Changes in Cyclin D1, cdk4, and Their Associated Molecules in Ischemic Pyramidal Neurons in Gerbil Hippocampus after Transient Ischemia and Neuroprotective Effects of Ischemic Preconditioning by Keeping the Molecules in the Ischemic Neurons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Groups
2.3. Surgery of IPC and TI
2.4. Western Blot
2.5. Tissue Preparation for Histological Study
2.6. Histochemistry Using Cresyl Violet
2.7. Fluoro-Jade B Histofluorescence
2.8. Immunohistochemistry
2.9. Double Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. CA1 Pyramidal Cells Died after TI and IPC Protected the Cells from TI
3.1.1. Finding by CV Histochemistry
3.1.2. Findings by NeuN Immunohistochemistry and F-J B Histofluorescence
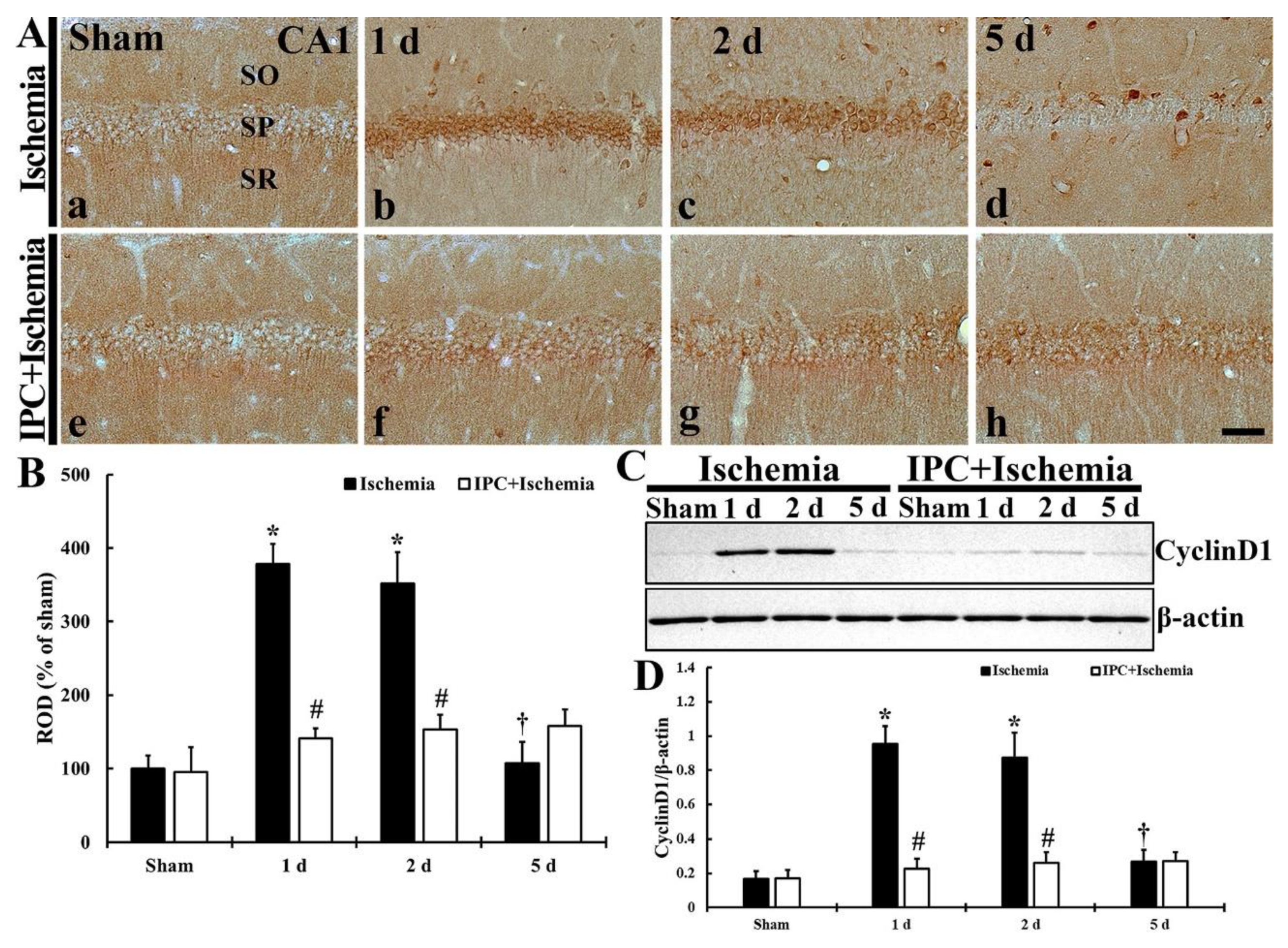
3.2. TI Altered Cyclin D1 Expression and IPC Protected the Change in CA1 Pyramidal Cells
3.2.1. Cyclin D1 Immunoreactivity
3.2.2. Cyclin D1 Protein Level
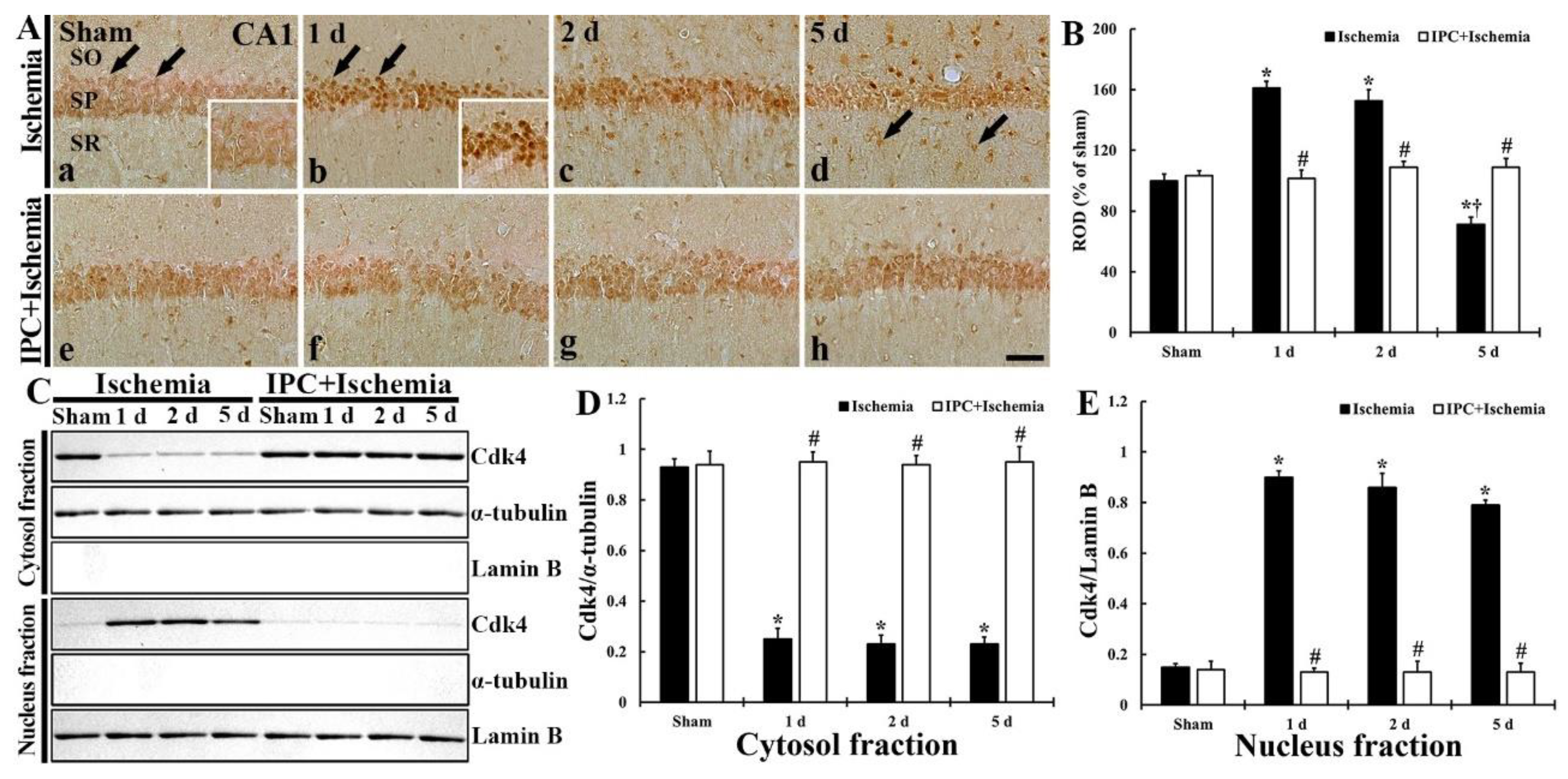
3.3. TI Altered cdk4 Expression and IPC Protected the Change in CA1 Pyramidal Cells
3.3.1. Cdk4 Immunoreactivity
3.3.2. Cdk4 Protein Level
3.3.3. New Expression of cdk4 in Astrocytes
3.4. TI Altered p16INK4a Expression and IPC Protected the Change in CA1 Pyramidal Cells
3.4.1. p16INK4a Immunoreactivity
3.4.2. p16INK4a Protein Level
3.5. TI Altered p-Rb Expression and IPC Protected the Change in CA1 Pyramidal Cells
3.5.1. p-RB Immunoreactivity
3.5.2. p-Rb Protein Level
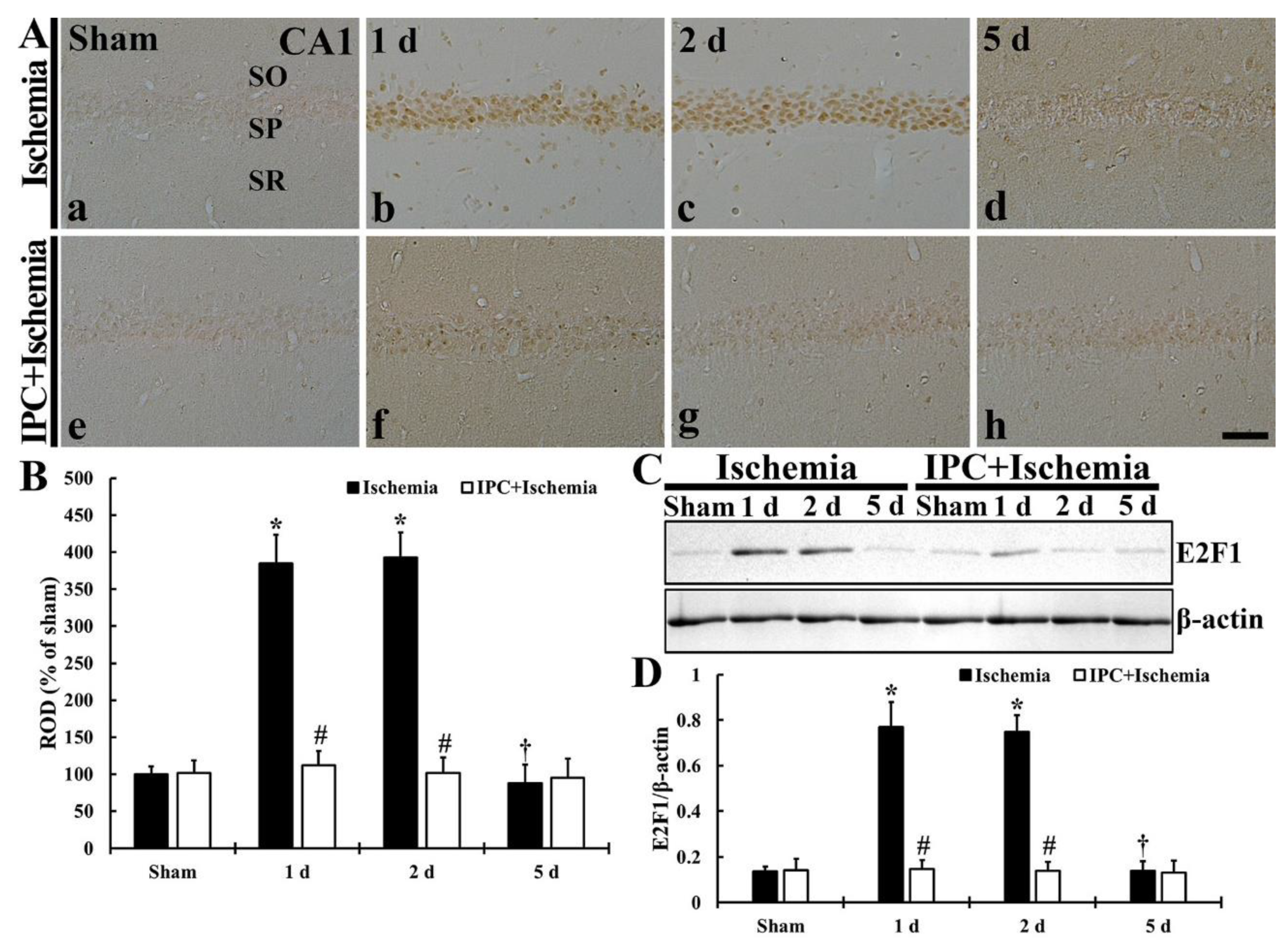
3.6. TI Altered E2F1 Expression and IPC Protected the Change in CA1 Pyramidal Cells
3.6.1. E2F1 Immunoreactivity
3.6.2. E2F1 Protein Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA1 | cornu ammonis 1 |
| CV | cresyl violet |
| Cdk4 | cyclin-dependent kinase 4 |
| E2F1 | E2 promoter binding factor 1 |
| F-JB | fluoro-jade B |
| GFAP | glial fibrillary acidic protein |
| Iba-1 | ionized calcium-binding adapter molecule 1 |
| IPC | ischemic preconditioning |
| NeuN | neuronal nuclear antigen |
| Rb | retinoblastoma |
| OD | optical density |
| ROD | relative optical density |
| SO | stratum oriens |
| SP | stratum pyramidale |
| SR | stratum radiatum |
| TI | transient ischemia. |
References
- Antonawich, F.J.; Fiore, S.M.; Welicky, L.M. Regulation of ischemic cell death by the lipoic acid-palladium complex, poly mva, in gerbils. Exp. Neurol. 2004, 189, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Endres, M.; Namura, S.; Shimizu-Sasamata, M.; Waeber, C.; Zhang, L.; Gomez-Isla, T.; Hyman, B.T.; Moskowitz, M.A. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J. Cereb. Blood Flow Metab. 1998, 18, 238–247. [Google Scholar] [CrossRef]
