Diabetic Foot: The Role of Fasciae, a Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
- Deep/muscular fasciae: are in contact with the muscles and are classified depending on their composition, orientation, and architecture as either aponeurotic and epimysial [4].
2. Role of the Deep Fasciae in the Diabetic Foot
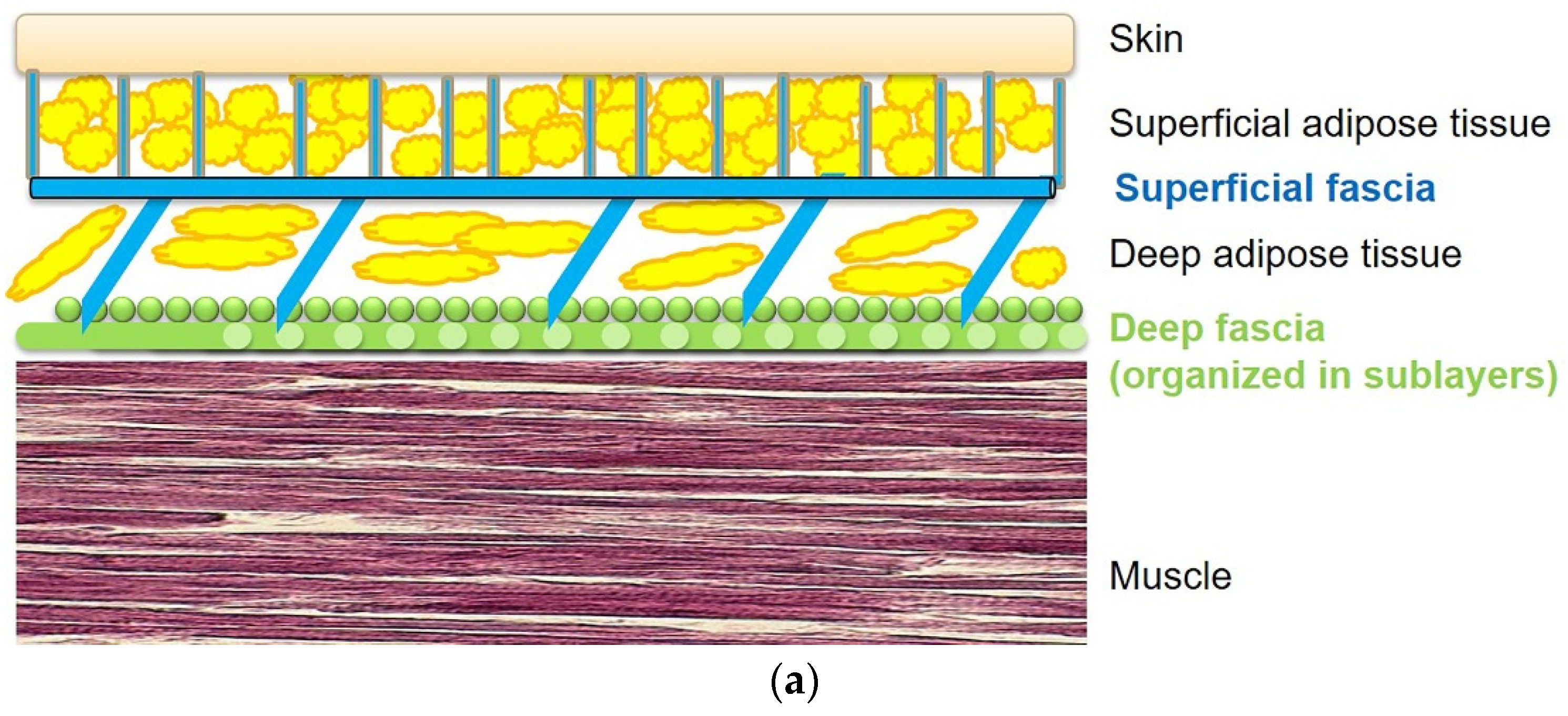
3. Role of the Superficial Fascia and Subcutaneous Tissue in the Diabetic Foot
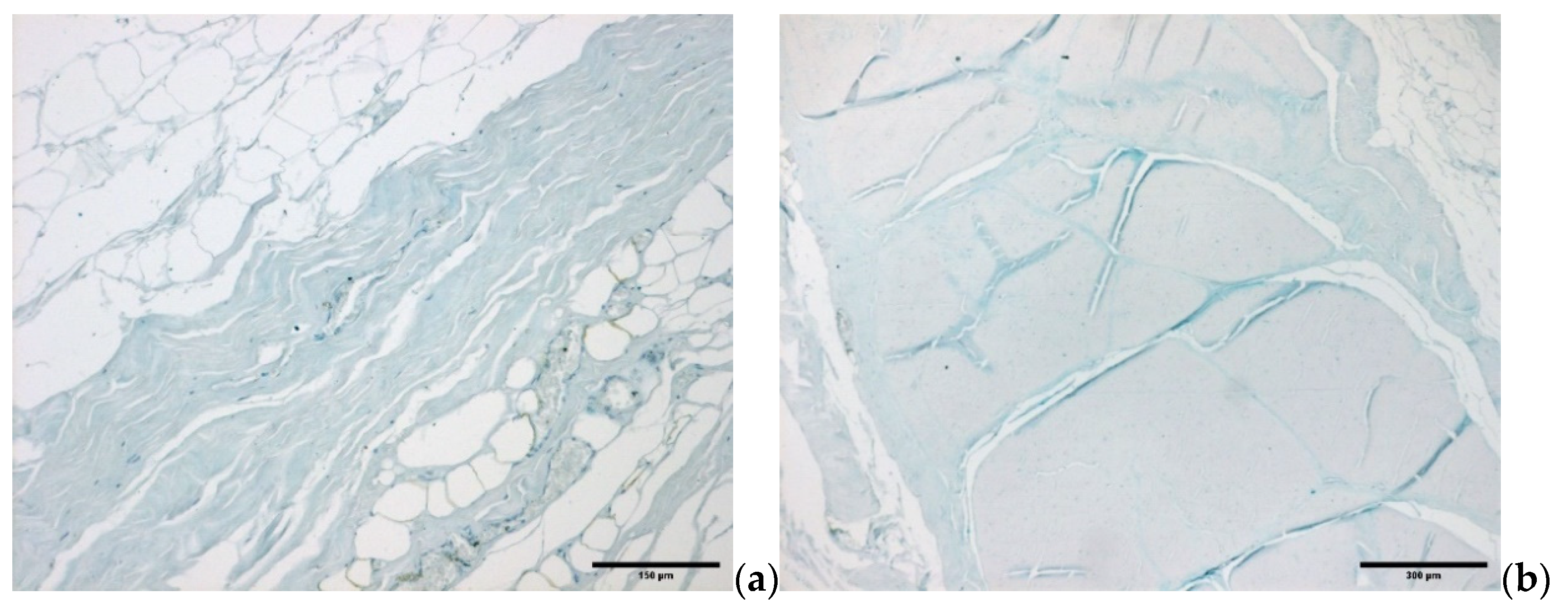
4. Microscopic Organization of Foot Fasciae and Their Possible Role in Diabetic Foot
4.1. Diabetic Foot: The Role of Cells
4.2. Diabetic Foot: Fascial ECM, Fibrous Component Role
4.3. Diabetic Foot: Fascial ECM, Water Component Role
4.4. Diabetic Foot: Fascial Nerve Elements Role
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Edmonds, M. Emerging drugs for diabetic foot ulcers. Expert Opin. Emerg. Drugs 2006, 11, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; Edmonds, M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs 2021, 81, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Pirri, C.; Fan, C.; Petrelli, L.; Guidolin, D.; De Caro, R.; Stecco, C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int. J. Mol. Sci. 2021, 22, 1411. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Corradin, M.; Macchi, V.; Morra, A.; Porzionato, A.; Biz, C.; De Caro, R. Plantar fascia anatomy and its relation-ship with Achilles tendon and paratenon. J Anat. 2013, 223, 665–676. [Google Scholar] [CrossRef]
- Bolton, N.R.M.; Smith, K.E.; Pilgram, T.K.; Mueller, M.J.; Bae, K.T. Computed tomography to visualize and quantify the plantar aponeurosis and flexor hallucis longus tendon in the diabetic foot. Clin. Biomech. 2005, 20, 540–546. [Google Scholar] [CrossRef]
- Bolívar, Y.A.; Munuera-Martínez, P.V.; Padillo, J.P. Relationship Between Tightness of the Posterior Muscles of the Lower Limb and Plantar Fasciitis. Foot Ankle Int. 2013, 34, 42–48. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Bowen, J.; Hall, J.; Tallis, A.; Tierney, E.; Freeman, D. Prevalence of Equinus in Diabetic versus Nondiabetic Patients. J. Am. Podiatr. Med Assoc. 2012, 102, 84–88. [Google Scholar] [CrossRef]
- Kiziltan, M.E.; Gunduz, A.; Kiziltan, G.; Akalin, M.A.; Uzun, N. Peripheral neuropathy in patients withdiabetic foot ulcers: Clinical and nerve conduction study. J. Neurol. Sci. 2007, 258, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Primadhi, R.A.; Herman, H. Diabetic foot: Which one comes first, the ulcer or the contracture? World J. Orthop. 2021, 12, 61–68. [Google Scholar] [CrossRef]
