Vocal Creativity in Elephant Sound Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Data Analysis
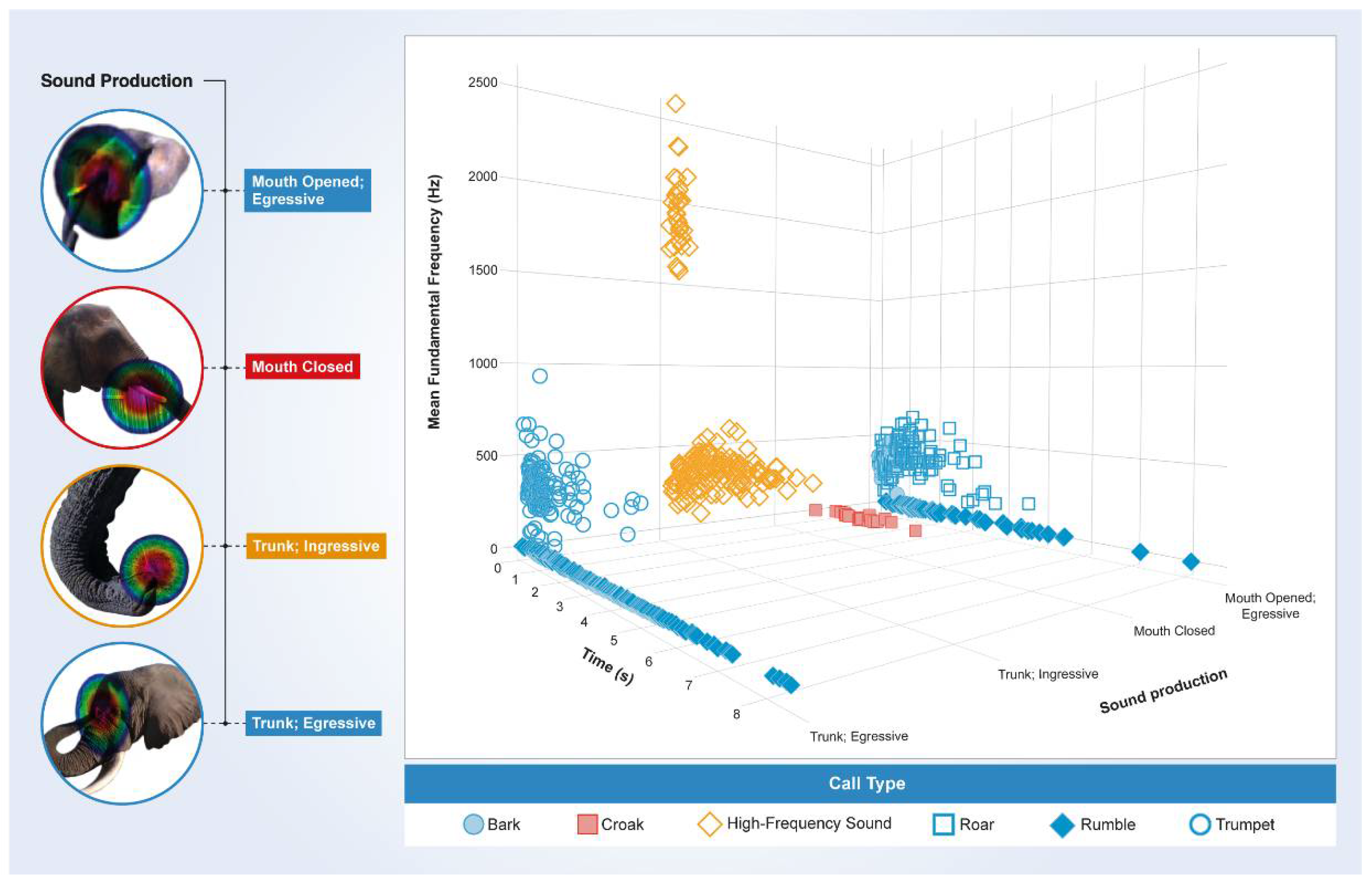
3. Results
3.1. Periodic Idiosyncratic Sounds
3.2. Aperiodic Idiosyncratic Sounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitch, W.T. The biology and evolution of language: ‘deep homology’ and the evolution of innovation. In The Cognitive Neurosciences; Gazzaniga, M.S., Ed.; MIT Press: Cambridge, CA, USA, 2009; pp. 873–883. [Google Scholar]
- Tyack, P. A taxonomy for vocal learning. Philos. Trans. R. Soc. B 2019, 375, 20180406. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.T.; Boeckx, C. Vocal learning: Beyond the continuum. PLoS Biol. 2020, 18, e3000627. [Google Scholar] [CrossRef] [Green Version]
- Poole, J.H.; Tyack, P.L.; Stoeger-Horwath, A.S.; Watwood, S. Elephants are capable of vocal learning. Nature 2005, 434, 455–456. [Google Scholar] [CrossRef]
- Stoeger, A.S.; Mietchen, D.; Sukhun, O.; de Silva, S.; Herbst, C.T.; Kwon, S.; Fitch, W.T. An Asian elephant imitates human speech. Curr. Biol. 2012, 22, 2144–2148. [Google Scholar] [CrossRef] [Green Version]
- Nair, B.R.; Balakrishnan, R.; Seelamantula, C.S.; Sukumar, R. Vocalizations of wild Asian elephants (Elephas maximus): Structural classification and social context. JASA 2009, 126, 2768–2778. [Google Scholar] [CrossRef] [Green Version]
- de Silva, S. Acousitc communication in the Asian elephant, Elephas maximus. Behavior 2010, 147, 825–852. [Google Scholar] [CrossRef]
- Berg, J.K. Vocalizations and associated behaviors of the African elephant (Loxodonta africana) in captivity. Z. Tierpsychol. 1983, 63, 63–79. [Google Scholar] [CrossRef]
- Leong, K.M.; Ortolani, A.; Burks, K.D.; Mellen, J.D.; Savage, A. Quantifying acoustic and temporal characteristics of vocalizations for a group of captive. African elephants Loxodonta africana. Bioacoustics 2003, 13, 213–231. [Google Scholar] [CrossRef]
- Soltis, J. Vocal communication in African elephants. Zoo Biol. 2010, 29, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Langbauer, W.R., Jr. Elephant communication. Zoo Biol. 2000, 19, 425–445. [Google Scholar] [CrossRef]
- Poole, J.H. Behavioral contexts of elephant acoustic communication. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H., Lee, P.C., Eds.; Chicago University Press: Chicago, IL, USA, 2011; pp. 125–161. [Google Scholar]
