Egg Incubation Mechanics of Giant Birds
Abstract
:Simple Summary
Abstract
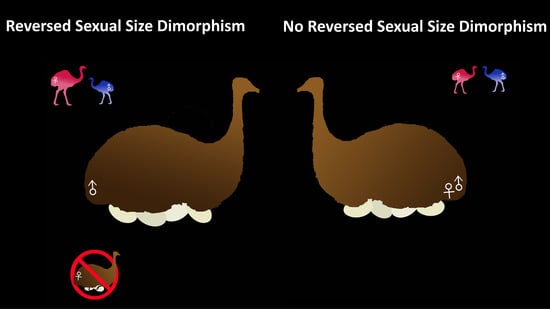
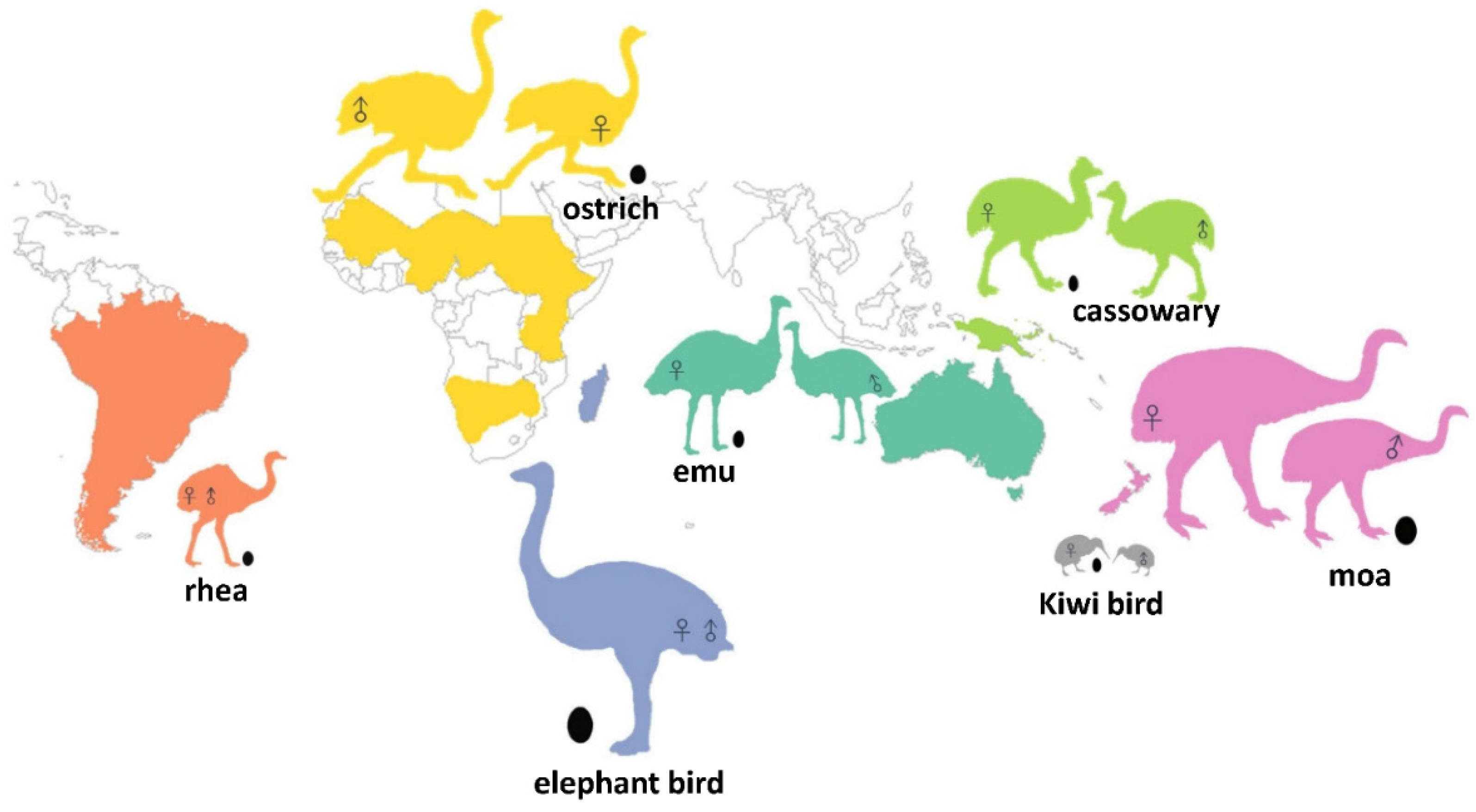
1. Introduction
2. Methods
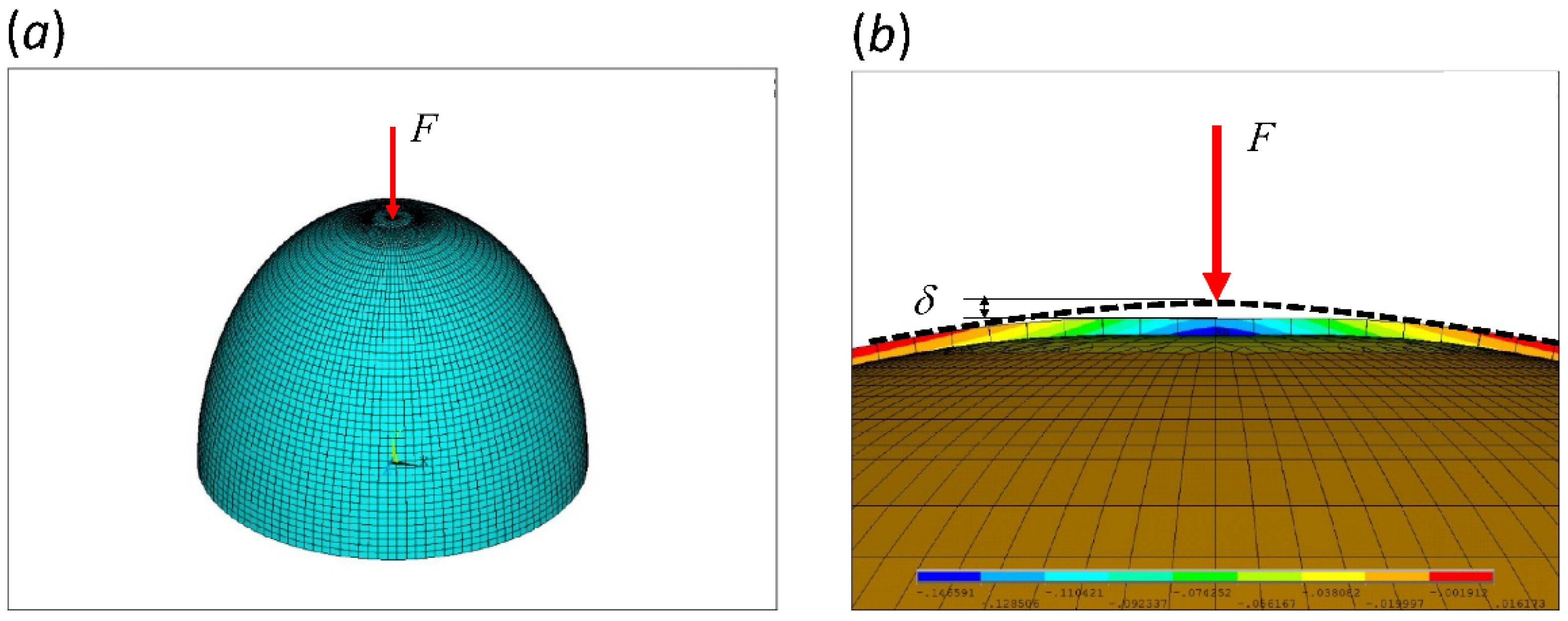
2.1. Finite Element Analysis (FEA)
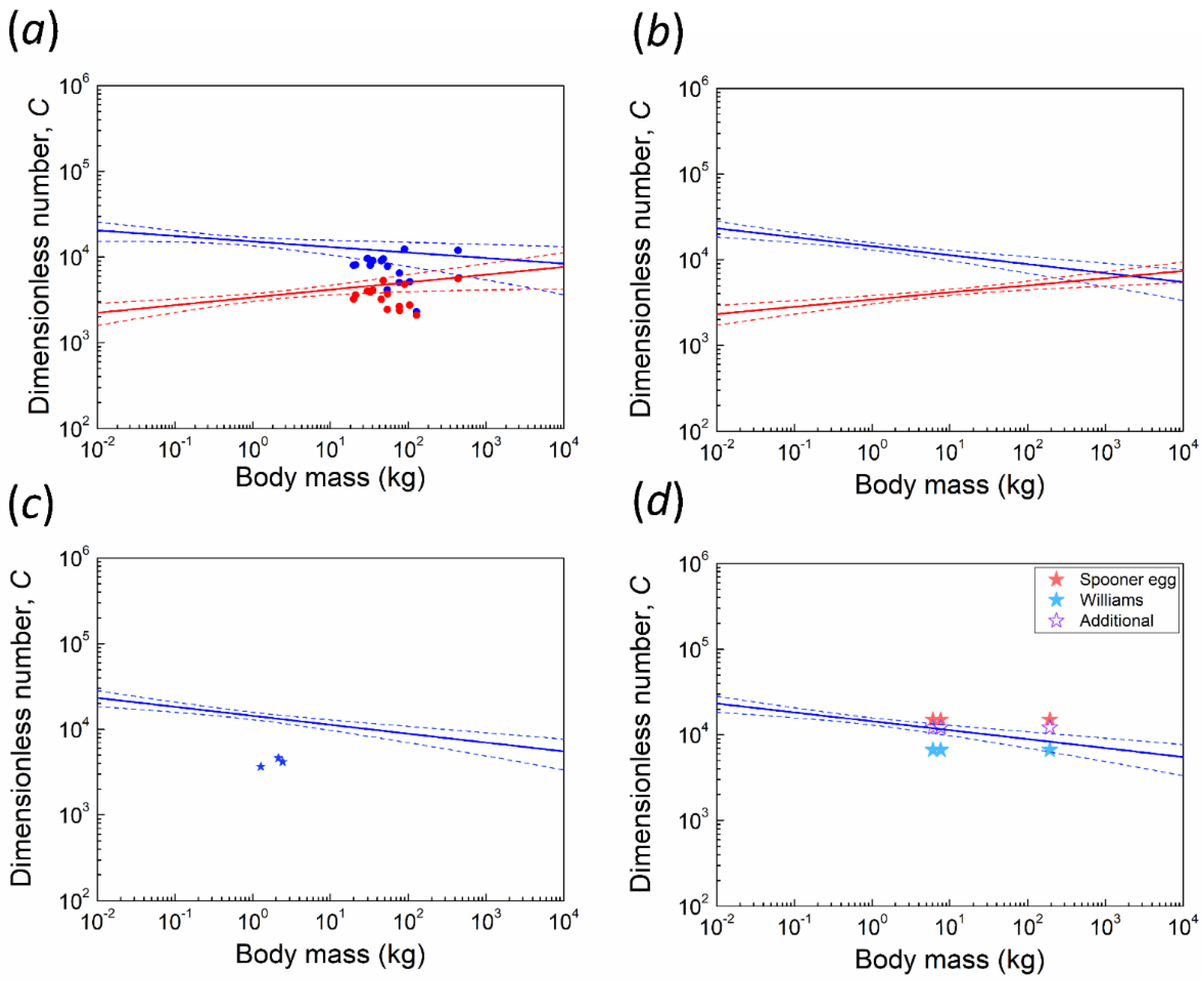
2.2. Dimensionless Number C, Critical Thickness, and the Factor of Safety
3. Results and Discussion
3.1. Kiwi and PGOM
3.2. Maximum Body Mass for Contact Incubation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brusatte, S.L.; O’Connor, J.K.; Jarvis, E.D. The Origin and Diversification of Birds. Curr. Biol. 2015, 25, R888–R898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeming, D.C. Avian Incubation: Behaviour, Environment, and Evolution; Oxford University Press: Oxford, UK, 2002; p. xiv. 421p. [Google Scholar]
- Juang, J.Y.; Chen, P.Y.; Yang, D.C.; Wu, S.P.; Yen, A.; Hsieh, H.I. The avian egg exhibits general allometric invariances in mechanical design. Sci. Rep. 2017, 7, 14205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawkins, R.; Wong, Y. The Ancestor’s Tale: A Pilgrimage to the Dawn of Evolution, 2nd ed.; Houghton Mifflin Harcourt: Boston, MA, USA, 2016; p. xxi. 771p. [Google Scholar]
- Murray, P.F.; Vickers-Rich, P. Magnificent Mihirungs: The Colossal Flightless Birds of the Australian Dreamtime; Indiana University Press: Bloomington, IN, USA, 2004. [Google Scholar]
- Hansford, J.P.; Turvey, S.T. Unexpected diversity within the extinct elephant birds (Aves: Aepyornithidae) and a new identity for the world’s largest bird. R. Soc. Open Sci. 2018, 5, 181295. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, K.J.; Llamas, B.; Soubrier, J.; Rawlence, N.J.; Worthy, T.H.; Wood, J.; Lee, M.S.; Cooper, A. Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 2014, 344, 898–900. [Google Scholar] [CrossRef] [Green Version]