- Yu, L.; Miao, H.; Hou, Y.; Zhang, B.; Guo, L. Neuroprotective effect of a20 on tnf-induced postischemic apoptosis. Neurochem. Res. 2006, 31, 21–32. [Google Scholar] [PubMed]
- Nitatori, T.; Sato, N.; Waguri, S.; Karasawa, Y.; Araki, H.; Shibanai, K.; Kominami, E.; Uchiyama, Y. Delayed neuronal death in the ca1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 1001–1011. [Google Scholar] [CrossRef]
- Colbourne, F.; Sutherland, G.R.; Auer, R.N. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J. Neurosci. 1999, 19, 4200–4210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohmura, A.; Nakajima, W.; Ishida, A.; Yasuoka, N.; Kawamura, M.; Miura, S.; Takada, G. Prolonged hypothermia protects neonatal rat brain against hypoxic-ischemia by reducing both apoptosis and necrosis. Brain Dev. 2005, 27, 517–526. [Google Scholar] [CrossRef]
- Liu, Y.; Kato, H.; Nakata, N.; Kogure, K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992, 586, 121–124. [Google Scholar] [CrossRef]
- Kirino, T.; Tsujita, Y.; Tamura, A. Induced tolerance to ischemia in gerbil hippocampal neurons. J. Cereb. Blood Flow Metab. 1991, 11, 299–307. [Google Scholar] [CrossRef]
- Nishi, S.; Taki, W.; Uemura, Y.; Higashi, T.; Kikuchi, H.; Kudoh, H.; Satoh, M.; Nagata, K. Ischemic tolerance due to the induction of hsp70 in a rat ischemic recirculation model. Brain Res. 1993, 615, 281–288. [Google Scholar] [CrossRef]
- Gidday, J.M. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006, 7, 437–448. [Google Scholar] [CrossRef]
- Stagliano, N.E.; Perez-Pinzon, M.A.; Moskowitz, M.A.; Huang, P.L. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J. Cereb. Blood Flow Metab. 1999, 19, 757–761. [Google Scholar] [CrossRef]
- Lehotsky, J.; Burda, J.; Danielisova, V.; Gottlieb, M.; Kaplan, P.; Saniova, B. Ischemic tolerance: The mechanisms of neuroprotective strategy. Anat. Rec. 2009, 292, 2002–2012. [Google Scholar] [CrossRef]
- Sun, F.; Johnson, S.R.; Jin, K.; Uteshev, V.V. Boosting endogenous resistance of brain to ischemia. Mol. Neurobiol. 2017, 54, 2045–2059. [Google Scholar] [CrossRef]
- Stetler, R.A.; Zhang, F.; Liu, C.; Chen, J. Ischemic tolerance as an active and intrinsic neuroprotective mechanism. Handb. Clin. Neurol. 2009, 92, 171–195. [Google Scholar] [PubMed]