- Soltis, J.; King, L.E.; Douglas-Hamilton, I.; Vollrath, F.; Savage, A. African elephant alarm calls distinguish between threats from humans and bees. PLoS ONE 2014, 9, e89403. [Google Scholar] [CrossRef]
- Soltis, J. Emotional communication in African elephants. In The Evolution of Emotional Communication: From Sounds in Nonhuman Mammals to Speech and Music in Man; Altenmüller, E., Schmidt, S., Zimmermann, E., Eds.; University Press: Oxford, UK, 2013; pp. 105–115. [Google Scholar]
- Padro, M.; Poole, J.H.; Stoeger, A.S.; Wrege, P.H.; O’Connel-Rodwell, C.E.; Padmalal, U.K.; de Silva, S. Differences in combinatorial calls among the three elephant species cannot be explained by phylogeny. Behav. Ecol. 2019, 30, 809–820. [Google Scholar] [CrossRef]
- McComb, K.; Reby, D.; Baker, L.; Moss, C.; Sayialel, S. Long-distance communication of acoustic cues to special identity in African elephants. Anim. Behav. 2003, 65, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Beeck, V.; Heilmann, G.; Kerscher, M.; Stoeger, A.S. A novel theory of Asian elephant high-frequency squeak production. BMC Biol. 2021, 19, 21. [Google Scholar] [CrossRef]
- Reichmuth, C.; Casey, C. Vocal learning in seals, sea lions, and walruses. Curr. Opin. Neurobiol. 2014, 28, 66–71. [Google Scholar] [CrossRef]
- Frankel, A.S. Sound production. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, G.G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1056–1071. [Google Scholar] [CrossRef]
- Schustermann, R.J.; Reichmuth, C. Novel sound production though contingency learning in the Pacific walrus (Odeobenus rosmarus divergens). Anim. Cogn. 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Pepperberg, I.M. Vocal learning in grey parrots: A brief review of perception, production, and cross-species comparison. Brain Lang. 2010, 115, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Patterson, D.K.; Pepperberg, I.M. A comparative study of human and parrot phonation: Acousitc and articulatory correlates of vowels. JASA 1994, 96, 634–648. [Google Scholar] [CrossRef]
- Patterson, D.K.; Pepperberg, I.M. Acousitc and articulatory correlates of stop consonantes in a parrot and a human subject. JASA 1998, 103, 2197–2215. [Google Scholar] [CrossRef] [PubMed]