- Birchard, G.F.; Deeming, D.C. Avian eggshell thickness: Scaling and maximum body mass in birds. J. Zool. 2009, 279, 95–101. [Google Scholar] [CrossRef]
- Worthy, T.H.; Holdaway, R.N. The Lost World of the Moa: Prehistoric Life of New Zealand, 1st ed.; Indiana University Press: Bloomington, IN, USA, 2002. [Google Scholar]
- Worthy, T.; Bunce, M.; Cooper, A.; Scofield, P. Dinornis—An insular oddity, a taxonomic conundrum reviewed. Monogr. Soc. Hist. Nat. Balear. 2005, 12, 377–390. [Google Scholar]
- Bunce, M.; Worthy, T.H.T.H.; Ford, T.; Hoppitt, W.; Willerslev, E.; Drummond, A.; Cooper, A. Extreme reversed sexual size dimorphism in the extinct New Zealand moa Dinornis. Nature 2003, 425, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Huynen, L.; Gill, B.J.; Millar, C.D.; Lambert, D.M. Ancient DNA reveals extreme egg morphology and nesting behavior in New Zealand’s extinct moa. Proc. Natl. Acad. Sci. USA 2010, 107, 16201–16206. [Google Scholar] [CrossRef] [Green Version]
- Grellet-Tinner, G.; Spooner, N.A.; Worthy, T.H. Is the “Genyornis” egg of a mihirung or another extinct bird from the Australian dreamtime? Quat. Sci. Rev. 2016, 133, 147–164. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.L.G. Genyornis Eggshell (Dromornithidae, Aves) from the Late Pleistocene of South-Australia. Alcheringa 1981, 5, 133–140. [Google Scholar] [CrossRef]
- Worthy, T.H.; Degrange, F.J.; Handley, W.D.; Lee, M.S.Y. