Pre-Existing Diabetes and COVID-Associated Hyperglycaemia in Patients with COVID-19 Pneumonia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population and Data Sources
2.2. Laboratory Variables
2.3. Definition of Diabetes
2.4. Statistical Analysis
3. Results
3.1. Study Participants
3.2. COVID-Associated Hyperglycaemia Was Associated with Specific Basal Characteristics
3.3. COVID-Associated Hyperglycaemia Was Associated with a Specific Clinical Laboratory Profile at Admission
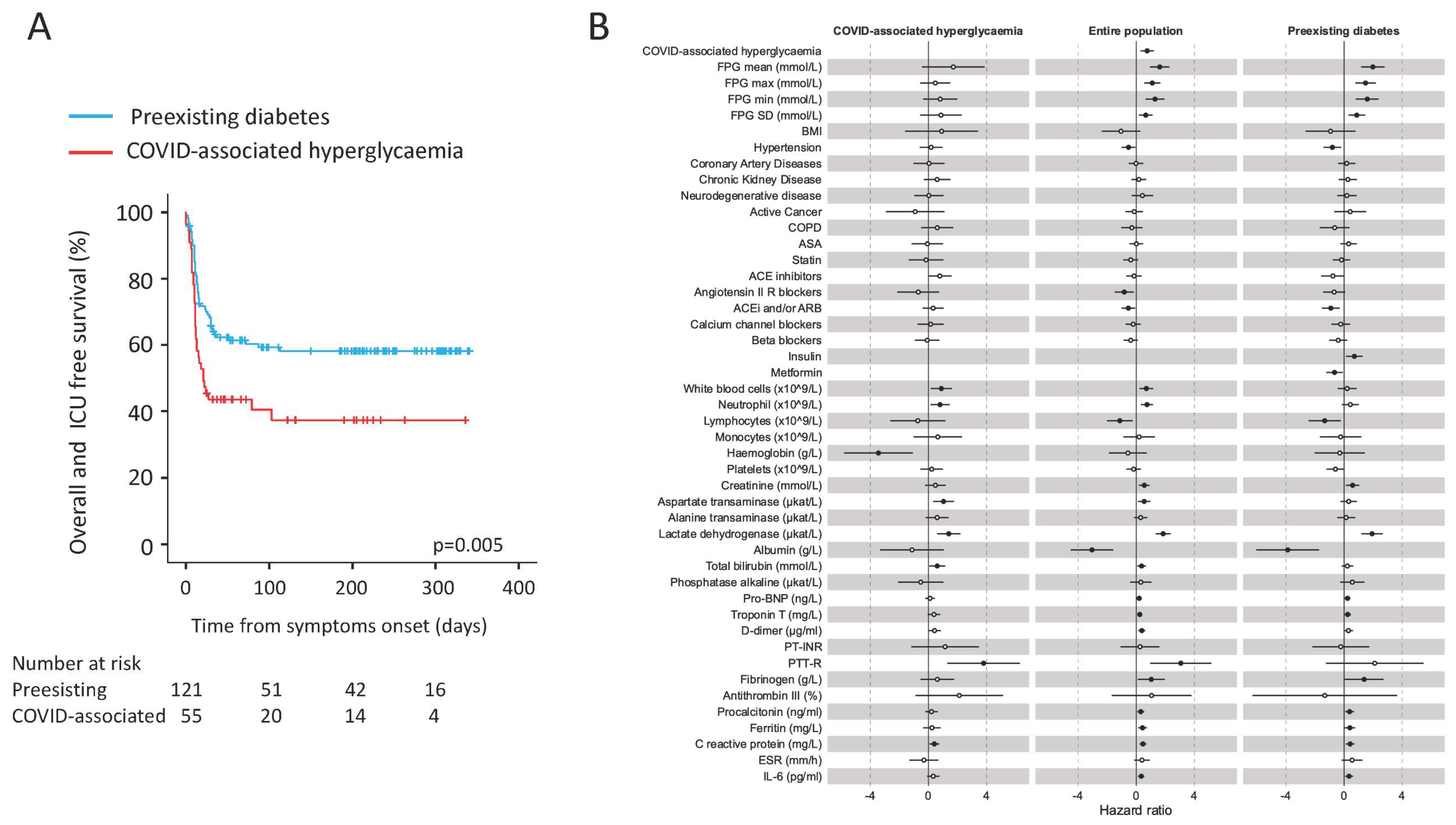
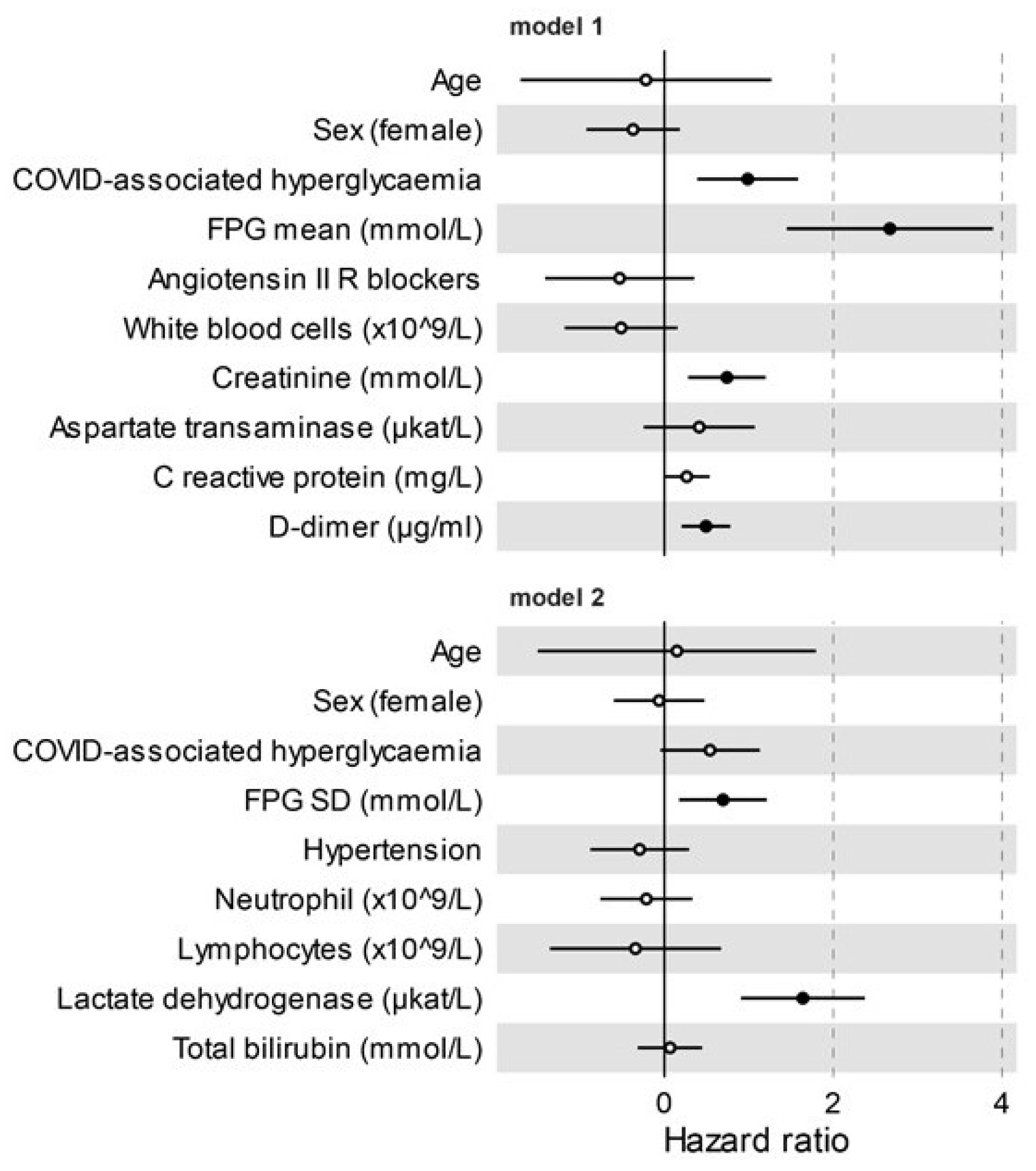
3.4. COVID-Associated Hyperglycaemia Was Associated with a Worse Clinical Outcome
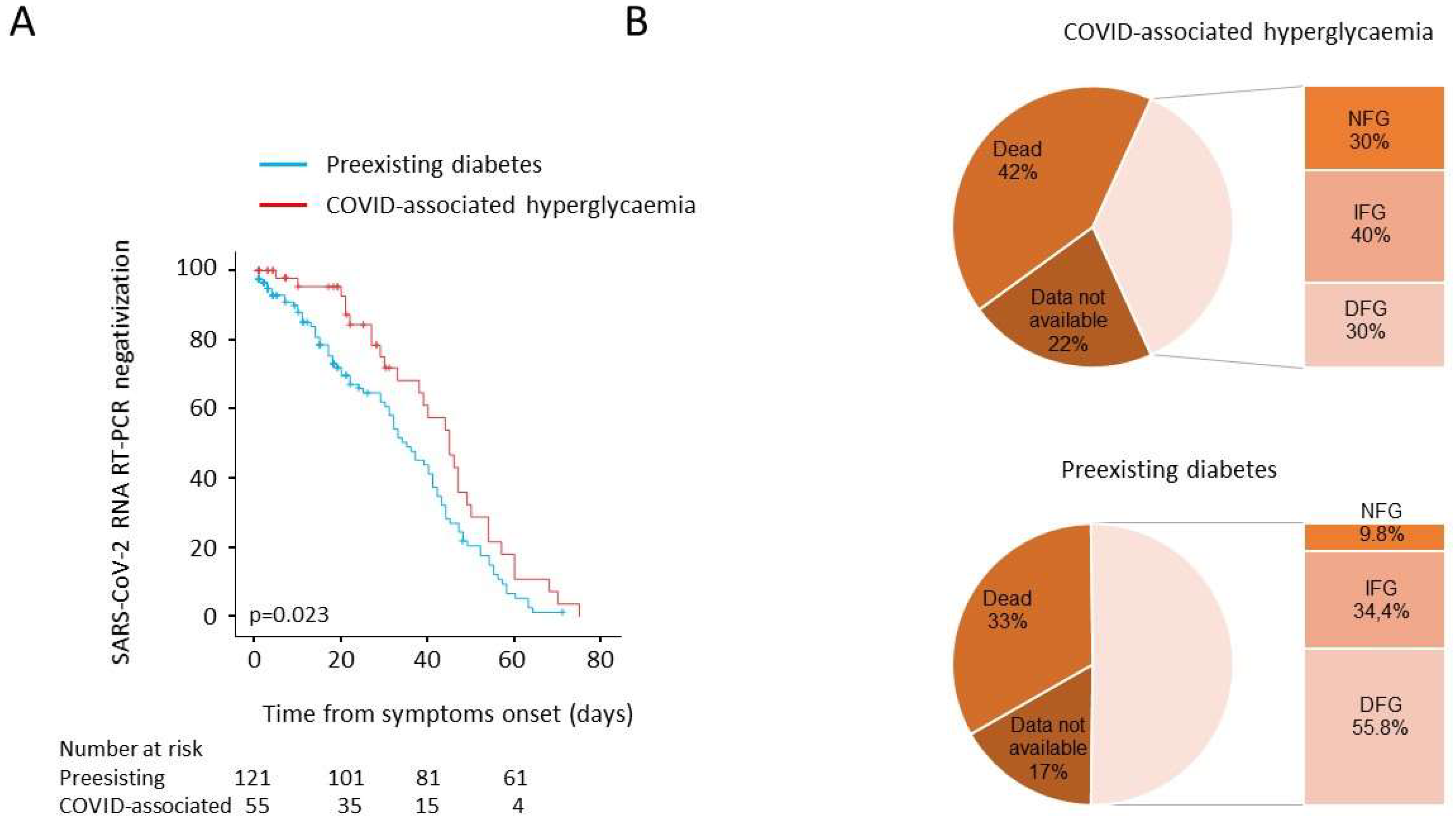
3.5. Post Discharge Follow-Up: Time to NT Swab Negativization and Persistence of Hyperglycaemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declarations
References
- Yang, J.; Feng, Y.; Yuan, M.; Yuan, S.; Fu, H.; Wu, B.; Sun, G.; Yang, G.; Zhang, X.; Wang, L. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef]
- Alraddadi, B.M.; Watson, J.T.; Almarashi, A.; Abedi, G.R.; Turkistani, A.; Sadran, M.; Housa, A.; Almazroa, M.A.; Alraihan, N.; Banjar, A. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; McAllister, D.A.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Risks of and risk factors for COVID-19 disease in people with diabetes: A cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Byrne, C.D.; Zheng, M.H.; Targher, G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in Covid-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Chen, J.; Zuo, X.; Zhang, H.; Deng, A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020, 22, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.J.; Ng, S.J.H.; Yeoh, E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 164, 108166. [Google Scholar] [CrossRef]
- Goldman, N.; Fink, D.; Cai, J.; Lee, Y.-N.; Davies, Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res. Clin. Pract. 2020, 166, 108291. [Google Scholar] [CrossRef]
- Hollstein, T.; Schulte, D.M.; Schulz, J.; Glück, A.; Ziegler, A.G.; Bonifacio, E.; Wendorff, M.; Franke, A.; Schreiber, S.; Bornstein, S.R. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: A case report. Nat. Metab. 2020, 2, 1021–1024. [Google Scholar] [CrossRef]