- Chao, C.Y.; Zheng, Y.-P.; Huang, Y.; Cheing, G. Biomechanical properties of the forefoot plantar soft tissue as measured by an optical coherence tomography-based air-jet indentation system and tissue ultrasound palpation system. Clin. Biomech. 2010, 25, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Zheng, Y.-P.; Cheing, G. Epidermal Thickness and Biomechanical Properties of Plantar Tissues in Diabetic Foot. Ultrasound Med. Biol. 2011, 37, 1029–1038. [Google Scholar] [CrossRef]
- Klaesner, J.W.; Hastings, M.K.; Zou, D.; Lewis, C.; Mueller, M.J. Plantar tissue stiffness in patients with diabetes mellitus and peripheral neuropathy. Arch. Phys. Med. Rehabil. 2002, 83, 1796–1801. [Google Scholar] [CrossRef]
- Piaggesi, A.; Romanelli, M.; Schipani, E.; Campi, F.; Magliaro, A.; Baccetti, F.; Navalesi, R.J. Hardness of plantar skin in dia-betic neuropathic feet. Diabetes Complicat. 1999, 13, 129–134. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tsai, W.-C.; Hsiao, T.-Y.; Tseng, F.-Y.; Shau, Y.-W.; Wang, C.-L.; Lin, S.-C. Diabetic effects on microchambers and macrochambers tissue properties in human heel pads. Clin. Biomech. 2009, 24, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, W.R.; Jahss, M.H.; Kummer, F.; Desai, P.; Gee, R.O.; Ricci, J.L. Histology and Histomorphometric Analysis of the Normal and Atrophic Heel Fat Pad. Foot Ankle Int. 1995, 16, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.F.; Davis, B.L.; Hardy, P.A. Characterization of the calcaneal fat pad in diabetic and nondiabetic patients using mag-netic resonance imaging. Magn. Reson. Imaging 1999, 17, 851–857. [Google Scholar] [CrossRef]
- Coffman, J.D.; A Cohen, R. Alpha-adrenergic and serotonergic mechanisms in the human digit. J. Cardiovasc. Pharmacol. 1988, 11, S49–S53. [Google Scholar]
- Henriksen, O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiol. Scand. Suppl. 1991, 603, 33–39. [Google Scholar]
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; De Caro, R.; Stecco, C. Fascia and soft tissues innervation in the human hip and their possible role in post-surgical pain. J. Orthop. Res. 2020, 38, 1646–1654. [Google Scholar] [CrossRef]
- Stecco, C.; Stern, R.; Porzionato, A.; Macchi, V.; Masiero, S.; Stecco, A.; De Caro, R. Hyaluronan within fascia in the etiology of myofascial pain. Surg. Radiol. Anat. 2011, 33, 891–896. [Google Scholar] [CrossRef]
- Ushiki, T. Collagen Fibers, Reticular Fibers and Elastic Fibers. A Comprehensive Understanding from a Morphological Viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinicalconsiderations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallegos, D.; Jiang, D.; Christ, S.; Ramesh, P.; Ye, H.; Wannemacher, J.; Gopal, S.K.; Yu, Q.; Aichler, M.; Walch, A.; et al. Patch repair of deep wounds by mobilized fascia. Nat. Cell Biol. 2019, 576, 287–292. [Google Scholar] [CrossRef]