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). R. Soc. Open Sci. 2017, 4, 170975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayfield, E.J.; Norman, D.B.; Horner, C.C.; Horner, J.R.; Smith, P.M.; Thomason, J.J.; Upchurch, P. Cranial design and function in a large theropod dinosaur. Nature 2001, 409, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Attard, M.R.G.; Wilson, L.A.B.; Worthy, T.H.; Scofield, P.; Johnston, P.; Parr, W.C.H.; Wroe, S. Moa diet fits the bill: Virtual reconstruction incorporating mummified remains and prediction of biomechanical performance in avian giants. Proc. R. Soc. B 2016, 283. [Google Scholar] [CrossRef] [Green Version]
- Button, D.J.; Rayfield, E.J.; Barrett, P.M. Cranial biomechanics underpins high sauropod diversity in resource-poor environments. Proc. R. Soc. B 2014, 281, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McHenry, C.R.; Wroe, S.; Clausen, P.D.; Moreno, K.; Cunningham, E. Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation. Proc. Natl. Acad. Sci. USA 2007, 104, 16010–16015. [Google Scholar] [CrossRef] [Green Version]
- Wroe, S.; Parr, W.C.H.; Ledogar, J.A.; Bourke, J.; Evans, S.P.; Fiorenza, L.; Benazzi, S.; Hublin, J.-J.; Stringer, C.; Kullmer, O.; et al. Computer simulations show that Neanderthal facial morphology represents adaptation to cold and high energy demands, but not heavy biting. Proc. R. Soc. B 2018, 285. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, D.F. Practical Methods of Estimating Volume and Fresh Weight of Bird Eggs. Auk 1979, 96, 73–77. [Google Scholar] [CrossRef]
- Dickison, M.R. The Allometry of Giant Flightless Birds. Ph.D. Thesis, Duke University, Durham, NC, USA, 2007. [Google Scholar]
- Gill, B.J. Eggshell characteristics of moa eggs (Aves: Dinornithiformes). J. R. Soc. N. Z. 2007, 37, 139–150. [Google Scholar] [CrossRef]
- Gill, B.J. A catalogue of moa eggs (Aves: Dinornithiformes). Rec. Auckl. Mus. 2006, 43, 55–80. [Google Scholar]