- Rafique, S.; Ahmed, F.W. A case of combined diabetic ketoacidosis and hyperosmolar hyperglycemic state in a patient with COVID-19. Cureus 2020, 12, e8965. [Google Scholar] [PubMed]
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-onset type 1 diabetes in children during COVID-19: Multicenter regional findings in the UK. Diabetes Care 2020, 43, e170–e171. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.I.; Griffin, G.D.; Simon, E.L. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am. J. Emerg. Med. 2020, 38, 2491.e2493–2491.e2494. [Google Scholar] [CrossRef]
- Soliman, A.; Al-Amri, M.; Ellithy, K.; Alaaraj, N.; Hamed, N.; De Sanctis, V. Newly-onset type 1 diabetes mellitus precipitated by COVID-19 in an 8-month-old infant. Acta Bio Med. Atenei Parm. 2020, 91, e2020046. [Google Scholar]
- Muniangi-Muhitu, H.; Akalestou, E.; Salem, V.; Misra, S.; Oliver, N.S.; Rutter, G.A. Covid-19 and Diabetes: A Complex Bidirectional Relationship. Front. Endocrinol. 2020, 11, 582936. [Google Scholar] [CrossRef]
- Ceriello, A. Hyperglycemia and COVID-19: What was known and what is really new? Diabetes Res. Clin. Pract. 2020, 167, 108383. [Google Scholar] [CrossRef]
- Hayden, M.R. An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of beta-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms. Cells 2020, 9, 2475. [Google Scholar] [CrossRef]
- Marchand, L.; Pecquet, M.; Luyton, C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020, 57, 1265–1266. [Google Scholar] [CrossRef]
- Liang, X.; Xu, J.; Xiao, W.; Shi, L.; Yang, H. The association of diabetes with COVID-19 disease severity: Evidence from adjusted effect estimates. Hormones 2021, 20, 409–414. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Fignani, D.; Licata, G.; Brusco, N.; Nigi, L.; Grieco, G.E.; Marselli, L.; Overbergh, L.; Gysemans, C.; Colli, M.L.; Marchetti, P. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front. Endocrinol. 2020, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020, 27, 125–136.e127. [Google Scholar] [CrossRef]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P. Read C SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 8, 1577–1591.e7. [Google Scholar] [CrossRef]