- S_Tools-STx Online Manual Acoustic Research Institute. Austrian Academy of Sciences. Available online: https://www.oeaw.ac.at/isf/das-institut/software/stx (accessed on 17 August 2018).
- Solomon Coder (Version Beta 11.01.22): A Simply Solution for Behavior Coding. Available online: http://solomoncoder.com/ (accessed on 20 September 2020).
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Fitch, W.T.; Neubauer, J.; Herzel, H. Calls out of chaos: The adaptive significance of nonlinear phenomena in mammalian vocal production. Anim. Behav. 2002, 63, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Boas, J.E.V.; Paulli, S. The Elephants Head. Studies in the Comparative Anatomy of the Indian Elephant and Other Mammals. Part I. The Facial Muscles and the Proboscis; Fischer: Berlin, Germany, 1908. [Google Scholar]
- Shoshani, J. Elephants: Majestic Creatures of the Wild; Rodale Press: Emmaus, PA, USA, 2000. [Google Scholar]
- Stoeger, A.S.; Baotic, A. Operant Control and call usage learning in African elephants. Phil. Trans. B 2021, 20200254. [Google Scholar] [CrossRef]
- Manabe, K.; Staddon, J.E.R.; Cleaveland, J.M. Control of vocal repertoire by reward in Budgerigars (Melopsittacus undulates). J. Comp. Psychol. 1997, 111, 50–62. [Google Scholar] [CrossRef]
- Reby, D.; Wyman, M.T.; Frey, R.; Passilongo, D.; Gilbert, J.; Locatelli, Y.; Charlton, B.D. Evidence of biphonation and source-filter interactions in the bugles of male North American wapiti (Cervis canadensis). Exp. Biol. 2016, 219, 1224–1236. [Google Scholar] [CrossRef] [Green Version]
- Tyack, P.L.; Miller, E.H. Vocal anatomy, acoustic communication and echolocation. In Marine Mammal Biology: An Evolutionary Approach; Hoetzel, R., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 142–184. [Google Scholar]
- Azola, A.; Palmer, J.; Mulheren, R.; Hofer, R.; Fischmeister, F.; Fitch, W.T.F. The physiology of oral whistling: A combined radiographic and MRI analysis. J. Appl. Physiol. 2018, 124, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, N.H. Acousitc Systems in Biology; Oxford Univeristy Press: New York, NY, USA, 1992. [Google Scholar]
- The Elephant Ethogram. Available online: https://www.elephantvoices.org/studies-a-projects/the-elephant-ethogram.html (accessed on 12 June 2021).