- Mlíkovsky, J. Eggs of extinct aepyornithids (Aves Aepyornithidae) of Madagascar: Size and taxonomic identity. Sylvia 2003, 39, 133–138. [Google Scholar]
- Grealy, A.; Phillips, M.; Miller, G.; Gilbert, M.T.P.; Rouillard, J.-M.; Lambert, D.; Bunce, M.; Haile, J. Eggshell palaeogenomics: Palaeognath evolutionary history revealed through ancient nuclear and mitochondrial DNA from Madagascan elephant bird (Aepyornis sp.) eggshell. Mol. Phylogenetics Evol. 2017, 109, 151–163. [Google Scholar] [CrossRef]
- Davies, S.J.J.F.; Bamford, M. Ratites and Tinamous: Tinamidae, Rheidae, Dromaiidae, Casuariidae, Apterygidae, Struthionidae; Oxford University Press: Oxford, UK, 2002; p. xxiii. 310p. [Google Scholar]
- Grzimek, B. Grzimek’s Animal Life Encyclopedia 8 Birds I Tinamous and Ratites to Hoatzins, 2nd ed.; Gale: Farmington Hills, MI, USA, 2003; Volume 8. [Google Scholar]
- Hume, J.P.; Walters, M. Extinct Birds; Bloomsbury Publishing: London, UK, 2012. [Google Scholar]
- Bunce, M.; Worthy, T.H.; Phillips, M.J.; Holdaway, R.N.; Willerslev, E.; Haile, J.; Shapiro, B.; Scofield, R.P.; Drummond, A.; Kamp, P.J.J.; et al. The evolutionary history of the extinct ratite moa and New Zealand Neogene paleogeography. Proc. Natl. Acad. Sci. USA 2009, 106, 20646–20651. [Google Scholar] [CrossRef] [Green Version]
- Holdaway, R.N.; Worthy, T.H. A reappraisal of the late quaternary fossil vertebrates of Pyramid Valley Swamp, North Canterbury, New Zealand. N. Z. J. Zool. 1997, 24, 69–121. [Google Scholar] [CrossRef]
- Huynen, L.; Millar, C.D.; Scofield, R.P.; Lambert, D.M. Nuclear DNA sequences detect species limits in ancient moa. Nature 2003, 425, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Grellet-Tinner, G.; Spooner, N.A.; Handley, W.D.; Worthy, T.H. The Genyornis Egg: Response to Miller et al.’s commentary on Grellet-Tinner et al., 2016. Quat. Sci. Rev. 2017, 161, 128–133. [Google Scholar] [CrossRef]
