and placed them in a plastic cup. Fifteen third instar nymphs were released into each plastic cup that were reared on seedlings and cultured soilless in a rearing box incubator at 26 ± 1 °C, 85 ± 10% R.H., 14 L:10 D). The LC50 dose of the third instar nymph to chlorpyrifos was measured and calculated after 72 h of being treated. Finally, the LC50 dose was used to screen the strain, and the number of rice materials and nymphs were increased (30 rice seedlings, as many nymphs as pests).
2.4. Quantitative PCR (RT-qPCR) for Insecticide Resistant-Related P450 Genes
Total RNA for RT-qPCR of the Lab-HN strain and XY17 fifth- (G5) and sixth (G6)-generation populations were extracted by the traditional method by Chen et al. [31]. The CDS sequences of ten insecticide resistant-related P450 genes (CYP6CS3, CYP6AX3, CYP408A3, CYP4CE3, CYP417A4, CYP4DD1, CYP18A1, CYP408A3, CYP6ER4, and CYP418A2) were obtained from the research of Yang et al. [27], which referred to the transcriptome of S. furcifera by Wang et al. [32]; one reference gene (RPL9) [33] from the Lab-HN strain and the XY17 population each were amplified through RT-qPCR, with eleven pairs of corresponding primers (Table S1). The mRNA levels of the P450 genes were measured by the methods of Wang et al. [34].
2.5. Cloning of CYP Genes Sequences
Full-length clones of P450 genes from resistant populations were adopted by the Kod-201 high-fidelity enzyme (200U Shanghai Toyang Textile Co., Ltd. (Japan)). The specific primers of two resistance-related P450 genes, CYP408A3 and CYP6CS3 [27], were designed based on the gene sequences from GenBank with Primer 3 online (Table S1). After the P450 gene was cloned, its concentration and bands were detected by gel electrophoresis, and the gel was recovered.
2.6. Analysis of CYP Genes DNA and Protein Sequences
The prediction tool I-TASSER online (http://zhanglab.ccmb.med.umich.edu/I-TASSER, accessed on 10 April 2021) was used to predict the protein structure. PyMol 2.3.4 software was used for mapping and analysis. A phylogenetic tree was constructed with MEGA 7.0 software (MEGA, Tempe, AZ, USA) based on a multiple alignment of the amino acid sequences performed by Clustal X 2.0 [35] program software and by adopting the neighbor-joining algorithm with the bootstrap values determined by 1000 replicates.
2.7. Preparation of dsRNA
Before microinjection, the template was amplified with T7-specific primers to prepare the template (Table S2). PCR products were analyzed on 1% agarose gel. Then, the PCR products were cloned and sequenced to confirm their identities and purified with VAHTS®® RNA Clean Beads (Vazyme Biotech Co., Ltd. Nanjing, China). The dsRNA was synthesized by using a MEGA script®® RNAi Kit (Thermo Fisher, Shanghai, China) according to the instruction manual and quantified by using an ultra-micro spectrophotometer (BIO-DL) at a wavelength of 260 nm. The calculated concentrations of the dsRNA are shown in Table S3.
2.8. dsRNA Microinjection
To better ensure the interference efficiency, we explored the conditions of the microinjection, and the results showed that the nymph had the highest mortality rate, with forty percent for the third instar, and a minimum mortality rate, with twenty percent for the fourth instar nymph of white-back planthopper that was injected with a dose of 40 nL.
To ensure the efficiency of RNA interference, we used a UMP3/Nanoliter2010 micro-injection device (World Precision Instruments, Sarasota, FL, USA) to insert dsRNA synthesized in vitro into the fourth instar nymph, according to the method of Zhang et al. [36]. In addition, the conditions for appropriate microinjection parameters have been explored and determined that the amount of 40 nL from the total of 150 ng resulted in a minimum mortality rate of 20% and an appropriate interference efficiency of 80%.
The fourth instar nymphs of the XY17 population were anaesthetized by CO2 for 30 s and placed on agarose plates, and then, a pointed brush was used to place WBPH in the grooves, prepared in advance. The dsRNA solutions of 150 ng were injected into third instar nymphs after selecting the right injection sites with the proper strength. Then, they were reared in a greenhouse with a temperature of 26 °C (±1 °C), photoperiod of 14:10 h (L:D), and relative humidity of 85% (±10%). After rearing for 2 h, each of the BPHs was checked, whether it survived or not, and the rice seedlings were renewed after every three or four days. In this way, four treatments, including the dsCYP408A3, dsCYP6CS3, mixture of dsCYP48A3 and dsCYP6CS3, and dsGFP diet (the blank control) were established with three replications. To assess the expression levels associated with each treatment, the whole bodies of the post-injection nymphs were collected at 24 h, 48 h, and 72 h for total RNA extraction and to calculate the reduction of the transcription levels of the P450 genes by using the RT-qPCR method, as above.
2.9. Assessment of Nymph Mortality and Cytochrome P450 Activity
To evaluate the RNAi efficacy of the CYP408A3 and CYP6CS3 genes in the susceptibility of the WBPH to chlorpyrifos, the injected nymphs (after 48 h of injection) were fed over the rice seedlings, treated with an LC50 dose of chlorpyrifos, and the mortality was recorded at 72 h post-treatment. Approximately, 30 injected nymphs were tested in each of the three replicates and the dsGFP as a control.