- Wan, L.; Jiang, D.; Correa-Gallegos, D.; Ramesh, P.; Zhao, J.; Ye, H.; Zhu, S.; Wannemacher, J.; Volz, T.; Rinkevich, Y. Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol. 2021, 97, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, H.; Nomura, T.; Hasegawa, M.; Tatsumi, C.; Imai, M.; Sakakibara, S.; Terashi, H. Use of synthetic serum-free medium for culture of human dermal fibroblasts to establish an experimental system similar to living dermis. Cytotechnology 2014, 67, 507–514. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Gullberg, D. The integrin-collagen connection--a glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Wu, C.; Bauer, J.; Juliano, R.; McDonald, J. The a5bl integrin fibronectin receptor, but not the a5 cytoplasmic domain, func-tions in an early and essential step in fbronectin matrix assembly. J. Biol. Chem. 1993, 268, 21883–21888. [Google Scholar] [CrossRef]
- Yoshizato, K.; Taira, T.; Yamamoto, N. Growth inhibition of human fibroblasts by reconstituted collagen fibrils. Biomed. Res. 1985, 6, 61–71. [Google Scholar] [CrossRef]
- Kono, T.; Tanii, T.; Furukawa, M.; Mizuno, N.; Kitajima, J.; Ishii, M.; Hamada, T.; Yoshizato, K. Cell cycle analysis of human dermal fibroblasts cultured on or in hydrated type I collagen lattices. Arch. Dermatol. Res. 1990, 282, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653. [Google Scholar] [CrossRef]
- Grillo, H.C.; Watts, G.T.; Gross, J. Studies in Wound Healing. I. Contraction and the Wound Contents. Plast. Reconstr. Surg. 1958, 22, 506. [Google Scholar] [CrossRef][Green Version]
- Watts, G.T.; Grillo, H.C.; Gross, J. Studies in Wound Healing: II. The Role of Granulation Tissue in Contraction. Plast. Reconstr. Surg. 1958, 22, 506–507. [Google Scholar] [CrossRef][Green Version]
- De Wever, O.; Westbroek, W.; Verloes, A.; Bloemen, N.; Bracke, M.; Gespach, C.; Bruyneel, E.; Mareel, M. Critical role of N-cadherin in myofibroblast invasion and migration in vitro stimulated by colon-cancer-cell-derived TGF-beta or wounding. J. Cell Sci. 2004, 117, 4691–4703. [Google Scholar] [CrossRef]
- Burke, J.P.; Cunningham, M.F.; Sweeney, C.; Docherty, N.G.; O’Connell, P.R. N-cadherin is overexpressed in Crohn’s stricture fibroblasts and promotes intestinal fibroblast migration. Inflamm. Bowel Dis. 2011, 17, 1665–1673. [Google Scholar] [CrossRef]
- Black, M.; Milewski, D.; Le, T.; Ren, X.; Xu, Y.; Kalinichenko, V.V.; Kalin, T.V. FOXF1 inhibits pulmonary fibrosis by prevent-ing CDH2-CDH11 cadherin switch in myofibroblasts. Cell Rep. 2018, 23, 442–458. [Google Scholar] [CrossRef]