- Shute, E.; Prideaux, G.J.; Worthy, T.H. Taxonomic review of the late Cenozoic megapodes (Galliformes: Megapodiidae) of Australia. R. Soc. Open Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, A.; Florijn, H.C.B.; Reis, P.M. Geometry-Induced Rigidity in Nonspherical Pressurized Elastic Shells. Phys. Rev. Lett. 2012, 109, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, E.N.; Sherman, V.R.; Pissarenko, A.; Rohrbach, S.D.; Fernandes, D.J.; Meyers, M.A. Nature’s technical ceramic: The avian eggshell. J. R. Soc. Interface 2017, 14, 20160804. [Google Scholar] [CrossRef] [Green Version]
- Kemps, B.; De Ketelaere, B.; Bamelis, F.; Govaerts, T.; Mertens, K.; Kamers, B.; Tona, K.; Decuypere, E.; De Baerdemaeker, J. Development of a Methodology for the Calculation of Young’s Modulus of Eggshell using Vibration Measurements. Biosyst. Eng. 2004, 89, 215–221. [Google Scholar] [CrossRef]
- Angst, D.; Buffetaut, E. Paleobiology of Giant Flightless Birds; ISTE Press—Elsevier: London, UK, 2018. [Google Scholar]
- Worthy, T.; Scofield, R. Twenty-first century advances in knowledge of the biology of moa (Aves: Dinornithiformes): A new morphological analysis and moa diagnoses revised. N. Z. J. Zool. 2012, 39, 87–153. [Google Scholar] [CrossRef]
- Yu, G.C.; Lam, T.T.Y.; Zhu, H.C.; Guan, Y. Two Methods for Mapping and Visualizing Associated Data on Phylogeny Using Ggtree. Mol. Biol. Evol. 2018, 35, 3041–3043. [Google Scholar] [CrossRef]