In order to evaluate whether two P450 genes (CYP408A3 and CYP6CS3) mediate the normal survival of white-back planthopper (WBPH), we used indoor sensitive strains as verification materials, a microinjection of dsRNA synthesized by two genes in vitro into the fourth innermost nymphs, and then transferred them to fresh rice seedlings for feeding. The survival rate within 72 h after injection was observed and statistically analyzed. The experiment was set up in three replicates with 20 nymphs injected into each treatment.
To verify the RNAi effect on the two P450 detoxification genes, P450 activity was determined in the 4th instar nymphs from the Lab-HN strain and the XY17-G6 population. The total P450 activity was detected by Rose et al. [37] and Wang et al. [38]. About 30 nymphs were selected and injected with PBS buffer for tissue grinding. After, the fish were centrifuged at 4 °C at 15,000× g for 15 min using a 5417R centrifuge (Eppendorf, Hamburg, Germany). Finally, the supernatant was absorbed as the total crude enzyme solution and stored at a low temperature for testing. Then, 100 μL of 4-nitroanisole (2 × 10−3 mol/L) and 90 μL of crude enzyme liquid were mixed in a cell culture plate, followed by incubation for 3 min at 30 °C in a water bath. Next, 10 μL of NADPH (9.6 × 10−3 mol/L) was added into the system for a reaction. The changes in the OD value at the excitation wavelength of 380 nm and emission wavelength of 460 nm were recorded at the intervals of 45 s for 15 min (Spectramax i3x, Meigu Molecular Instruments (Shanghai) Co., Ltd. Shanghai, China). Finally, the specific activity of P450s was calculated using the standard curve of 7-hydroxycoumarin. The total protein content of the enzyme solution was determined by the Bradford [39] method while using bovine albumin as a standard.
2.10. Data Analysis
A Probit analysis was conducted by using the statistical analysis system (POLO 2.0) to calculate the slope, LC50, 95% CI, and χ2 values of chlorpyrifos to the susceptible strain and XY17 population for determining their resistance levels [34,40]. The relative expression of the P450 genes was calculated based on the 2−ΔΔCT method [41]. The relative expression levels of the resistant-related P450 genes and mortality ratio were compared by using an analysis of variance (ANOVA), followed by a Student’s t-test for multiple comparisons (p < 0.05, 0.01), conducted by using SPSS (Microsoft 10.0)version 20.0 software.
3. Results
3.1. Expression Profiles of P450 mRNAs in Lab-HN and XY17 Populations of Sogatella furcifera
In insects, P450s play an important role in the metabolism of endogenous substances (such as juvenile hormones and ecdysis hormones) and exogenous substances (such as chemical pesticides). To analyze the molecular function of ten P450 genes in the development of resistance to chlorpyrifos, RT-qPCR was used to detect their expression levels in the resistant populations (G5 and G6). The results showed that the expression levels of the CYP408A3 and CYP417A4 genes were significantly enhanced in the XY17-G5 population 27.85-fold (p < 0.001) and 12.10-fold (p < 0.01), respectively, as compared to the Lab-HN strain. The relative expression levels of CYP6FJ3 and CYP6CS3 were 5.9- to 10.0-fold and were significant with the other genes (p < 0.05). For the other genes (CYP6ER4 and CYP4CE3), the expression levels were slightly below the above genes (p < 0.05) and indicated 3.08-fold and 3.50-fold, respectively, while the P450 genes of CYP18A1, CYP6AX3, and CYP4DD1 showed the minimum distribution in their expression levels, ranging from 1.34- to 2.38-fold (Figure 1A). For the gene CYP6CS3, its expression level was the highest in the third instar nymph population of XY17-G6, which was 26.13-fold, and the CYP408A3 also had a high expression level (8.32-fold (p < 0.01)). The genes of CYP4CE3 and CYP6ER4 were still elevated at 3.64- and 4.01-fold, respectively, which was significantly higher than the other three genes: CYP417A4, CYP18A1, and CYP4DD1, ranging from 1.02- to 1.16-fold (Figure 1B). Therefore, CYP408A3, CYP6CS3, and CYP6ER4 were significantly overexpressed in the XY17 G5 and G6-resistant populations. It can be speculated that the above genes may be related to the development of S. furcifera resistance to chlorpyrifos. Finally, the two genes of CYP408A3 and CYP6CS3 with the highest upregulation observed of the 10 genes that were screened were selected as the target genes for functional verification.
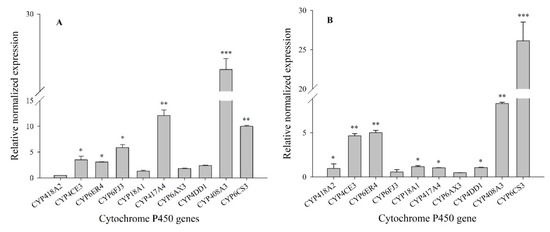
Figure 1.
Relative expression qualities of select insecticide resistant-related P450 genes in the 3rd instar nymph resistant populations selected by a LC50 dose of chlorpyrifos for 5 generations (A) and 6 generations (B) compared to the Lab-HN strain. The relative normalized expression was presented as the mean of three replications ± SE. The F9, 20 values of the relative expression qualities of insecticide resistant-related P450 genes in the 3rd instar nymph of the XY17-G5 (A) and XY17-G6 (B) field populations were 378.54 and 100.745, and the p-values were = 0.0001 < 0.01. *, **, and *** showed significance at the 0.05, 0.01, and 0.001 levels with a Student’s t-test, respectively.
3.2. Characterization of Full-Length Amino Acid Sequences of Two Cloned P450 Genes
With multiple protein sequences alignments, there are four specific conserved motifs in the two cytochrome P450 genes (Figure 2). The heme–iron ligand signatures of the cytochrome P450 cysteine FxxGxRxCxG (CYP408A3: 448–457 and CYP6CS3: 447–456) were demonstrated in the lamina of the two cytochrome P450 proteins.