- Coate, K.C.; Cha, J.; Shrestha, S.; Wang, W.; Gonçalves, L.M.; Almaça, J.; Kapp, M.E.; Fasolino, M.; Morgan, A.; Dai, C.; et al. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020, 32, 1028–1040.e1024. [Google Scholar] [CrossRef]
- Kusmartseva, I.; Wu, W.; Syed, F.; Van Der Heide, V.; Jorgensen, M.; Joseph, P.; Tang, X.; Candelario-Jalil, E.; Yang, C.; Nick, H. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020, 32, 1041–1051.e1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 31, 1068–1077.e1063. [Google Scholar] [CrossRef]
- Akarsu, C.; Karabulut, M.; Aydin, H.; Sahbaz, N.A.; Dural, A.C.; Yegul, D.; Peker, K.D.; Ferahman, S.; Bulut, S.; Dönmez, T. Association between acute pancreatitis and COVID-19: Could pancreatitis be the missing piece of the puzzle about increased mortality rates? J. Investig. Surg. 2020, 1–7. [Google Scholar] [CrossRef]
- Wu, L.; Girgis, C.M.; Cheung, N.W. COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin. Endocrinol. 2020, 93, 390–393. [Google Scholar] [CrossRef]
- Lampasona, V.; Secchi, M.; Scavini, M.; Bazzigaluppi, E.; Brigatti, C.; Marzinotto, I.; Davalli, A.; Caretto, A.; Laurenzi, A.; Martinenghi, S.; et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: An observational cohort study. Diabetologia 2020, 63, 2548–2558. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, S.; Chen, T.; Cui, Z.; Shi, N.; Zhong, X.; Qiu, K.; Zhang, J.; Zeng, T.; Chen, L.; et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes. Metab. 2020, 22, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, P.; Zhang, S.; Song, S.; Wang, Z.; Ma, Y.; Xu, J.; Wu, F.; Duan, L.; Yin, Z.; et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: A multi-centre retrospective study. Diabetologia 2020, 63, 2102–2111. [Google Scholar] [CrossRef]
- Fadini, G.P.; Morieri, M.L.; Boscari, F.; Fioretto, P.; Maran, A.; Busetto, L.; Bonora, B.M.; Selmin, E.; Arcidiacono, G.; Pinelli, S.; et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res. Clin. Pract. 2020, 168, 108374. [Google Scholar] [CrossRef]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020, 167, 108382. [Google Scholar] [CrossRef]