- Boas, J.E.V.; Paulli, S. The Elephant’s Head: Studies in the Comparative Anatomy of the Organs of the Head of the Indian Elephant and Other Mammals, Part II; Fischer: Berlin, Germany, 1925. [Google Scholar]
- Zann, R.A. The Zebra Finch: A Synthesis of Field and Laboratory Studies; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- O’Connell, L.A.; Hofmann, H.A. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J. Comp. Neurol. 2011, 519, 3599–3639. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, P.K. Human speech and birdsong: Communication and the social brain. Proc. Natl. Acad Sci. USA 2003, 100, 9645–9646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, H. Birdsong and singing behavior. Ann. N. Y. Acad. Sci. 2004, 1016, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Matheson, L.E.; Sakata, J.T. Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc. Natl. Acad Sci. USA 2016, 113, 6641–6646. [Google Scholar] [CrossRef] [Green Version]
- Theofanopoulou, C.; Boeckx, C.; Jarvis, E.D. A hypothesis on a role of oxytocin in the social mechanisms of speech and vocal learning. Proc. Biol. Sci. 2017, 284, 20170988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, M.H.; King, A.P.; West, M.J. Social interaction shapes babbling: Testing parallels between birdsong and speech. Proc. Natl. Acad Sci. USA 2003, 100, 8030–8035. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, P.K. Is speech learning ‘gated’ by the social brain? Dev. Sci. 2007, 10, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Syal, S.; Finlay, B.L. Thinking outside the cortex: Social motivation in the evolution and development of language. Dev. Sci. 2011, 14, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Carouso-Peck, S.; Goldstein, H.M. Female social feedback reveals non-imitative mechanisms of vocal learning in Zebra Finches. Curr. Biol. 2019, 29, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepperberg, I.M. Social influences on the acquisition of human-based codes in parrots and nonhuman primates. In Social Influences on Vocal Development; Snowdon, C.T., Hausberger, M., Eds.; Cambrigde University Press: Cambrigde, UK, 1997; pp. 157–177. [Google Scholar]
- Pepperberg, I.M. Human speech: Its learning and use by Grey parrots. In Nature’s Music; Marler, P., Slabbekoorn, H., Eds.; Elsevier: London, UK, 2004; pp. 363–373. [Google Scholar]