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investi-gation. Front. Physiol. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Fede, C.; Macchi, V.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial glid-ing regulation. Clin. Anat. 2018, 31, 667–676. [Google Scholar] [CrossRef]
- Dawidowicz, J.; Matysiak, N.; Szotek, S.; Maksymowicz, K. Telocytes of Fascial Structures. Adv. Exp. Med. Biol. 2016, 913, 403–424. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibro-plasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Suzuki, N.; Yokoyama, F.; Nomizu, M. Functional Sites in the Laminin Alpha Chains. Connect. Tissue Res. 2005, 46, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.; Monti, E.; Bondí, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Me-chanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef]
- Kang, L.; Ayala, J.E.; Lee-Young, R.S.; Zhang, Z.; James, F.D.; Neufer, P.D.; Pozzi, A.; Zutter, M.M.; Wasserman, D.H. Di-et-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin al-pha2beta1 in mice. Diabetes 2011, 60, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Berria, R.; Wang, L.; Richardson, D.K.; Finlayson, J.; Belfort, R.; Pratipanawatr, T.; De Filippis, E.A.; Kashyap, S.; Mandarino, L.J. Increased collagen content in insulin-resistant skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E560–E565. [Google Scholar] [CrossRef] [PubMed]
- Arkkila, P.E.T.; Rönnemaa, T.; Koskinen, P.J.; Kantola, I.M.; Seppänen, E.; Viikari, J.S.A. Biochemical markers of type III and I collagen: Association with retinopathy and neuropathy in Type 1 diabetic subjects. Diabet. Med. 2001, 18, 816–821. [Google Scholar] [CrossRef]
- Lehti, T.M.; Silvennoinen, M.; Kivela, R.; Kainulainen, H.; Komulainen, J. E_ects of streptozotocin-induced diabetes and physical training on gene expression of extracellular matrix proteins in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E900–E907. [Google Scholar] [CrossRef]
- Pérez-Panero, A.J.; Ruiz-Muñoz, M.; Cuesta-Vargas, A.I.; Gónzalez-Sánchez, M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: A systematic review. Medicine 2019, 98, e16877. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Fessel, G.; Li, Y.; Diederich, V.; Guizar-Sicairos, M.; Schneider, P.; Sell, D.R.; Monnier, V.M.; Snedeker, J.G. Advanced Glycation End-Products Reduce Collagen Molecular Sliding to Affect Collagen Fibril Damage Mechanisms but Not Stiffness. PLoS ONE 2014, 9, e110948. [Google Scholar] [CrossRef]