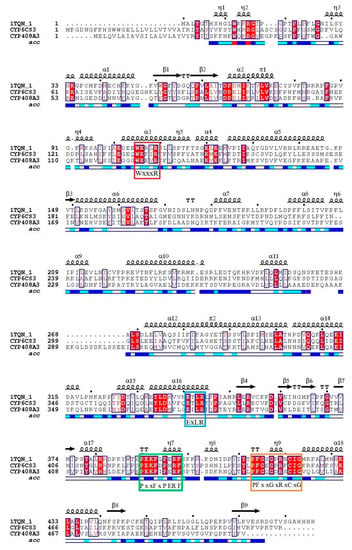
Figure 2.
Graph of sequence alignment in three cytochrome P450 genes. The 1TQN-1 sequence is a reference sequence for predicting the function of two amino acid sequences of the P450 gene. The red box located in helix 4 is a conserved sequence, and the amino acid sequence is WxxxR; the blue box located in helix 15 is the conserved sequence, with the amino acid sequence ExLR; the green box of the amino acid sequence is PxxFxPE/DRF; the orange box located in random coil 13 (13, heme binding) is a conserved sequence; and the amino acid sequence is FxxGxxxCxG.
To further verify the diversity of the cytochrome P450 genes of S. furcifera during the resistance formation process, we compared the existing P450 sequence in the NCBI library with the evolutionary tree, as shown in Figure 3. The phylogenetic tree showed that CYP6CS3 and CYP408A3 were most closely related with CYP6CS2v1 from L. striatellus and CYP408A1v2 from N. lugens, respectively.
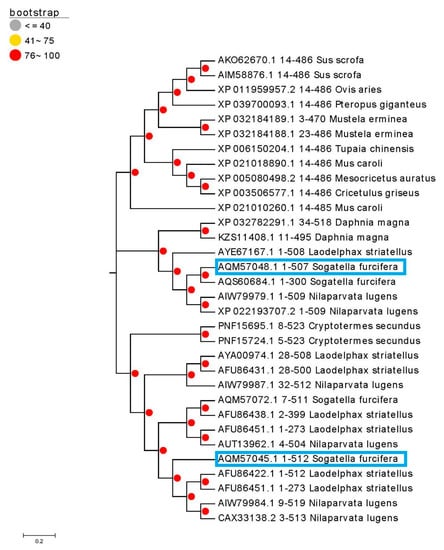
Figure 3.
Phylogenetic relationship among CYP6CS3 and CYP408A3, other insect P450s. Two selected and validated P450 genes have been labeled with blue boxes (The AOM57048.1 represents the P450 gene CYP408A3 and the AOM57045.1 represents the CYP6CS3). The GenBank accession numbers are shown before the P450 names. The phylogenetic tree was inferred using Neighbor-Joining. The scale bar indicates 0.1 amino acid substitutions per site. The percentage of the replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The phylogenetic analyses were conducted in MEGA 7.0.
3.3. Interaction of the Tertiary Structure of CYP6CS3 and CYP408A3 with Chlorpyrifos
The optimal three-dimensional structure of the cytochrome P450 gene was predicted using I-TASSER online simulation (Figure 4A,B). The precheck results showed that 98.3%~98.9% of the amino acid residues in the three-dimensional (3D) structure of the cytochrome P450 genes were in the reasonable area of the Ramachandran point diagram, which theoretically indicated that the 3D structure of the two cytochrome P450 genes was reliable. After making a prediction about the optimization of the three-dimensional structure, we carried out the docking of the chlorpyrifos agent molecules with the model. The CYP6CS3 and CYP408A3 domain consists of several active amino acid residues and is located near the entrance of the active sac and at the heme-binding region. The total core value between CYP6CS3 and chlorpyrifos was 6.3297 (crash of −1.4145). The total core value between CYP408A3 and chlorpyrifos was 6.2386 (crash of −1.5198). In addition, the active structural cavity of the CYP6CS3 protein of the P450 gene was significantly larger than that of the CYP408A3 protein, and the molecular inclusion of chlorpyrifos was stronger, which provided more suitable conditions for the metabolic interpretation of chlorpyrifos.
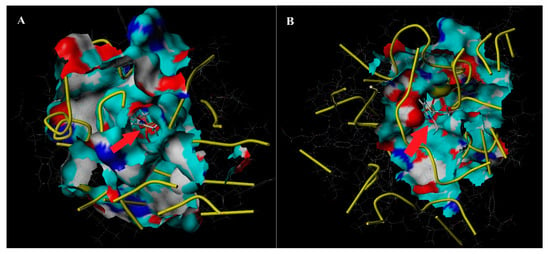
Figure 4.
The docking structure with chlorpyrifos of CYP6CS3 and CYP408A3. (A) CYP6CS3 domain and chlorpyrifos. (B) CYP408A3 domain and chlorpyrifos. The molecular cartoons were drawn with PyMol 2.3.4 software. The molecular model of chlorpyrifos was embedded in the active cavity of the P450 protein molecule, and the size of the opening of the active cavity affected the metabolic activity of the corresponding P450 protein (the active cavity is indicated by the red arrow).