- Musser, W.B.; Bowles, A.E.; Grebner, D.M.; Crance, J.L. Differences in acoustic features of vocalizations produced by killer whales cross-socialized with bottlenose dolphins. J. Acoust. Soc. Am. 2014, 136, 1990–2002. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, D.Y.; Liao, D.A.; Ghazanfar, A.A. Vocal learning via social reinforcement by infant marmoset monkeys. Curr. Biol. 2017, 27, 1844–1852. [Google Scholar] [CrossRef] [Green Version]
- Moss, C.J.; Poole, J.H. Relationship and social structure in African elephants. In Primate Social Relationships: An Integrated Approach; Hinde, R.A., Ed.; Blackwell Scientific: Hoboken, NJ, USA, 1983; pp. 315–325. [Google Scholar]
- Fishlock, V.; Lee, P.C. Forest elephants: Fission-fusion and social arenas. Anim. Behav. 2013, 85, 357–363. [Google Scholar] [CrossRef]
- De Silva, S.; Ranjeewa, A.D.G.; Kryazhimskiy, S. The dynamics of social networks among female Asian elephants. BMC Ecol. 2011, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlstead, K.; Paris, S.; Brown, J.L. Good keeper-elephant relationships in North American zoos are mutually beneficial to welfare. Appl. Anim. Behav. Sci. 2019, 211, 103–111. [Google Scholar] [CrossRef]
- Hart, L.A. The Asian elephants-driver partnership: The drivers’ perspective. Appl. Anim. Behav. Sci. 1994, 40, 297–312. [Google Scholar] [CrossRef]
- Rossman, Z.T.; Padfield, C.; Young, D.; Hart, L.A. Elephant-initiated interactions with humans: Individual differences and specific preferences in captive African elephants (Loxodonta Africana). Front. Vet. Sci. 2017, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- ASAB/ABS. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2015, 99, 1–9. [Google Scholar] [CrossRef]



| High-Frequency Sound | |||
| Parameter | Jabu (N = 92) | Morula (N = 50) | Sawu (N = 37) |
| F0 start ± SD (Hz) | 554.66 ± 156.02 | 541.48 ± 299.22 | 2141.31 ± 385.78 |
| F0 mid ± SD (Hz) | 454.20 ± 71.669 | 404.08 ± 279.84 | 1854.62 ± 331.98 |
| F0 end ± SD (Hz) | 350.20 ± 100.82 | 313.72 ± 202.56 | 1672.32 ± 398.29 |
| F0 minimum ± SD (Hz) | 345.90 ± 99.43 | 299.20 ± 204.13 | 1696.34 ± 269.33 |
| F0 maximum ± SD (Hz) | 578.70 ±140.89 | 563.16 ± 332.78 | 2161.57 ± 392.13 |
| F0 mean ± SD (Hz) | 444.69 ± 59.87 | 391.80 ± 242.74 | 1859.90 ± 285.14 |
| Peak frequency ± SD (Hz) | 1182.03 ± 223.53 | 406.08 ± 253.35 | 2011.16 ± 330.32 |
| Duration ± SD (s) | 2.43 ± 0.93 | 1.17 ± 0.59 | 0.58 ± 0.17 |