- Kirino, T.; Nakagomi, T.; Kanemitsu, H.; Tamura, A. Ischemic tolerance. Adv. Neurol. 1996, 71, 505–511. [Google Scholar]
- Nakamura, H.; Katsumata, T.; Nishiyama, Y.; Otori, T.; Katsura, K.; Katayama, Y. Effect of ischemic preconditioning on cerebral blood flow after subsequent lethal ischemia in gerbils. Life Sci. 2006, 78, 1713–1719. [Google Scholar] [CrossRef]
- Della-Morte, D.; Guadagni, F.; Palmirotta, R.; Ferroni, P.; Testa, G.; Cacciatore, F.; Abete, P.; Rengo, F.; Perez-Pinzon, M.A.; Sacco, R.L.; et al. Genetics and genomics of ischemic tolerance: Focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics 2012, 13, 1741–1757. [Google Scholar] [CrossRef] [PubMed]
- Kardesoglu, E.; Isilak, Z.; Uz, O.; Yiginer, O. Ischemic conditioning: A current concept in reducing reperfusion injury. Chin. Med. J. 2011, 124, 480. [Google Scholar] [PubMed]
- Pan, W.; Cox, S.; Hoess, R.H.; Grafstrom, R.H. A cyclin d1/cyclin-dependent kinase 4 binding site within the c domain of the retinoblastoma protein. Cancer Res. 2001, 61, 2885–2891. [Google Scholar] [PubMed]
- Fajas, L. Re-thinking cell cycle regulators: The cross-talk with metabolism. Front. Oncol. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Stoica, B.A.; Byrnes, K.R.; Faden, A.I. Cell cycle activation and cns injury. Neurotox. Res. 2009, 16, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yang, S.H.; Liu, R.; Brun-Zinkernagel, A.M.; Koulen, P.; Simpkins, J.W. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and alzheimer’s disease-like tauopathy in female rats. J. Biol. Chem. 2004, 279, 22684–22692. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, J.; Iyirhiaro, G.O.; Park, D.S. Cell cycle machinery and stroke. BBA Mol. Basis Dis. 2007, 1772, 484–493. [Google Scholar] [CrossRef]
- Broughton, B.R.S.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, E331–E339. [Google Scholar] [CrossRef]
- Sumrejkanchanakij, P.; Tamamori-Adachi, M.; Matsunaga, Y.; Eto, K.; Ikeda, M.A. Role of cyclin d1 cytoplasmic sequestration in the survival of postmitotic neurons. Oncogene 2003, 22, 8723–8730. [Google Scholar] [CrossRef][Green Version]
- Kranenburg, O.; van der Eb, A.J.; Zantema, A. Cyclin d1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996, 15, 46–54. [Google Scholar] [CrossRef]