3.4. Functional Analysis of CYP6CS3 and CYP408A3 via RNAi
To elucidate the functions of CYP6CS3 and CYP408A3, two P450 genes in S. furcifera in mediating the development of chlorpyrifos resistance, the relative expression levels were detected via RT-qPCR in the fourth instar nymphs of the XY17-G6 population at 24 h, 48 h, and 72h after the dsRNA injection. The results showed, after the injection, that the expression of CYP6CS3 and CYP408A3 decreased within 48 h, as compared to the dsGFP injection, in which the most obvious results, which was 93.45% for injected CYP6CS3 and 80.34% for CYP408A3 at 72 h (Figure 5), respectively (p < 0.01), were observed. In addition, the mRNA expression level of the injection containing the mixture of the two genes was also significantly decreased, which was stable at about 94% (p < 0.01).
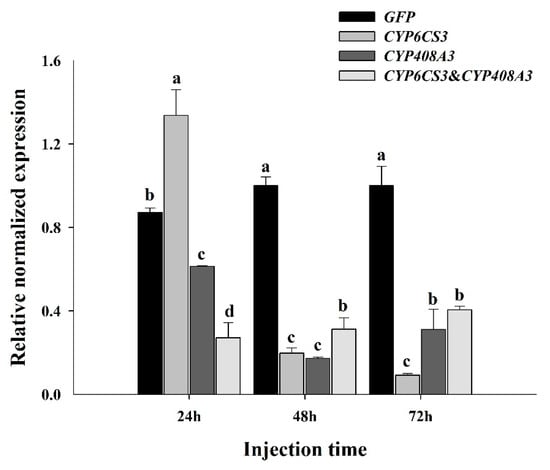
Figure 5.
Functional analysis of CYP6CS3 and CYP408A3 by RNAi. The relative expression of CYP6CS3 and CYP408A3 in the fourth instar nymphs injected with dsCYP6CS3, dsCYP408A3, or the mixture of dsCYP6CS3 and dsCYP408A3 in each period compared with dsGFP. Significant differences are indicated by different letters, for example, a, b, c, and d (p < 0.05, with a Student’s t-test).
The larvae that survived well in each treatment were selected at 48 h post-dsRNA injection, and the P450s enzyme activities were determined. The results showed that the P450 activity level of the XY17-G6 population (3.42 nmol∙min−1∙mg pro−1) and XY17-dsGFP treatment (2.96 nmol∙min−1∙mg pro−1) were consistent and significantly higher than that of the Lab-HN strain (2.42 nmol∙min−1∙mg pro−1) (p > 0.05). In target gene interference processing, the P450 activity in the larvae of the dsCYP6CS3 treatment (2.08 nmol∙min−1∙mg pro−1) showed a significant reduction. For the dsCYP408A3 (2.24 nmol∙min−1∙mg pro−1) and mixture of dsCYP6CS3 and dsCYP408A3 (2.29 nmol∙min−1∙mg pro−1) injection treatments, they also showed a significant reduction compared with the other treatments, but it was slightly higher than that of the dsCYP6CS3 treatment (Table 1).

Table 1.
P450 activity of the Lab-HN strain and XY17 population 48 h after RNAi.
To assess the efficiency of RNAi, the survived fourth instar nymphs after 48 h of injection, containing the dsRNA of CYP6CS3 and CYP408A3, were dealt with LC50 of chlorpyrifos. The results showed that the mortality rate was increased significantly in the RNA interference treatment group compared with the treatments of injecting dsGFP (51.67%) and G6-Blank control (48.9%) generation (p < 0.01). Among the treatments of RNA interference, the mixture of dsCYP6CS3 and dsCYP408A3 and sole dsCYP6CS3 had the highest mortality (93.33% and 91.67%, respectively) at 72 h after injection (p < 0.05), followed by the treatment of sole dsCYP408A3 reaching 69.33% (Figure 6).

Figure 6.
Mortality at 72 h of the dsRNA-injected fifth instar nymphs after being unexposed to the chlorpyrifos in the HN-Lab and exposed to the chlorpyrifos in the XY17-G6 population. Significant differences are indicated by different letters, for example, a, b, and c (p < 0.05, with a Student’s t-test).
In order to evaluate whether the selected genes were necessary for the survival of the test insect, we injected dsRNA into the indoor sensitive population to observe the mortality rate of the test insect. The results of the functional necessity validation of the two P450 genes in the HN-Lab showed that the nymphs in the CYP408A3 and CYP6CS3 injection groups had good survival compared with the blank control group (95%). The survival rate of the nymphs in the dsGFP and dsCYP408A3 injection group was 93.3%. The survival rate of the dsCYP6CS3 injection group was 91.6% (Figure 6).
All results suggested that the RNAi-mediated silencing of CYP6CS3 increased the susceptibility of the XY17-G6 population to chlorpyrifos.
4. Discussion
Chlorpyrifos, as a commonly utilized effective insecticide, has been used in China for more than 15 years [42]. It can be used for effective control of rice pests because of its high capability of internal absorption and acts as an acetylcholinesterase inhibitor in insects [13]. Like all traditional pesticides, under the conditions of excessive or irregular use, chlorpyrifos resistance is still produced in various rice pests, including S. furcifera [43,44]. Under natural conditions, there are many possible reasons for the development of resistance in S. furcifera [45]. However, the increase of the metabolic activities of the different detoxification enzymes is still an important factor, promoting the development of insecticidal resistance. The reports of Xu et al. showed that three P450 genes were significantly overexpressed (6.87–12.14-fold) in the chlorpyrifos resistant population of L. striatellus [29]. In addition, it was proved by a RNAi experiment that the resistance in the indoor resistant population of Nilaparvata lugens against clothianidin was caused by the overexpression of the P450 gene CYP6ER1 [46]. According to the research of our lab, P450 CYP6ER4 was screened by RT-qPCR for its field resistance to the chlorpyrifos S. furcifera population, and RNA interference was used to verify that the gene was mainly involved in the generation of resistance to chlorpyrifos in the population [47]. In the present research, we detected the upregulation of six CYP genes among ten tested in the XY17-resistant population, which proved that the P450 detoxification enzyme could mediate the metabolic resistance of the white-backed planthopper to chlorpyrifos.