- Cariou, B.; Pichelin, M.; Goronflot, T.; Gonfroy, C.; Marre, M.; Raffaitin-Cardin, C.; Thivolet, C.; Wargny, M.; Hadjadj, S.; Gourdy, P. Phenotypic characteristics and prognosis of newly diagnosed diabetes in hospitalized patients with COVID-19: Results from the CORONADO study. Diabetes Res. Clin. Pract. 2021, 175, 108695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Zhang, J.; Cao, Y.; Zhao, X.; Yu, N.; Gao, Y.; Ma, J.; Zhang, H.; Zhang, J.; et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: A single-centre, retrospective, observational study in Wuhan. Diabetes Obes. Metab. 2020, 22, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Desai, R.; Gupta, U.; Prakash, A.; Jain, A.; Aggarwal, A. Admission Hyperglycemia in Non-diabetics Predicts Mortality and Disease Severity in COVID-19: A Pooled Analysis and Meta-summary of Literature. SN Compr. Clin. Med. 2020, 2, 2161–2166. [Google Scholar] [CrossRef]
- Sathish, T.; Kapoor, N.; Cao, Y.; Tapp, R.J.; Zimmet, P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes. Metab. 2021, 23, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Murrells, T.; Mulnier, H.; Sinclair, A.J. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 659–660. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 1409. [Google Scholar]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Beziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Lönnrot, M.; Lynch, K.F.; Larsson, H.E.; Lernmark, Å.; Rewers, M.J.; Törn, C.; Burkhardt, B.R.; Briese, T.; Hagopian, W.A.; She, J.-X. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: The TEDDY study. Diabetologia 2017, 60, 1931–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hippich, M.; Holthaus, L.; Assfalg, R.; Zapardiel-Gonzalo, J.; Kapfelsperger, H.; Heigermoser, M.; Haupt, F.; Ewald, D.A.; Welzhofer, T.C.; Marcus, B.A.; et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med 2021, 2, 149–163. [Google Scholar] [CrossRef]
- Sardu, C.; D’Onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia 2020, 63, 2486–2487. [Google Scholar] [CrossRef]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metab. 2021, 33, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020, 32, 437–446.e435. [Google Scholar] [CrossRef]
- Sathish, T.; Tapp, R.J.; Cooper, M.E.; Zimmet, P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2020, 47, 101204. [Google Scholar] [CrossRef]