- Li, Y.; Chopp, M.; Powers, C.; Jiang, N. Immunoreactivity of cyclin d1/cdk4 in neurons and oligodendrocytes after focal cerebral ischemia in rat. J. Cereb. Blood Flow Metab. 1997, 17, 846–856. [Google Scholar] [CrossRef]
- Timsit, S.; Rivera, S.; Ouaghi, P.; Guischard, F.; Tremblay, E.; Ben-Ari, Y.; Khrestchatisky, M. Increased cyclin d1 in vulnerable neurons in the hippocampus after ischaemia and epilepsy: A modulator of in vivo programmed cell death? Eur. J. Neurosci. 1999, 11, 263–278. [Google Scholar] [CrossRef]
- Sakurai, M.; Hayashi, T.; Abe, K.; Itoyama, Y.; Tabayashi, K.; Rosenblum, W.I. Cyclin d1 and cdk4 protein induction in motor neurons after transient spinal cord ischemia in rabbits. Stroke 2000, 31, 200–207. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Chopp, M.; Powers, C. Granule cell apoptosis and protein expression in hippocampal dentate gyrus after forebrain ischemia in the rat. J. Neurol. Sci. 1997, 150, 93–102. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.H.; Maharjan, S.; Park, J.A.; Choi, K.S.; Park, H.; Jeong, Y.; Ahn, J.H.; Kim, I.H.; Lee, J.C.; et al. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J. Neuroinflamm. 2017, 14, 122. [Google Scholar] [CrossRef]
- Guo, P.; Jin, Z.; Wu, H.; Li, X.; Ke, J.; Zhang, Z.; Zhao, Q. Effects of irisin on the dysfunction of blood-brain barrier in rats after focal cerebral ischemia/reperfusion. Brain Behav. 2019, 9, e01425. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regen. Res. 2019, 14, 1394–1403. [Google Scholar] [PubMed]
- Seo, W.J.; Ahn, J.H.; Lee, T.K.; Kim, B.; Lee, J.C.; Park, J.H.; Yoo, Y.H.; Shin, M.C.; Cho, J.H.; Won, M.H.; et al. High fat diet accelerates and exacerbates microgliosis and neuronal damage/death in the somatosensory cortex after transient forebrain ischemia in gerbils. Lab. Anim. Res. 2020, 36, 28. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Merchenthaler, I. Estrogen prevents the loss of ca1 hippocampal neurons in gerbils after ischemic injury. Neuroscience 2003, 116, 851–861. [Google Scholar] [CrossRef]
- Stanojlovic, M.; Gusevac, I.; Grkovic, I.; Zlatkovic, J.; Mitrovic, N.; Zaric, M.; Horvat, A.; Drakulic, D. Effects of chronic cerebral hypoperfusion and low-dose progesterone treatment on apoptotic processes, expression and subcellular localization of key elements within akt and erk signaling pathways in rat hippocampus. Neuroscience 2015, 311, 308–321. [Google Scholar] [CrossRef]