Currently, it has been accepted by pesticide toxicity researchers that the development of pesticide resistance is caused by the controlled overexpression of resistance-related detoxification genes such as P450 under continuous pesticide stress [48,49,50,51]. However, there are a large number of P450 genes that may be combined by several genes when they perform various functions in insects [45]. Zhang et al. [24] found that the twelve P450 genes were observed for upregulation in the imidacloprid-resistant N. lugens population. Four P450 genes, CYP6AY1, CYP6ER1, CYP4CE1, and CYP6CW1, played important roles in imidacloprid resistance after RNAi and in vitro recombination of the corresponding proteins. One more research showed that nine P450 genes were upregulated and three P450 genes were downregulated under the LD85 dose of Imidacloprid and Cycloxaprid [27]. Ali et al. [15] also found that sixteen P450s and one GST gene were significantly overexpressed in the dinotefuran-resistant strain (22-fold) two-fold higher than the susceptible strain. In our study, while detecting the spatial and temporal expression of ten resistant P450 genes, among which four P450 genes in the CYP6 Clade (CYP6ER4, CYP6FJ3, CYP6AX3, and CYP6CS3); four P450 genes in the CYP4 Clade (CYP4CE3, CYP417A2, CYP4DD1, and CYP408A3); and one in the CYP2 Clade (CYP18A1) were upregulated in the XY17-resistant population, especially for CYP417A2, CYP408A3, CYP6ER4, and CYP6CS3. These results are consistent with the mechanism proposed by Okey that there are different expression levels of multiple P450 genes during the development of resistance [52]. As a mature sequencing technology, RNA-Seq provides real-time and stable technical support for researchers in the process of mining drug resistance-related genes [53]. However, for transcriptome sequencing, materials with a stable genetic background are required. Our experimental design was defective due to the different backgrounds of the chlorpyrifos-resistant population in the field and sensitive population in the room. In the future, we will use a two-way screening method to screen sensitive and resistant populations, so as to better use transcriptome sequencing to reveal the expression of resistance genes. RNAi is an effective technique with high specificity in the last decade and is being widely used to investigate the gene functions in insects [54,55]. We investigated the contribution of multiple P450 genes that were expressed significantly in the formation of pesticide resistance and their interactions by the RNAi technique. When the expression levels of CYP6CS3 and CYP408A3 were suppressed by applying the injection of dsCYP6CS3, dsCYP408A3 and dsCYP6CS3 and dsCYP408A3, the mortality of the larvae treated with the LC50 of chlorpyrifos increased significantly, especially for the injection having a mixture of two genes, which showed higher mortality rates, and this phenomenon was consistent with the research results of Lu et al. [45]. Moreover, the total P450 activity of every treatment showed a significant reduction as compared with the control, but for the mixed injection treatment, it was slightly lower than the single gene interference effect, probably because we used the half-concentration of a single gene from the total injection when we injected the dsRNA mixture. However, there was a slight downregulation of the P450 enzyme activity in the dsGFP injection group, which may be due to the fact that the nymphs themselves needed a certain recovery time after the microinjection, so the P450 enzyme activity in vivo was in a relatively unstable state. It was worth considering about these two P450 genes may not be necessary for the survival of S. furcifera, and due to that, the treated nymphs almost never died after we injected dsRNA alone into the HN-Lab population without exposure to chlorpyrifos. These results indicated that the development of resistance in the XY17 population is mediated by the two or more pairs of multiple P450 genes, and there is a cascade regulation and synergistic effect between multiple genes that still need to be further studied to clarify and explore the regulatory mechanisms.
The P450s are multi-enzymatic complexes and have a long history; the diversity of the protein structure determines the diversity of the function. The structural differences of the insect cytochrome P450 protein can reflect the evolutionary process of the P450 gene. At present, as an inherent functional domain of the P450 gene, the heme-binding domain FxxGxRxCxG is the main target to explore the structure and function of the P450 gene [56]. Our research showed that the conserved region was located near the typical active lumen and the molecular modeling display and that structure of chlorpyrifos was surrounded by the active site of CYP6CS3. The protein has a predicted oval active site structure, large volume, and large substrate channel, enabling chlorpyrifos to adapt to active site cavities. The spacious cavity of the P450 enzyme allows larger molecules to access the oxygen bound to the heme in the reaction center and brings the substrate closer to the binding site [57]. Our results are close to the research of Rupasinghe [58], in which a comparison of the homology models for CYP688 and CYP321A1 described their substrate-binding cavities, which were predicted to be more spacious in these two enzymes that metabolize a wider range of compounds. Therefore, we hypothesized that CYP6CS3 can present a greater metabolic ability than CYP408A3 [59,60]. This result also confirmed the phenomenon that dsCYP6CS3 had a high interference efficiency and a high degree of P450 enzyme activity decline mediated by CYP6CS3. Moreover, the P450 gene is ubiquitous in all species. Although its gene functions are diverse, the P450 gene forms some conserved regions in the process of species adaptation and evolution to maintain a physiological balance for insects when they respond to external stress. In our research, the phylogenetic tree comparison showed that the two selected resistance-related P450 genes were close to their homologous resistance genes. The results showed that gene CYP6CS3 was most closely related with CYP6CS2v1, which is from the deltamethrin-resistant L. striatellus strain [61], and CYP408A3 was most closely related with CYP408A1v2 from the N. lugens strain [62], respectively. From the function of the related subfamily genes, it can be analyzed that the two P450 genes selected by us have a high possibility of benig involved in the formation of metabolic resistance in resistant populations.