- Sardu, C.; D’Onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Outcomes in Patients with Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care 2020, 43, 1408–1415. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Baral, R.; White, M.; Vassiliou, V.S. Effect of Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19: A Systematic Review and Meta-analysis of 28,872 Patients. Curr. Atheroscler. Rep. 2020, 22, 61. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, Y.; Hong, Y. Decreased Mortality of COVID-19 with Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients With Hypertension: A Meta-Analysis. Hypertension 2020, 76, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J.; Pan, L.Y.; Jiang, H.Y. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol. Res. 2020, 158, 104927. [Google Scholar] [CrossRef]
- Barochiner, J.; Martinez, R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 1244–1252. [Google Scholar] [CrossRef]
- Crouse, A.B.; Grimes, T.; Li, P.; Might, M.; Ovalle, F.; Shalev, A. Metformin Use Is Associated With Reduced Mortality in a Diverse Population With COVID-19 and Diabetes. Front. Endocrinol. 2021, 11, 1081. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.; Sun, C.; Zhang, H.; Qu, G.; Chen, Y.; Cheng, C.; Chen, E.L.; Ahmed, M.A.; Kim, K.Y.; et al. The Association Between Hypoglycemic Agents and Clinical Outcomes of COVID-19 in Patients with Diabetes: A Systematic Review and Meta-Analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407. [Google Scholar] [CrossRef]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, C.; Sun, Y.; Wang, D.W. Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab. 2021, 33, 65–77.e62. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Knighton, P.; Zaccardi, F.; Bakhai, C.; Barron, E.; Holman, N.; Kar, P.; Meace, C.; Sattar, N.; Sharp, S. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: A nationwide observational study in England. Lancet Diabetes Endocrinol. 2021, 9, 293–303. [Google Scholar] [CrossRef]