- O’Hare, M.J.; Kushwaha, N.; Zhang, Y.; Aleyasin, H.; Callaghan, S.M.; Slack, R.S.; Albert, P.R.; Vincent, I.; Park, D.S. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J. Neurosci. 2005, 25, 8954–8966. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, I.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Cho, G.S.; Tae, H.J.; Chen, B.H.; Yan, B.C.; Yoo, K.Y.; et al. Ischemic preconditioning protects hippocampal pyramidal neurons from transient ischemic injury via the attenuation of oxidative damage through upregulating heme oxygenase-1. Free Radic. Biol. Med. 2015, 79, 78–90. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, J.H.; Kim, I.H.; Cho, G.S.; Ahn, J.H.; Tae, H.J.; Choi, S.Y.; Cho, J.H.; Kim, D.W.; Kwon, Y.G.; et al. Neuroprotection of ischemic preconditioning is mediated by thioredoxin 2 in the hippocampal ca1 region following a subsequent transient cerebral ischemia. Brain Pathol. 2017, 27, 276–291. [Google Scholar] [CrossRef]
- Nedergaard, M.; Jakobsen, J.; Diemer, N.H. Autoradiographic determination of cerebral glucose content, blood flow, and glucose utilization in focal ischemia of the rat brain: Influence of the plasma glucose concentration. J. Cereb. Blood Flow Metab. 1988, 8, 100–108. [Google Scholar] [CrossRef]
- Neigh, G.N.; Glasper, E.R.; Kofler, J.; Traystman, R.J.; Mervis, R.F.; Bachstetter, A.; DeVries, A.C. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in ca1 pyramidal cells and selectively alters acquisition of spatial memory. Eur. J. Neurosci. 2004, 20, 1865–1872. [Google Scholar] [CrossRef]
- Kirino, T.; Sano, K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Lee, J.C.; Kim, D.W.; Kim, B.; Sim, H.; Kim, J.D.; Ahn, J.H.; Park, J.H.; Lee, C.H.; Won, M.H.; et al. Ischemia-reperfusion under hyperthermia increases heme oxygenase-1 in pyramidal neurons and astrocytes with accelerating neuronal loss in gerbil hippocampus. Int. J. Mol. Sci. 2021, 22, 3963. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Kassell, N.F.; Lee, K.S. Induction of ischemic tolerance and antioxidant activity by brief focal ischemia. Neuroreport 1997, 8, 847–851. [Google Scholar] [CrossRef]
- Guegan, C.; Levy, V.; David, J.P.; Ajchenbaum-Cymbalista, F.; Sola, B. C-jun and cyclin d1 proteins as mediators of neuronal death after a focal ischaemic insult. Neuroreport 1997, 8, 1003–1007. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoo, K.Y.; Choi, J.H.; Park, O.K.; Hwang, I.K.; Choi, S.Y.; Kim, D.H.; Won, M.H. Cyclin d1 immunoreactivity changes in ca1 pyramidal neurons and dentate granule cells in the gerbil hippocampus after transient forebrain ischemia. Neurol. Res. 2011, 33, 93–100. [Google Scholar] [CrossRef]