- Wu, X.; Monnier, V.M. Enzymatic deglycation of proteins. Arch. Biochem. Biophys. 2003, 419, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.R.; Twigg, S.M. Fibrosis in diabetes complications: Pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008, 4, 575–596. [Google Scholar]
- Li, Y.; Fessel, G.; Georgiadis, M.; Snedeker, J. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013, 32, 169–177. [Google Scholar] [CrossRef]
- Gautieri, A.; Passini, F.S.; Silvan, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Griffin, C.T.; Wang, S.S.; Bissell, D.M. Role of CD44 in epithelial wound repair: Migration of rat hepatic stellate cells utilizes hyaluronic acid and CD44v6. J. Biol. Chem. 2005, 280, 15398–15404. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Mccarthy, J.B.; Yazdani, A.; Turley, E.A. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed Res. Int. 2014, 2014, 103923. [Google Scholar] [CrossRef]
- Fede, C.; Angelini, A.; Stern, R.; Macchi, V.; Porzionato, A.; Ruggieri, P.; De Caro, R.; Stecco, C. Quantification of hyaluronan in human fasciae: Variations with function and anatomical site. J. Anat. 2018, 233, 552–556. [Google Scholar] [CrossRef]
- Stecco, A.; Macchi, V.; Masiero, S.; Porzionato, A.; Tiengo, C.; Stecco, C.; Delmas, V.; De Caro, R. Pectoral and femoral fasci-ae: Common aspects and regional specializations. Surg. Radiol. Anat. 2009, 31, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Macchi, V.; Porzionato, A.; Morra, A.; Parenti, A.; Stecco, A.; Delmas, V.; De Caro, R. The ankle retinacula: Mor-phological evidence of the proprioceptive role of the fascial system. Cells Tissues Organs 2010, 192, 200–210. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Sensitivity of the Fasciae to the Endocannabinoid System: Production of Hyaluronan-Rich Vesicles and Potential Peripheral Effects of Cannabinoids in Fascial Tissue. Int. J. Mol. Sci. 2020, 21, 2936. [Google Scholar] [CrossRef]
- Viola, M.; Vigetti, D.; Karousou, E.; D’Angelo, M.L.; Caon, I.; Moretto, P.; De Luca, G.; Passi, A. Biology and biotechnology of hyaluronan. Glycoconj. J. 2015, 32, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Elee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Forgacs, G.; Newman, S.A.; Hinner, B.; Maier, C.W.; Sackmann, E. Assembly of collagen matrices as a phase transition re-vealed by structural and rheologic studies. Biophys. J. 2003, 84, 1272–1280. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on Hyaluronan Behavior in Aqueous Solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Racine, R.R.; Hennig, M.J.P.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.; Bril, V.; Freeman, R.; Malik, R.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Bönhof, G.J.; Herder, C.; Strom, A.; Papanas, N.; Roden, M.; Ziegler, D. Emerging biomarkers, tools, and treatments for dia-betic polyneuropathy. Endocr. Rev. 2019, 40, 153–192. [Google Scholar] [CrossRef]