- Kosiborod, M.; Berwanger, O.; Koch, G.G.; Martinez, F.; Mukhtar, O.; Verma, S.; Chopra, V.; Javaheri, A.; Ambery, P.; Gasparyan, S.B.; et al. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure because of COVID-19: Design and rationale for the DARE-19 study. Diabetes Obes. Metab. 2021, 23, 886–896. [Google Scholar] [CrossRef]
| Pre-Existing Diabetes | COVID-Associated Hyperglycaemia | p | Missing | |
|---|---|---|---|---|
| N | 121 | 55 | ||
| Age, years | 71 (62–77.5) | 69 (54–78) | 0.324 | 0 |
| Sex, male [N (%)] | 84 (71.2) | 37 (63.8) | 0.387 | 0 |
| BMI | 29.16 (26.12–33.8) | 26.52 (23.87–30.12) | 0.014 | 20 |
| 23 (20.5) | 14 (31.8) | 0.036 | |
| 36 (32.1) | 19 (43.2) | ||
| 53 (47.3) | 11 (25) | ||
| Ethnicity [N (%)] | 0.998 | 0 | ||
| 106 (87.6) | 48 (87.3) | ||
| 7 (5.8) | 3 (5.5) | ||
| 4 (3.3) | 2 (3.6) | ||
| 4 (3.3) | 2 (3.6) | ||
| Comorbidities [N (%)] | 0 | |||
| 91 (75.2) | 32 (58.2) | 0.033 | |
| 35 (28.9) | 6 (10.9) | 0.012 | |
| 16 (13.2) | 5 (9.1) | 0.617 | |
| 32 (26.4) | 7 (12.7) | 0.05 | |
| 23 (19) | 3 (5.5) | 0.021 | |
| 7 (5.8) | 8 (14.5) | 0.078 | |
| Preadmission treatment [N (%)] | 0 | |||
| 71 (58.7) | 0 | ||
| 12 (9.9) | 0 | ||
| 3 (2.5) | 0 | ||
| 3 (2.5) | 0 | ||
| 1 (0.8) | 0 | ||
| 14 (11.6) | 0 | ||
| 3 (2.5) | 0 | ||
| 4 (3.3) | 0 | ||
| 41 (33.9) | 0 | ||
| 47 (38.8) | 7 (12.7) | <0.001 | |
| 44 (36.4) | 6 (10.9) | 0.001 | |
| 28 (23.1) | 11 (20) | 0.699 | |
| 28 (23.1) | 6 (10.9) | 0.065 | |
| 54 (44.6) | 17 (30.9) | 0.099 | |
| 35 (28.9) | 9 (16.4) | 0.091 | |
| 43 (35.5) | 13 (23.6) | 0.162 | |
| Admission to the hospital | 0 | |||
| Median time from symptoms to admission, days | 6 (2–9.5) | 7 (3–10) | 0.484 | |
| Symptoms at onset [N (%)] | ||||
| 93 (78.2) | 46 (85.2) | 0.31 | |
| 79 (66.4) | 37 (68.5) | 0.86 | |
| 46 (38.7) | 21 (38.9) | 0.99 | |
| 35 (29.4) | 10 (18.5) | 0.14 | |
| 19 (17.3) | 8 (14.8) | 0.82 | |
| 17 (14.3) | 8 (14.8) | 0.99 | |
| 19 (16) | 7 (13) | 0.82 | |
| 12 (10.1) | 1 (1.9) | 0.066 | |
| 12 (10.1) | 2 (3.7) | 0.23 | |
| 16 (13.4) | 6 (11.1) | 0.81 | |
| 5 (4.2) | 6 (11.1) | 0.10 | |
| 9 (7.6) | 6 (11.1) | 0.56 | |
| 8 (6.7) | 5 (9.3) | 0.55 | |
| 5 (4.2) | 2 (3.7) | 0.99 | |
| 2 (1.9) | 0 | 0.99 |
| Pre-Existing Diabetes | COVID-Associated Hyperglycaemia | Missing | ||
|---|---|---|---|---|
| N | 121 | 55 | p | |
| Clinical outcomes | 0 | |||