- Wiessner, C.; Brink, I.; Lorenz, P.; Neumann-Haefelin, T.; Vogel, P.; Yamashita, K. Cyclin d1 messenger rna is induced in microglia rather than neurons following transient forebrain ischaemia. Neuroscience 1996, 72, 947–958. [Google Scholar] [CrossRef]
- Small, D.L.; Monette, R.; Fournier, M.C.; Zurakowski, B.; Fiander, H.; Morley, P. Characterization of cyclin d1 expression in a rat global model of cerebral ischemia. Brain Res. 2001, 900, 26–37. [Google Scholar] [CrossRef]
- Kato, H.; Takahashi, A.; Itoyama, Y. Cell cycle protein expression in proliferating microglia and astrocytes following transient global cerebral ischemia in the rat. Brain Res. Bull. 2003, 60, 215–221. [Google Scholar] [CrossRef]
- Osuga, H.; Osuga, S.; Wang, F.; Fetni, R.; Hogan, M.J.; Slack, R.S.; Hakim, A.M.; Ikeda, J.E.; Park, D.S. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 2000, 97, 10254–10259. [Google Scholar] [CrossRef]
- Love, S. Neuronal expression of cell cycle-related proteins after brain ischaemia in man. Neurosci. Lett. 2003, 353, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, J.; Iyirhiaro, G.; Aleyasin, H.; Rios, M.; Vincent, I.; Callaghan, S.; Bland, R.J.; Slack, R.S.; During, M.J.; Park, D.S. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 14080–14085. [Google Scholar] [CrossRef] [PubMed]
- Katchanov, J.; Harms, C.; Gertz, K.; Hauck, L.; Waeber, C.; Hirt, L.; Priller, J.; von Harsdorf, R.; Bruck, W.; Hortnagl, H.; et al. Mild cerebral ischemia induces loss of cyclin-dependent kinase inhibitors and activation of cell cycle machinery before delayed neuronal cell death. J. Neurosci. 2001, 21, 5045–5053. [Google Scholar] [CrossRef] [PubMed]
- Zindy, F.; Soares, H.; Herzog, K.H.; Morgan, J.; Sherr, C.J.; Roussel, M.F. Expression of ink4 inhibitors of cyclin d-dependent kinases during mouse brain development. Cell Growth Differ. 1997, 8, 1139–1150. [Google Scholar] [PubMed]
- Watanabe, G.; Pena, P.; Shambaugh, G.E., III; Haines, G.K., III; Pestell, R.G. Regulation of cyclin dependent kinase inhibitor proteins during neonatal cerebella development. Brain Res. Dev. Brain Res. 1998, 108, 77–87. [Google Scholar] [CrossRef]