Based on the results of our study, we speculated that the P450 genes CYP408A3 and CYP6CS3, especially CYP6CS3, play an important role in S. furcifera against chlorpyrifos resistance. Additionally, further investigation is needed to determine which P450 is the key gene involved in the development of resistance in white-backed planthoppers. Our results lay the foundation for further exploring the functions of CYP408A3 and CYP6CS3 in resistance formation through prokaryotic expression, such as in vitro prokaryotic expression and eukaryotic expression, and the function of the two genes can also be verified by further gene knockouts.
5. Conclusions
In this study, we detected the P450 gene expression in a field chlorpyrifos-resistant population screened for six generations with a LD50 dose (XY-17), and two selected P450 genes were verified by RNAi. Our results showed that the P450 gene CYP6CS3 may play an important role in the development of resistance to chlorpyrifos in this population. This work could help to deepen the understanding of chlorpyrifos resistance research in white-back planthoppers (WBPH) and could also inform decision-making for the development and management of chlorpyrifos resistance in wild WBPH.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10080795/s1: Table S1 are RT-qPCR and full-length primers of insecticide resistant-related P450 genes, and Table S2 are dsRNA synthesis primers of insecticide resistant-related P450 genes. The primers of the insecticide resistant-related P450 genes used in this study. Table S3. The dsRNA synthesis concentration of the P450 gene.
Author Contributions
Conceived and designed the experiments: Y.R. and X.W. Performed the experiments; X.W. and Y.R. Analyzed the data: C.G., X.L., and Y.Z. Contributed reagents/materials/analysis tools: L.S. and Y.H. Drafted and revised the manuscript: X.W., Y.R., H.A., and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key R&D Program of China (2018YFD0200300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available in publicly accessible repositories within the bibliography.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sogawa, K. Vulnerability to insect pests in Chinese hybrid rice. Agric. Tech. 2001, 56, 398–402. [Google Scholar]
- Zhou, G.H.; Wen, J.J.; Cai, D.J. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family reoviridae. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Cheng, Z.N.; Li, S.; Gao, R.Z.; Sun, F.; Liu, W.C.; Zhou, G.H.; Wu, J.X.; Zhou, X.P.; Zhou, Y.J. Distribution and genetic diversity of southern rice black-streaked dwarf virus in China. Virol. J. 2013, 10, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukura, K.; Towata, T.; Sakai, J.; Onuki, M.; Okuda, M.; Matsumura, M. Dynamics of Southern rice black-streaked dwarf virus in rice and implication for virus acquisition. Phytopathology 2013, 5, 509–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.G.; Xiang, X.; Yu, H.L.; Liu, S.H.; Yin, Y.; Cui, P.; Wu, Y.Q.; Yang, J.; Jiang, C.X.; Yang, Q.F. Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2018, 146, 71–79. [Google Scholar] [CrossRef]
- Liu, M.G.; Jiang, C.X.; Mao, M.; Liu, C.; Li, Q.; Wang, X.G.; Yang, Q.F.; Wang, H.J. Effect of the insecticide dinotefuran on the ultrastructure of the flight muscle of female Sogatella furcifera (Hemiptera: Delphacidae). J. Econ. Entomol. 2017, 110, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, J.Y.; Liang, A.; Wang, F.H. Transcriptome profiling and dimorphic expression of sex-related genes in fifth-instar nymphs of Sogatella furcifera, an important rice pest. Genomics 2020, 112, 1105–1111. [Google Scholar] [CrossRef]
- Matsumura, M.; Takeuchi, H.; Satoh, M.; Sanada-Morimura, S.; Otuka, A.; Watanabe, T.; Thanh, D.V. Species-specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in east and southeast Asia. Pest Manag. Sci. 2008, 64, 1115–1121. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Shao, Y.; Yang, B.J.; Liu, Z.W. Fipronil resistance in the white-backed planthopper (Sogatella furcifera): Possible resistance mechanisms and cross-resistance. Pest Manag. Sci. 2010, 66, 121–125. [Google Scholar] [CrossRef]
- Matsumura, M.; Sanada-Morimura, S.; Otuka, A.; Ohtsu, R.; Sakumoto, S.; Takeuchia, H.; Satoha, M. Insecticide susceptibilities in populations of two rice planthoppers, Nilaparvata lugens and Sogatella furcifera, immigrating into Japan in the period 2005–2012. Pest Manag. Sci. 2014, 70, 615–622. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, W.; Zhang, S.; Wu, S.F.; Ban, L.F.; Su, J.Y.; Gao, C.F. Susceptibility of Sogatella furcifera and Laodelphax striatellus (Hemiptera: Delphacidae) to six insecticides in China. J. Econ. Entomol. 2014, 107, 1916–1922. [Google Scholar] [CrossRef]