| % biphonation | 75.6 | 63.3 | 43.2 |
| % subharmonics | 47.2 | 12.2 | 43.6 |
| % frequency jumps | 29.3 | 8.2 | 16.2 |
| % chaos | 3.3 | ---- | 13.5 |
| Throb Sound | |||
| Jabu (N = 92) | Morula (N = 50) | ||
| Peak frequency ± SD (Hz) | 82.79 ± 50.38 | 128.56 ± 14.34 | |
| Duration ± SD (s) | 0.47 ± 0.054 | 0.51 ± 0.086 | |
| Oral Burst | |||
| Drumbo (N = 15) | Mogli (N = 10) | Sawu (N = 12) | |
| Peak frequency ± SD (Hz) | 468 ± 21.99 | 461 ± 23.56 | 445 ± 78.86 |
| Duration ± SD (s) | 1.11 ± 0.255 | 1.01 ± 0.192 | 0.58 ± 0.247 |
| Individual | Sound Type | Sound Emission | Respiratory Phase | Description |
|---|---|---|---|---|
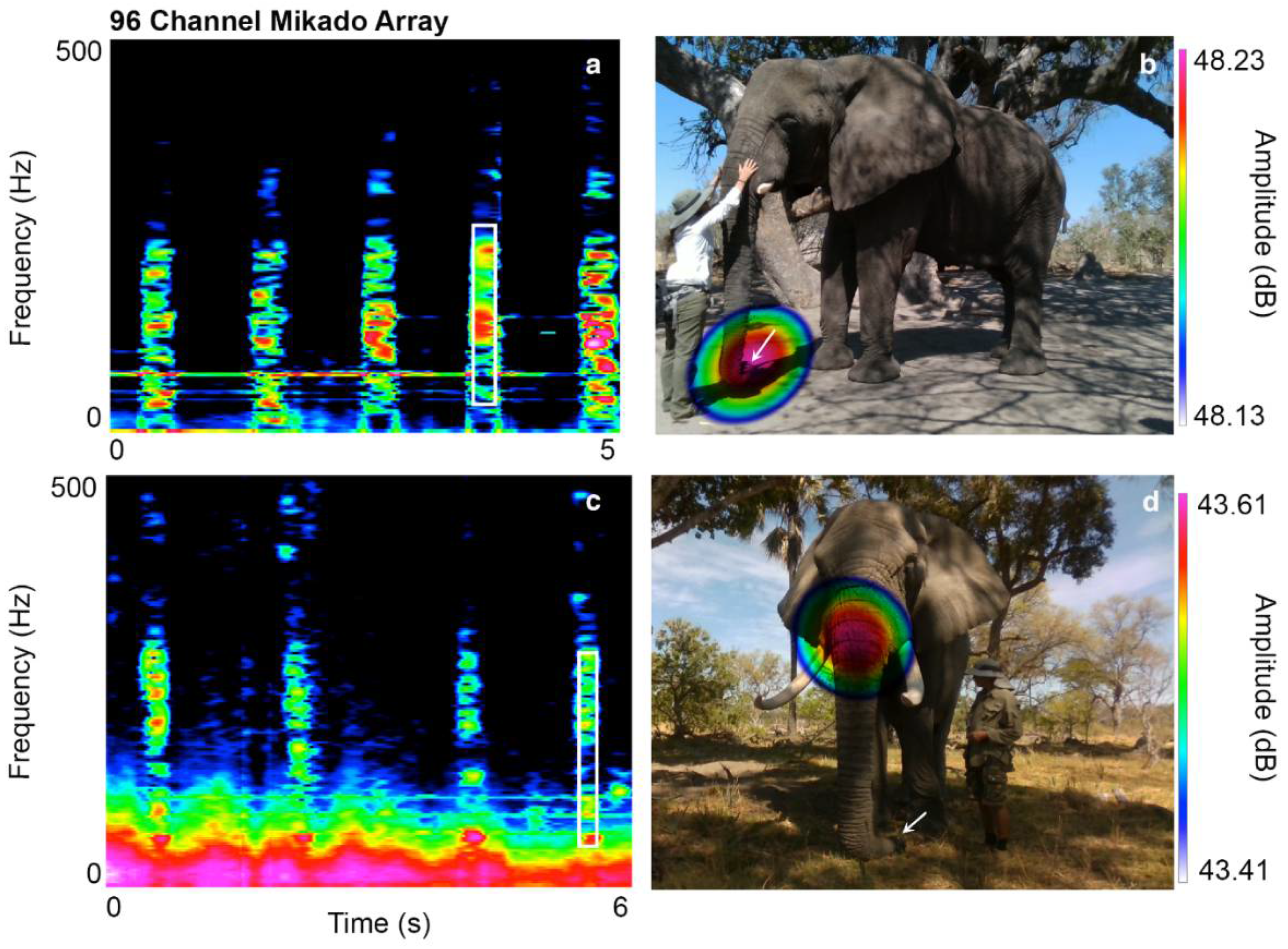
| Jabu | HFS | Trunk tip | Ingressive sound | Tilts the tip of his trunk to the left, while stiffening and closing the left nasal tube. |
| Morula | HFS | Trunk tip | Ingressive sound | Tilts the tip of her trunk upwards, stiffening and closing the left nasal tube. |
| Sawu | HFS | Trunk tip | Ingressive sound | Tilts the tip of her trunk slightly to the right, stiffening and closing the right nasal tube. |
| Jabu | Throb sound | Trunk base | Egressive sound | Contractions of musculus nasalis. |
| Morula | Throb sound | Trunk tip | Egressive sound | Contractions of the maxillo labialis at the trunk base. |
| Mogli | Oral burst | Mouth | Egressive sound | Vibration of soft palate: air blocked by a posterior obstruction of the oral chamber, then abruptly released, causing a burst of sound. |
| Drumbo | Oral burst | Mouth | Egressive sound | Not known, most likely similar to Mogli. |
| Sawu | Oral burst | Mouth | Egressive sound | Not known, most likely similar to Mogli. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoeger, A.S.; Baotic, A.; Heilmann, G. Vocal Creativity in Elephant Sound Production. Biology 2021, 10, 750. https://doi.org/10.3390/biology10080750
Stoeger AS, Baotic A, Heilmann G. Vocal Creativity in Elephant Sound Production. Biology. 2021; 10(8):750. https://doi.org/10.3390/biology10080750
Chicago/Turabian StyleStoeger, Angela S., Anton Baotic, and Gunnar Heilmann. 2021. "Vocal Creativity in Elephant Sound Production" Biology 10, no. 8: 750. https://doi.org/10.3390/biology10080750
APA StyleStoeger, A. S., Baotic, A., & Heilmann, G. (2021). Vocal Creativity in Elephant Sound Production. Biology, 10(8), 750. https://doi.org/10.3390/biology10080750