- Connell-Crowley, L.; Harper, J.W.; Goodrich, D.W. Cyclin d1/cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell 1997, 8, 287–301. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, X.; Ren, Q.G.; Yi, C.J.; Yu, Z.Y.; Xie, X.W.; Wang, W. The involvement of upregulation and translocation of phospho-rb in early neuronal apoptosis following focal cerebral ischemia in rats. Neurochem. Res. 2009, 34, 1113–1119. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, Q.G.; Zhang, Z.H.; Zhou, K.; Yu, Z.Y.; Luo, X.; Wang, W. Phospho-rb mediating cell cycle reentry induces early apoptosis following oxygen-glucose deprivation in rat cortical neurons. Neurochem. Res. 2012, 37, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Morris, E.J.; Bremner, R.; Keramaris, E.; Padmanabhan, J.; Rosenbaum, M.; Shelanski, M.L.; Geller, H.M.; Greene, L.A. Involvement of retinoblastoma family members and e2f/dp complexes in the death of neurons evoked by DNA damage. J. Neurosci. 2000, 20, 3104–3114. [Google Scholar] [CrossRef]
- Trimarchi, J.M.; Lees, J.A. Sibling rivalry in the e2f family. Nat. Rev. Mol. Cell Biol. 2002, 3, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Mealing, G.A.; Paris, J.; Lou, A.; Edwards, A.; Hou, S.T.; MacManus, J.P.; Hakim, A.M.; Morley, P. Attenuation of neurotoxicity in cortical cultures and hippocampal slices from e2f1 knockout mice. J. Neurochem. 2001, 78, 316–324. [Google Scholar] [CrossRef] [PubMed]
- MacManus, J.P.; Jian, M.; Preston, E.; Rasquinha, I.; Webster, J.; Zurakowski, B. Absence of the transcription factor e2f1 attenuates brain injury and improves behavior after focal ischemia in mice. J. Cereb. Blood Flow Metab. 2003, 23, 1020–1028. [Google Scholar] [CrossRef]
- MacManus, J.P.; Koch, C.J.; Jian, M.; Walker, T.; Zurakowski, B. Decreased brain infarct following focal ischemia in mice lacking the transcription factor e2f1. Neuroreport 1999, 10, 2711–2714. [Google Scholar] [CrossRef]
- Cuomo, O.; Casamassa, A.; Brancaccio, P.; Laudati, G.; Valsecchi, V.; Anzilotti, S.; Vinciguerra, A.; Pignataro, G.; Annunziato, L. Sumoylation of sodium/calcium exchanger in brain ischemia and ischemic preconditioning. Cell Calcium 2020, 87, 102195. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, S.; Bigdeli, M.R. Anti-excitotoxic effects of cannabidiol are partly mediated by enhancement of ncx2 and ncx3 expression in animal model of cerebral ischemia. Eur. J. Pharmacol. 2017, 794, 270–279. [Google Scholar] [CrossRef]