- Yu, G.C.; Smith, D.K.; Zhu, H.C.; Guan, Y.; Lam, T.T.Y. GGTREE: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oskam, C.L.; Haile, J.; McLay, E.; Rigby, P.; Allentoft, M.E.; Olsen, M.E.; Bengtsson, C.; Miller, G.H.; Schwenninger, J.-L.; Jacomb, C.; et al. Fossil avian eggshell preserves ancient DNA. Proc. R. Soc. B 2010, 277, 1991–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, W.A. The Kiwi. Sci. Am. 1978, 239, 132–142. [Google Scholar] [CrossRef]
- Deeming, D.C.; Birchard, G.F. Why were extinct gigantic birds so small? J. Avian Biol. 2008, 1, 187–194. [Google Scholar] [CrossRef]
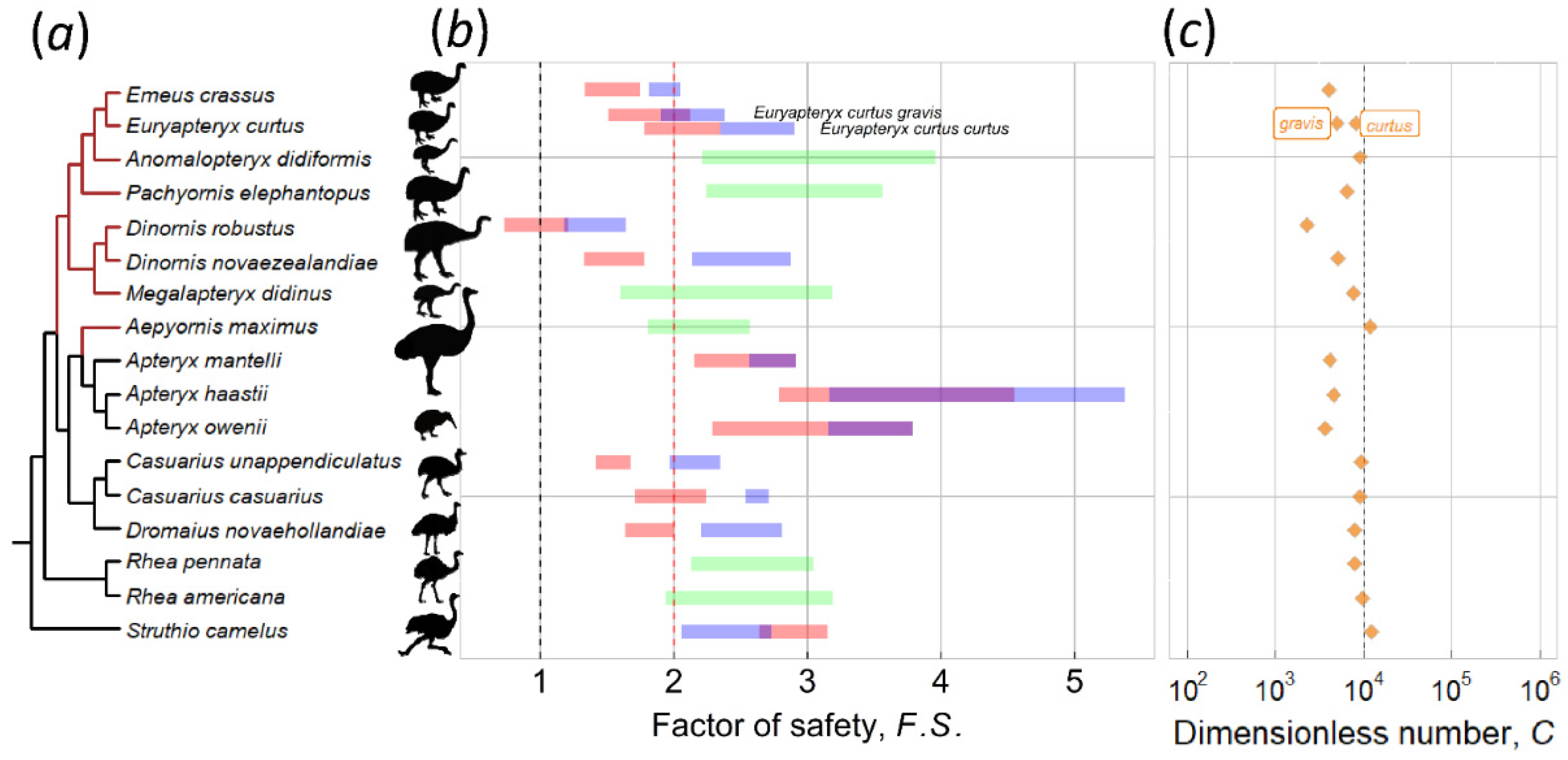
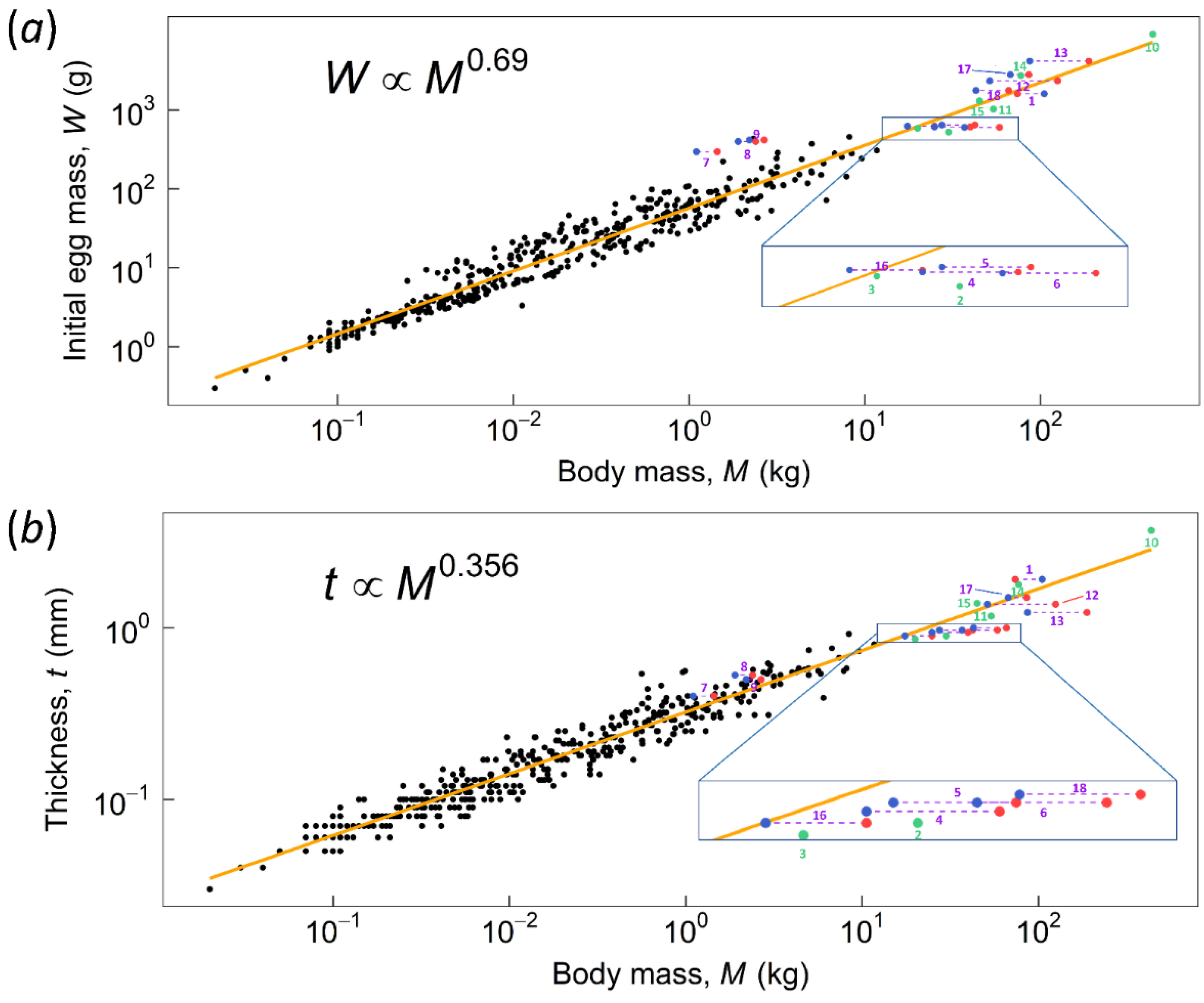
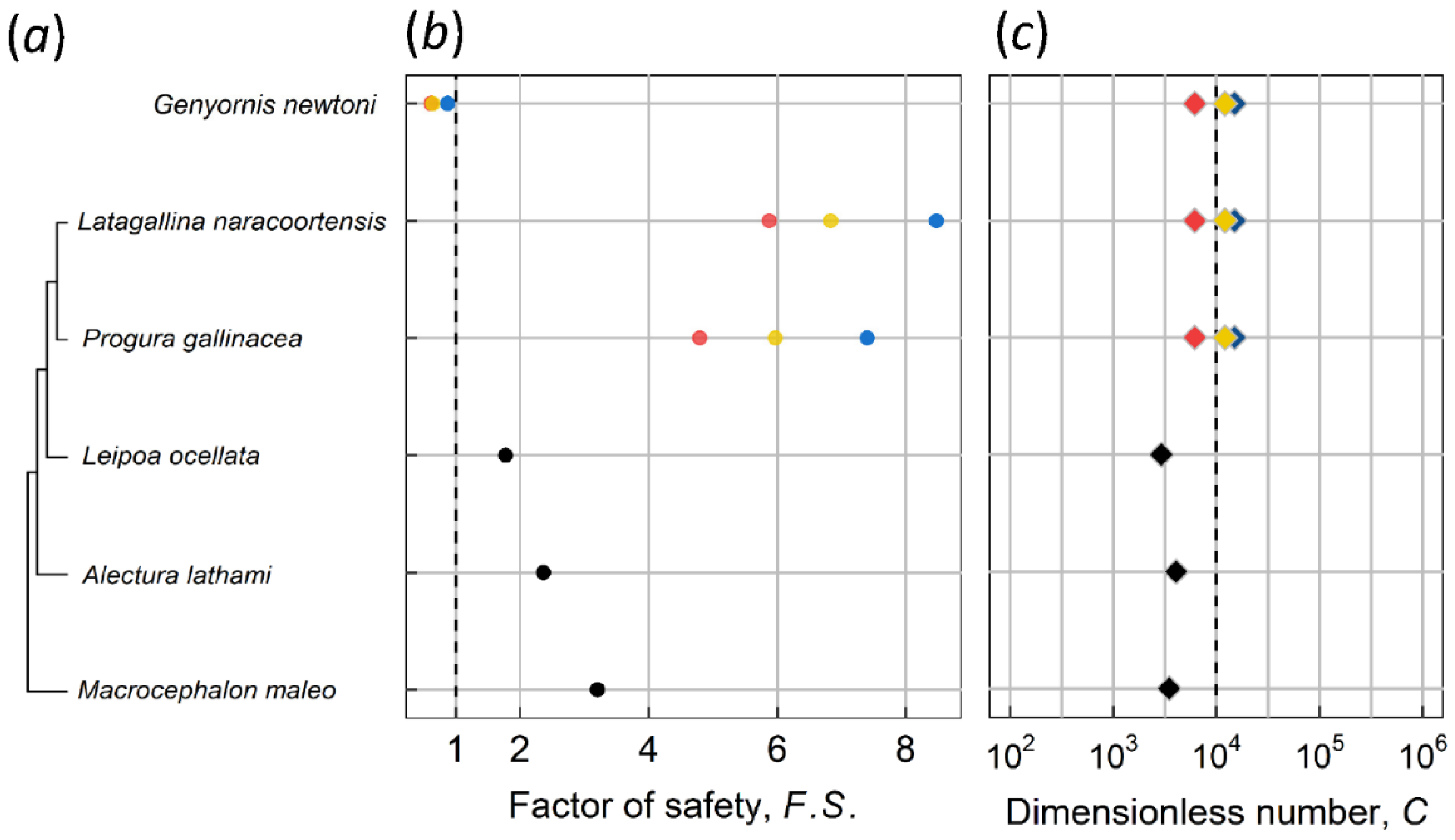
| Species | Egg Length, B (mm) | Egg Width, A (mm) | Shell Thickness, t (mm) | Egg Mass, W (g) | Body Mass, M (kg) | ESR | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| FBM (Max.) | FBM (Min.) | MBM (Max.) | MBM (Min.) | |||||||
| 1 Struthio camelus d | 158 | 131 | 1.92 | 1600 | 85 | 63 | 130 | 80 | 1.79% | [3,12] |
| 2 Rhea americana e | 128 | 86 | 0.9 | 525 | 40 | 20 | 1.75% | [3,28] | ||
| 3 Rhea pennata e | 126 | 92 | 0.86 | 584 | 25 | 15 | 2.92% | [22,28] | ||
| 4 Dromaius novaehollandiae b | 136 | 89 | 0.94 | 610 | 45 | 35 | 30 | 20 | 1.88% | [3,12] |
| 5 Casuarius casuarius a | 135 | 92 | 0.97 | 644 | 50 | 35 | 30 | 25 | 1.84% | [3,12] |
| 6 Casuarius unappendiculatus a | 136 | 90 | 0.97 | 604 | 64.35 | 52.65 | 40.7 | 33.3 | 1.26% | [22,27] |
| 7 Apteryx owenii c | 110 | 70 | 0.4 | 295 | 1.9 | 1 | 1.3 | 0.9 | 23.17% | [22,28] |
| 8 Apteryx haastii c | 123 | 77 | 0.53 | 400 | 3.3 | 1.5 | 2.6 | 1.2 | 18.59% | [22,28] |
| 9 Apteryx mantelli c | 125 | 78 | 0.5 | 417 | 3.27 | 2.09 | 2.59 | 1.82 | 17.06% | [22,27] |
| 10 Aepyornis maximus f | 303 | 224 | 3.7 | 9120 | 541 | 334 | 2.08% | [6,25,26] | ||
| 11 Megalapteryx didinus g | 160 | 108 | 1.17 | 1023 | 80 | 28 | 1.89% | [23,30] | ||