- Mense, S.; Hoheisel, U. Evidence for the existence of nociceptors in rat thoracolumbar fascia. J. Bodyw. Mov. Ther. 2016, 20, 623–628. [Google Scholar] [CrossRef]
- Kumar, C.G.S.; Rajagopal, K.V.; Hande, M.; Maiya, A.G.; Mayya, S.S. Intrinsic foot muscle and plantar tissue changes in type 2 diabetes mellitus. J. Diabetes 2015, 7, 850–857. [Google Scholar] [CrossRef]
- Papachristou, S.; Pafili, K.; Papanas, N. Skin AGEs and diabetic neuropathy. BMC Endocr. Disord. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Ashrafi, M.; Baguneid, M.; Bayat, A. The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm. Venereol. 2016, 96, 587–594. [Google Scholar] [CrossRef]
- Fede, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Pirri, C.; Fan, C.; De Caro, R.; Stecco, C. Evidence of a new hidden neural network into deep fasciae. Sci. Rep. 2021, 11, 12623. [Google Scholar] [CrossRef] [PubMed]
- Magerl, W.; Thalacker, E.; Vogel, S.; Schleip, R.; Klein, T.; Treede, R.-D.; Schilder, A. Tenderness of the Skin after Chemical Stimulation of Underlying Temporal and Thoracolumbar Fasciae Reveals Somatosensory Crosstalk between Superficial and Deep Tissues. Life (Basel) 2021, 11, 370. [Google Scholar] [CrossRef]
- Taguchi, T.; Hoheisel, U.; Mense, S. Dorsal horn neurons having input from low back structures in rats. Pain 2008, 138, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.A.; Ryals, J.M.; Feldman, E.L.; Wright, D.E. Abnormal Muscle Spindle Innervation and Large-Fiber Neuropathy in Diabetic Mice. Diabetes 2008, 57, 1693–1701. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Fede, C.; Pirri, N.; Petrelli, L.; Fan, C.; De Caro, R.; Stecco, C. Diabetic Foot: The Role of Fasciae, a Narrative Review. Biology 2021, 10, 759. https://doi.org/10.3390/biology10080759
Pirri C, Fede C, Pirri N, Petrelli L, Fan C, De Caro R, Stecco C. Diabetic Foot: The Role of Fasciae, a Narrative Review. Biology. 2021; 10(8):759. https://doi.org/10.3390/biology10080759
Chicago/Turabian StylePirri, Carmelo, Caterina Fede, Nina Pirri, Lucia Petrelli, Chenglei Fan, Raffaele De Caro, and Carla Stecco. 2021. "Diabetic Foot: The Role of Fasciae, a Narrative Review" Biology 10, no. 8: 759. https://doi.org/10.3390/biology10080759
APA StylePirri, C., Fede, C., Pirri, N., Petrelli, L., Fan, C., De Caro, R., & Stecco, C. (2021). Diabetic Foot: The Role of Fasciae, a Narrative Review. Biology, 10(8), 759. https://doi.org/10.3390/biology10080759