| Median follow up, days (95%CI) | 222 (202–242) | 190 (98–282) | 0.065 | |
| After admission [N (%)] | ||||
| 79 (65.3) | 32 (58.2) | ||
| 7 (8.9) | 4 (12.5) | 0.008 | |
| 14 (17.7) | 1 (3.1) | ||
| 51 (64.6) | 17 (53.1) | ||
| 7 (8.9) | 10 (31.3) | ||
| 42 (34.7) | 23 (41.8) | ||
| 11 (26.2) | 11 (47.8) | 0.103 | |
| Median hospital stay, days | 15.5 (7–29) | 20 (13–31) | 0.098 | |
| Adverse clinical outcome [N (%)] | 49 (40.5) | 33 (60) | 0.022 | |
| Swab negativization, days (95%CI) | ||||
| Median time from symptoms, | 35 (29–41) | 45 (37.5–52.5) | 0.023 | 0 |
| Fasting glucose (mmol/L) | ||||
| 8.38 (6.55–10.99) | 7.97 (7.43–9.19) | 0.882 | 0 |
| 11.04 (8.21–14.62) | 9.49 (7.93–11.59) | 0.109 | |
| 6.1 (4.85–8.1) | 7.04 (5.38–7.71) | 0.238 | |
| 2.44 (1.3–3.64) | 1.78 (1.01–3) | 0.158 | |
| - N° of determinations | 3 (2–7) | 2 (1–7) | 0.043 | |
| Glycated haemoglobin | 49.5 (42.25–57.75) | 37 (36–47) | 0.005 | 111 |
| Laboratory at admission *: | ||||
| White blood cells (×109/L) | 7.1 (5.4–10.4) | 9.7 (6.1–14.3) | 0.004 | 0 |
| 5.2 (3.9–8.1) | 8.4 (4.85–12.9) | 0.003 | 2 |
| 0.9 (0.6–1.3) | 0.9 (0.6–1.35) | 0.55 | 2 |
| 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 0.5 | 0 |
| 12.5 (10.75–13.5) | 12.9 (11.5–14.6) | 0.042 | 0 |
| 227 (166–298) | 228 (160–344) | 0.62 | 0 |
| Creatinine (µmol/L) | 95.5 (74.3–134.8) | 93.7 (76.9–137.9) | 0.99 | 0 |
| Aspartate transaminase (µkat/L) | 0.63 (0.42–1.14) | 0.95 (0.65–1.39) | 0.001 | 0 |
| Alanine transaminase (µkat/L) | 0.53 (0.3–0.93) | 0.78 (0.47–1.24) | 0.012 | 0 |
| Lactate dehydrogenase (µkat/L) | 5.92 (4.42–8.17) | 8.04 (5.35–16.63) | 0.001 | 0 |
| Albumin (g/L) | 27.9 (24.48–30.98) | 26.5 (22.7–29.7) | 0.17 | 57 |
| Total bilirubin (µmol/L) | 9.23 (5.39–14.87) | 11.62 (8.16–18.81) | 0.054 | 5 |
| Phosphatase alkaline (µkat/L) | 1.27 (0.97–1.92) | 1.26 (0.95–1.81) | 0.65 | 29 |
| Pro-BNP (ng/L) | 529 (288–1663) | 682 (182–2429) | 0.72 | 64 |
| Troponin T (µg/L) | 19 (11.42–45.57) | 26.6 (11.02–88.67) | 0.29 | 44 |
| D-dimer (µg/mL) | 1.69 (0.68–3.83) | 2.88 (1.04–6.62) | 0.18 | 39 |
| PT-INR | 1.12 (1.04–1.2350) | 1.19 (1.06–1.29) | 0.11 | 15 |
| PTT-R | 1 (0.92–1.1) | 0.98 (0.91–1.01) | 0.83 | 15 |
| Fibrinogen (g/L) | 607 (483–746) | 570 (433–741) | 0.70 | 85 |