| 12 Dinornis novaezealandiae g | 190 | 150 | 1.375 | 2343 | 160 | 91 | 69 | 34 | 2.65% | [10,23,24] |
| 13 Dinornis robustus g | 240 | 178 | 1.23 | 4167 | 275 | 102 | 113 | 61 | 3.03% | [10,23,24] |
| 14 Pachyornis elephantopus g | 221 | 150 | 1.79 | 2725 | 106 | 49 | 3.52% | [23,29] | ||
| 15 Anomalopteryx didiformis g | 165 | 120 | 1.39 | 1302 | 64 | 26 | 2.89% | [23,30] | ||
| 16 Euryapteryx curtus curtus g | 121 | 97 | 0.9 | 624 | 30 | 20 | 20 | 15 | 2.94% | [12,23] |
| 17 Euryapteryx curtus gravis g | 205 | 158 | 1.5 | 2804 | 105 | 67 | 80 | 55 | 3.65% | [12,23] |
| 18 Emeus crassus g | 179 | 134 | 1 | 1761 | 80 | 52 | 50 | 36 | 3.23% | [23,24,31,32] |
| Egg Specimen | Species | Egg Length, B (mm) | Egg Breath, A (mm) | Shell Thickness, t (mm) | Egg Mass, W (g) | Body Mass, M (kg) | ESR | Ref. |
|---|---|---|---|---|---|---|---|---|
| Williams | Genyornis newtoni | 155 | 125 | 1.15 | 1327 | 192 | 0.69% | [14,33] |
| Latagallina naracoortensis | 155 | 125 | 1.15 | 1327 | 6.1 | 21.75% | [13,14,34] | |
| Progura gallinacea | 155 | 125 | 1.15 | 1327 | 7.7 | 17.23% | [13,14,34] | |
| Spooner Egg | Genyornis newtoni | 126 | 97 | 1.3 | 650 | 192 | 0.34% | [13,33] |
| Latagallina naracoortensis | 126 | 97 | 1.3 | 650 | 6.1 | 10.66% | [13,34] | |
| Progura gallinacea | 126 | 97 | 1.3 | 650 | 7.7 | 8.44% | [13,34] |
| Species | Common Name | Egg Length, B (mm) | Egg Width, A (mm) | Shell Thickness, t (mm) | Egg Mass, W (g) | Body Mass, M (g) | Dimensionless Number, C | Factor of Safety, F.S. |
|---|---|---|---|---|---|---|---|---|
| Diomedea Exulans | Wandering Albatross | 129.5 | 79.7 | 0.58 | 455.0 | 8190 | 3185 | 2.16 |
| Phoebastria Nigripes | Black-footed Albatross | 108.2 | 69.2 | 0.50 | 286.0 | 3195 | 6328 | 4.81 |