- Mu, X.C.; Zhang, W.; Wang, L.X.; Zhang, S.; Zhang, K.; Gao, C.F.; Wu, S.F. Resistance monitoring and cross-resistance patterns of three rice planthoppers, Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus to dinotefuran in China. Pestic. Biochem. Physiol. 2016, 134, 8–13. [Google Scholar] [CrossRef]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.X.; Jin, D.C.; Li, W.H.; Cheng, Y.; Li, F.L.; Ye, Z.C. Monitoring trends in insecticide resistance of field populations of Sogatella furcifera (Hemiptera: Delphacidae) in Guizhou Province, China, 2012–2015. J. Econ. Entomol. 2017, 110, 641–650. [Google Scholar] [CrossRef]
- Ali, E.; Mao, K.K.; Liao, X.; Jin, R.H.; Li, J.H. Cross-resistance biochemical characterization of buprofezin resistance in the white-backed planthopper, Sogatella furcifera (Horváth). Pestic. Biochem. Physiol. 2019, 158, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, S.H.; Wang, X.G.; Zhang, Y.M.; Gong, C.W.; Chen, L.; Zhang, S.R.; Shen, L.T. Sublethal effects of sulfoxaflor on population projection and development of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Crop Prot. 2019, 120, 97–102. [Google Scholar] [CrossRef]
- Yu, L. New York state will phase out chlorpyrifos in 2021. J. Agrochem. Mark. 2019, 10, 49. [Google Scholar]
- Yang, G. Vietnam will ban Ethyl Chlorpyrifos and Fipronil after 2 years later. Pestic. Mark. News 2019, 6, 48. [Google Scholar]
- Bhattacharyya, A.; Bhaumik, A.; Rani, P.U.; Mandal, S.; Epidi, T.T. Nano-particles-A recent approach to insect pest control. Afr. J. Biotechnol. 2010, 24, 3489–3493. [Google Scholar]
- Su, J.Y.; Wang, Z.W.; Zhang, K.; Tian, X.G.; Yin, Y.Q.; Zhao, X.Q.; Shen, A.; Gao, C.F. Study on insecticide resistance of white-backed planthopper. Fla. Entomol. Soc. 2013, 96, 948–956. [Google Scholar] [CrossRef]
- Mohan, M.; Gujar, G.T. Local variation in susceptibility of the diamondback moth, Plutella xylostella (Linnaeus) to insecticides and role of detoxification enzymes. Crop Prot. 2009, 22, 495–504. [Google Scholar] [CrossRef]
- Lai, T.C.; Li, J.; Su, J.Y. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Wang, X.G.; Huang, Q.; Hao, Q.; Ran, S.; Wu, Y.Q.; Cui, P.; Yang, J.; Jiang, C.X.; Yang, Q.F. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Prot. 2018, 106, 110–116. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, Y.X.; Sun, H.H.; Liu, Z.W. Metabolic imidacloprid resistance in the brown planthopper, Nilaparvata lugens, relies on multiple P450 enzymes. Int. J. Mol. Sci. 2016, 79, 50–56. [Google Scholar] [CrossRef]
- Gao, B.L.; Wu, J.; Huang, S.J.; Mu, L.F.; Han, Z.J. Insecticide resistance in field populations of Laodelphax striatellus Fallén (Homoptera: Delphacidae) in China and its possible mechanisms. Int. J. Pest Manag. 2008, 54, 13–19. [Google Scholar] [CrossRef]
- Bao, H.B.; Gao, H.L.; Zhang, Y.X.; Fan, D.; Fang, J.; Liu, Z. The roles of CYP6AY1 and CYP6ER1 in imidacloprid resistance in the brown planthopper: Expression levels and detoxification efficiency. Pestic. Biochem. Physiol. 2016, 129, 70–74. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zhang, Y.X.; Yang, B.J.; Fang, J.C.; Liu, Z.W. Transcriptomic responses to different doses of cycloxaprid involved in detoxification and stress response in the whitebacked planthopper, Sogatella furcifera. Entomol. Exp. Appl. 2016, 158, 248–257. [Google Scholar] [CrossRef]
- Liao, X.; Jin, R.G.; Zhang, X.L.; Ali, E.; Mao, K.K.; Xu, P.F.; Li, J.H.; Wan, H. Characterization of sulfoxaflor resistance in the brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2019, 75, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, M.; Han, Z.J. Biochemical and molecular characterisation and cross-resistance in field and laboratory chlorpyrifos-resistant strains of Laodelphax striatellus (Hemiptera: Delphacidae) from eastern China. Pest Manag. Sci. 2014, 70, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.K.; Zhang, X.L.; Ali, E.; Liao, X.; Jin, R.H.; Ren, Z.J.; Wan, H.; Li, J.H. Characterization of nitenpyram resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhang, Y.; Yang, R.; Zhang, S.; Xu, X.; Jiang, C.X. The population growth, development and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) under the sublethal dose of triflumezopyrim. Chemosphere 2020, 247, 125865. [Google Scholar] [CrossRef]
- Wang, L.; Tang, N.; Gao, X.L.; Chang, Z.X.; Zhang, L.G.; Zhou, G.H.; Guo, D.Y.; Zeng, Z.; Li, W.J.; Akinyemi, I.A.; et al. Genome sequence of a rice pest, the white-backed planthopper (Sogatella furcifera). GigaScience 2017, 6, 1–9. [Google Scholar]
- An, X.K.; Hou, M.L.; Liu, Y.D. Reference gene selection and evaluation for gene expression studies using RT-qPCR in the white-backed Planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Econ. Entomol. 2016, 109, 879. [Google Scholar] [CrossRef]
- Wang, X.G.; Ruan, Y.W.; Gong, C.W.; Xiang, X.; Xu, X.; Zhang, Y.M.; Shen, L.T. Transcriptome analysis of Sogatella furcifera (Homoptera: Delphacidae) in response to sulfoxaflor and functional verification of resistance-related P450 genes. Int. J. Mol. Sci. 2019, 20, 4573. [Google Scholar] [CrossRef] [Green Version]