| Antithrombin III (%) | 91 (83.7–100.2) | 89.5 (76.2–110) | - | 142 |
| Procalcitonin (ng/mL) | 0.61 (0.31–1.38) | 0.95 (0.49–3.58) | 0.014 | 42 |
| Ferritin (µg/L) | 823 (462–1499) | 1254 (585–2433) | 0.063 | 40 |
| C reactive protein (mg/L) | 93.5 (28.35–172.25) | 113.6 (31.1–204.9) | 0.39 | 0 |
| ESR (mm/h) | 73 (39–106) | 68 (48–87) | - | 98 |
| IL-6 (pg/mL) | 56.4 (23.1–173) | 59.8 (27.12–163.25) | 0.96 | 74 |
| Humoral immune response [N (%)] | ||||
| Sampling time from symptoms, days | 9 (4.75–13) | 10 (7–17.75) | 0.20 | 27 |
| 3 (3) | 0 (0) | 0.55 | |
| 4 (4) | 4 (8.3) | 0.27 | |
| 53 (52.5) | 27 (56.3) | 0.73 | |
| 56 (55.4) | 29 (60.4) | 0.60 | |
| 57 (56.4) | 34 (70.8) | 0.11 | |
| 60 (59.4) | 31 (64.6) | 0.59 | |
| 79 (78.2) | 35 (72.9) | 0.54 | |
| 76 (75.2) | 42 (87.5) | 0.13 | |
| 66 (65.3) | 35 (72.9) | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurenzi, A.; Caretto, A.; Molinari, C.; Bazzigaluppi, E.; Brigatti, C.; Marzinotto, I.; Mercalli, A.; Melzi, R.; Nano, R.; Tresoldi, C.; et al. Pre-Existing Diabetes and COVID-Associated Hyperglycaemia in Patients with COVID-19 Pneumonia. Biology 2021, 10, 754. https://doi.org/10.3390/biology10080754
Laurenzi A, Caretto A, Molinari C, Bazzigaluppi E, Brigatti C, Marzinotto I, Mercalli A, Melzi R, Nano R, Tresoldi C, et al. Pre-Existing Diabetes and COVID-Associated Hyperglycaemia in Patients with COVID-19 Pneumonia. Biology. 2021; 10(8):754. https://doi.org/10.3390/biology10080754
Chicago/Turabian StyleLaurenzi, Andrea, Amelia Caretto, Chiara Molinari, Elena Bazzigaluppi, Cristina Brigatti, Ilaria Marzinotto, Alessia Mercalli, Raffaella Melzi, Rita Nano, Cristina Tresoldi, and et al. 2021. "Pre-Existing Diabetes and COVID-Associated Hyperglycaemia in Patients with COVID-19 Pneumonia" Biology 10, no. 8: 754. https://doi.org/10.3390/biology10080754
APA StyleLaurenzi, A., Caretto, A., Molinari, C., Bazzigaluppi, E., Brigatti, C., Marzinotto, I., Mercalli, A., Melzi, R., Nano, R., Tresoldi, C., Landoni, G., Ciceri, F., Lampasona, V., Scavini, M., & Piemonti, L. (2021). Pre-Existing Diabetes and COVID-Associated Hyperglycaemia in Patients with COVID-19 Pneumonia. Biology, 10(8), 754. https://doi.org/10.3390/biology10080754