| Phoebetria Palpebrata | Light-mantled Albatross | 104.0 | 64.5 | 0.48 | 243.0 | 3150 | 5428 | 3.95 |
| Macronectes Giganteus | Southern Giant Petrel | 104.4 | 65.9 | 0.58 | 237.0 | 4395 | 10,865 | 5.61 |
| Aptenodytes Patagonicus | King Penguin | 104.5 | 75.8 | 0.80 | 306.0 | 11,751 | 13,741 | 4.00 |
| Cygnus Columbianus | Tundra Swan | 106.9 | 68.2 | 0.76 | 280.0 | 6750 | 12,655 | 2.41 |
| Pinguinus Impennis | Great auk | 124.0 | 75.8 | 0.74 | 372.0 | 5000 | 8299 | 7.56 |
| Rhea Americana | Greater Rhea | 128.0 | 86.0 | 0.90 | 525.0 | 23,000 | 9637 | 2.80 |
| Casuarius Casuarius | Southern Cassowary | 135.0 | 92.1 | 0.97 | 644.0 | 44,000 | 9108 | 2.12 |
| Dromaius Novaehollandiae | Emu | 136.0 | 89.0 | 0.94 | 610.0 | 34,200 | 8043 | 2.02 |
| Apteryx Australis | Southern Brown Kiwi | 125.8 | 78.5 | 0.50 | 434.0 | 2330 | 3924 | 2.79 |
| Macrocephalon Maleo | Maleo | 105.6 | 61.7 | 0.38 | 222.0 | 1564 | 3488 | 3.20 |
| Gymnogyps Californianus | California Condor | 110.2 | 66.7 | 0.92 | 280.0 | 8450 | 17,911 | 6.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, A.; Wu, H.-J.; Chen, P.-Y.; Yu, H.-T.; Juang, J.-Y. Egg Incubation Mechanics of Giant Birds. Biology 2021, 10, 738. https://doi.org/10.3390/biology10080738
Yen A, Wu H-J, Chen P-Y, Yu H-T, Juang J-Y. Egg Incubation Mechanics of Giant Birds. Biology. 2021; 10(8):738. https://doi.org/10.3390/biology10080738
Chicago/Turabian StyleYen, An, Hsiao-Jou Wu, Pin-Yi Chen, Hon-Tsen Yu, and Jia-Yang Juang. 2021. "Egg Incubation Mechanics of Giant Birds" Biology 10, no. 8: 738. https://doi.org/10.3390/biology10080738
APA StyleYen, A., Wu, H.-J., Chen, P.-Y., Yu, H.-T., & Juang, J.-Y. (2021). Egg Incubation Mechanics of Giant Birds. Biology, 10(8), 738. https://doi.org/10.3390/biology10080738