- Lee, J.C.; Cho, J.H.; Kim, I.H.; Ahn, J.H.; Park, J.H.; Cho, G.S.; Chen, B.H.; Shin, B.N.; Tae, H.J.; Park, S.M.; et al. Ischemic preconditioning inhibits expression of na(+)/h(+) exchanger 1 (nhe1) in the gerbil hippocampal ca1 region after transient forebrain ischemia. J. Neurol. Sci. 2015, 351, 146–153. [Google Scholar] [CrossRef]
- Lee, J.C.; Tae, H.J.; Kim, I.H.; Cho, J.H.; Lee, T.K.; Park, J.H.; Ahn, J.H.; Choi, S.Y.; Bai, H.C.; Shin, B.N.; et al. Roles of hif-1alpha, vegf, and nf-kappab in ischemic preconditioning-mediated neuroprotection of hippocampal ca1 pyramidal neurons against a subsequent transient cerebral ischemia. Mol. Neurobiol. 2017, 54, 6984–6998. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-K.; Kim, D.W.; Lee, J.-C.; Park, C.W.; Sim, H.; Ahn, J.H.; Park, J.H.; Shin, M.C.; Cho, J.H.; Lee, C.-H.; et al. Changes in Cyclin D1, cdk4, and Their Associated Molecules in Ischemic Pyramidal Neurons in Gerbil Hippocampus after Transient Ischemia and Neuroprotective Effects of Ischemic Preconditioning by Keeping the Molecules in the Ischemic Neurons. Biology 2021, 10, 719. https://doi.org/10.3390/biology10080719
Lee T-K, Kim DW, Lee J-C, Park CW, Sim H, Ahn JH, Park JH, Shin MC, Cho JH, Lee C-H, et al. Changes in Cyclin D1, cdk4, and Their Associated Molecules in Ischemic Pyramidal Neurons in Gerbil Hippocampus after Transient Ischemia and Neuroprotective Effects of Ischemic Preconditioning by Keeping the Molecules in the Ischemic Neurons. Biology. 2021; 10(8):719. https://doi.org/10.3390/biology10080719
Chicago/Turabian StyleLee, Tae-Kyeong, Dae Won Kim, Jae-Chul Lee, Cheol Woo Park, Hyejin Sim, Ji Hyeon Ahn, Joon Ha Park, Myoung Cheol Shin, Jun Hwi Cho, Choong-Hyun Lee, and et al. 2021. "Changes in Cyclin D1, cdk4, and Their Associated Molecules in Ischemic Pyramidal Neurons in Gerbil Hippocampus after Transient Ischemia and Neuroprotective Effects of Ischemic Preconditioning by Keeping the Molecules in the Ischemic Neurons" Biology 10, no. 8: 719. https://doi.org/10.3390/biology10080719
APA StyleLee, T.-K., Kim, D. W., Lee, J.-C., Park, C. W., Sim, H., Ahn, J. H., Park, J. H., Shin, M. C., Cho, J. H., Lee, C.-H., Won, M.-H., & Choi, S. Y. (2021). Changes in Cyclin D1, cdk4, and Their Associated Molecules in Ischemic Pyramidal Neurons in Gerbil Hippocampus after Transient Ischemia and Neuroprotective Effects of Ischemic Preconditioning by Keeping the Molecules in the Ischemic Neurons. Biology, 10(8), 719. https://doi.org/10.3390/biology10080719