- Lertkiatmongkol, P.; Jenwitheesuk, E.; Rongnoparut, P. Homology modeling of mosquito cytochrome P450 enzymes involved in pyrethroid metabolism, insights into differences in substrate selectivity. BMC Res. Notes 2011, 4, 321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Y.; Wang, Y.; Yang, Y.; Cang, X.; Liu, Z. Expression induction of P450 genes by imidacloprid in Nilaparvata lugens: A genome-scale analysis. Pestic. Biochem. Physiol. 2016, 132, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.L.; Barbhaiya, L.; Roe, R.M. Cytochrome P450-associated insecticide resistance and the development of biochemical diagnostic assays in Heliothis virescens. Pestic. Biochem. Physiol. 1995, 51, 178–191. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, C.; Long, G.Y.; Yang, H.; Jin, D.C. Sublethal effects of buprofezin on development, reproduction, and chitin synthase 1 gene (SfCHS1) expression in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia Pac. Entomol. 2018, 21, 585–591. [Google Scholar] [CrossRef]
- Bradford, M.M.A. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 25, 248–256. [Google Scholar] [CrossRef]
- Robertson, J.L.; Preisler, H.K. Pesticide Bioassays with Arthropods. Fla. Entomol. 2008, 91, 510–511. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR, the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Preetha, G.; Stanley, J.; Suresh, S.; Samiyappan, R. Risk assessment of insecticides used in rice on miridbug, Cyrtorhinus lividipennis Reuter, the important predator of brown planthopper, Nilaparvata lugens (Stål.). Chemosphere 2010, 80, 498–503. [Google Scholar] [CrossRef]
- Owolade, O.; Ogunleti, D. Bffects of Titanium Dioxide on The Diseases, Development and yield ofedible cowpea. J. Plant. Prot. Res. 2008, 48, 3. [Google Scholar] [CrossRef]
- Li, W.H.; Mao, K.K.; Liu, C.Y.; Gong, P.P.; Xu, P.F.; Wu, G.; Le, W.; Wan, H.; You, H.; Li, J.H. Resistance monitoring and assessment of the control failure likelihood of insecticides in field populations of the whitebacked planthopper Sogatella furcifera (Horváth). Crop Prot. 2020, 127, 104973. [Google Scholar] [CrossRef]
- Liu, N.N.; Li, M.; Gong, Y.H.; Liu, F.; Li, T. Cytochrome P450s-Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Mao, K.; Liao, X.; Xu, P.; Li, Z.; Ali, E.; Li, J. Overexpression of CYP6ER1 associated with clothianidin resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 154, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.W.; Wang, X.G.; Xiang, X. Status of insecticide resistance and biochemical characterization of chlorpyrifos resistance in Sogatella furcifera (Horváth) in Sichuan Province, China. Pestic. Biochem. Physiol. 2020, 171, 104723. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. 2008, 38, 634–644. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef]
- Okey, A.B. Enzyme induction in the cytochrome P-450 system. Pharmacol. Ther. 1990, 45, 241–298. [Google Scholar] [CrossRef]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.X.; Liu, Y.B.; Rong, W.H. RNA-seq and its application in transcriptome research. Genetics 2011, 33, 1191–1202. [Google Scholar]
- Zhu, K.Y. RNA interference: A powerful tool in entomological research and a novel approach for insect pest management. Insect Sci. 2013, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.H.; Wu, M.; Pu, J.; Feng, A.; Zhang, Q.; Han, Z.J. A functional study of two dsRNA binding protein genes in Laodelphax striatellus. Pest Manag. Sci. 2013, 69, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Yocum, G.D.; Rinehart, J.P. Hormonal control of diapause. Compr. Insect Mol. Sci. 2005, 3, 615–650. [Google Scholar]
- Stiborová, M.; Indra, R.; Frei, E.; Schmeiser, H.H.; Eckschlager, T.; Adam, V.; Heger, Z.; Arlt, V.M.; Martínek, V. Cytochrome b5 plays a dual role in the reaction cycle of cytochrome P450 3A4 during oxidation of the anticancer drug ellipticine. Monatsh. Chem. 2017, 148, 1983–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupasinghe, S.G.; Wen, Z.M.; Chiu, T.L.; Schuler, M.A. Helicoverpa zea CYP6B8 and CYP321A1: Different molecular solutions to the problem of metabolizing plant toxins and insecticides. Protein Eng. Des. Sel. 2007, 20, 615–624. [Google Scholar] [CrossRef]
- Cui, S.F.; Wang, L.; Ma, L.; Geng, X.Q. P450-mediated detoxification of botanicals in insects. Phytoparasitica 2016, 44, 585–599. [Google Scholar] [CrossRef]
- Thomas, J.H. Rapid birth-death evolution specific to xenobiotic cytochrome P450 genes in vertebrates. PLoS Genet. 2007, 3, e6. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wu, M.; Han, Z.J. Overexpression of Multiple Detoxification Genes in Deltamethrin Resistant Laodelphax striatellus (Hemiptera: Delphacidae) in China. PLoS ONE 2013, 8, e79443. [Google Scholar] [CrossRef] [PubMed]
- Lao, S.H.; Huang, X.H.; Huang, H.J.; Liu, C.W.; Zhang, C.X.; Bao, Y.Y. Genomic and transcriptomic insights into the cytochrome P450 monooxygenase gene repertoire in the rice pest brown planthopper, Nilaparvata lugens. Genomics 2015, 106, 301–309. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).