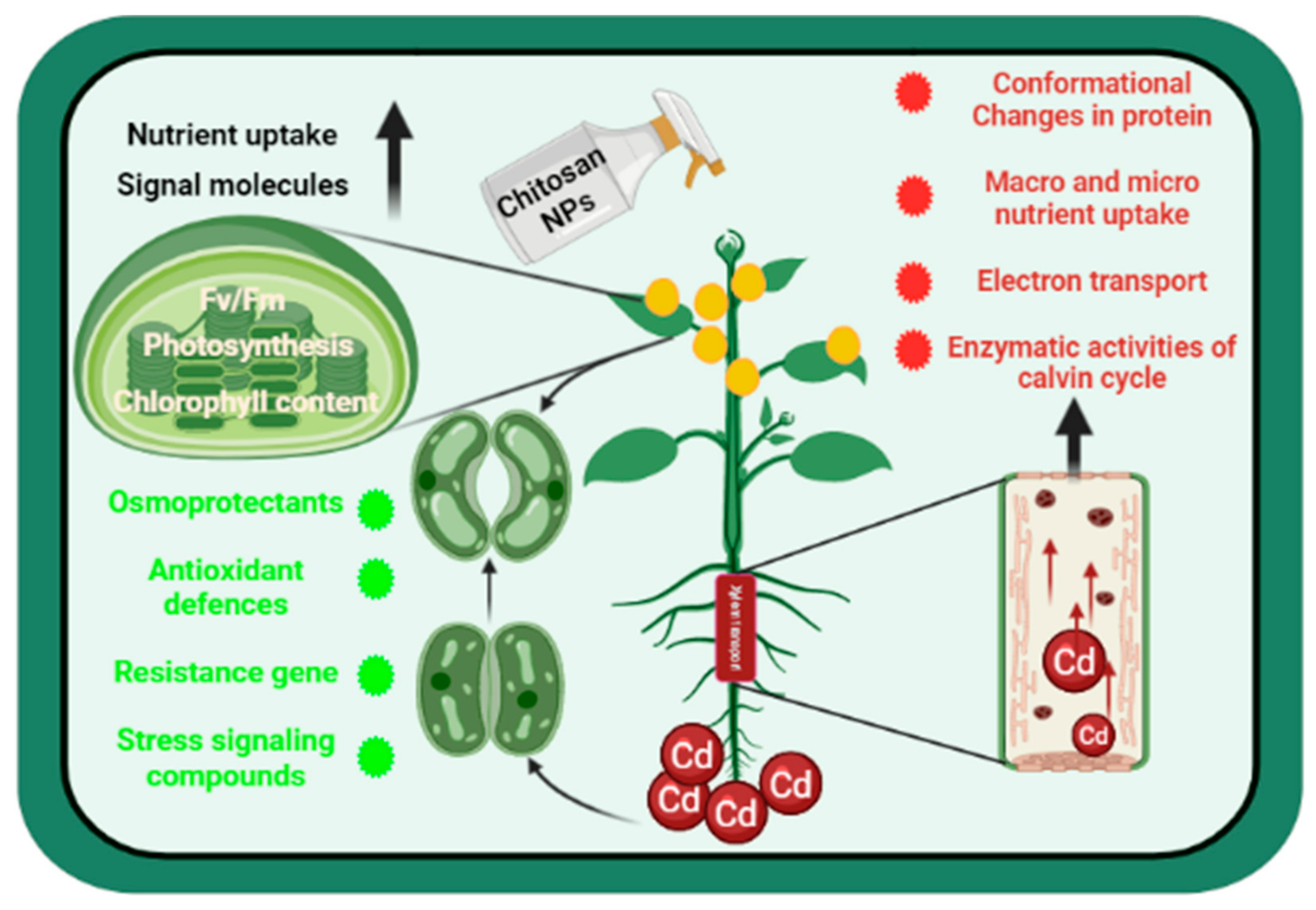
Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle
2.2. Solanum lycopersicum Cultural and Treatment Pattern
2.3. Shoot and Root Dry Weight
2.4. SPAD Index Measurement (Chlorophyll Content Estimation)
2.5. Chlorophyll Fluorescence
2.6. Leaf Gas Exchange Characteristics
2.7. Lipid Peroxidation (MDA) and Hydrogen Peroxide (H2O2) Determination
2.8. Antioxidant Enzymes
2.9. Glutathione and Ascorbic Acid
2.10. Leaf Protein and Proline
2.11. Plant Cd Concentration
2.12. Statistical Analysis
3. Results
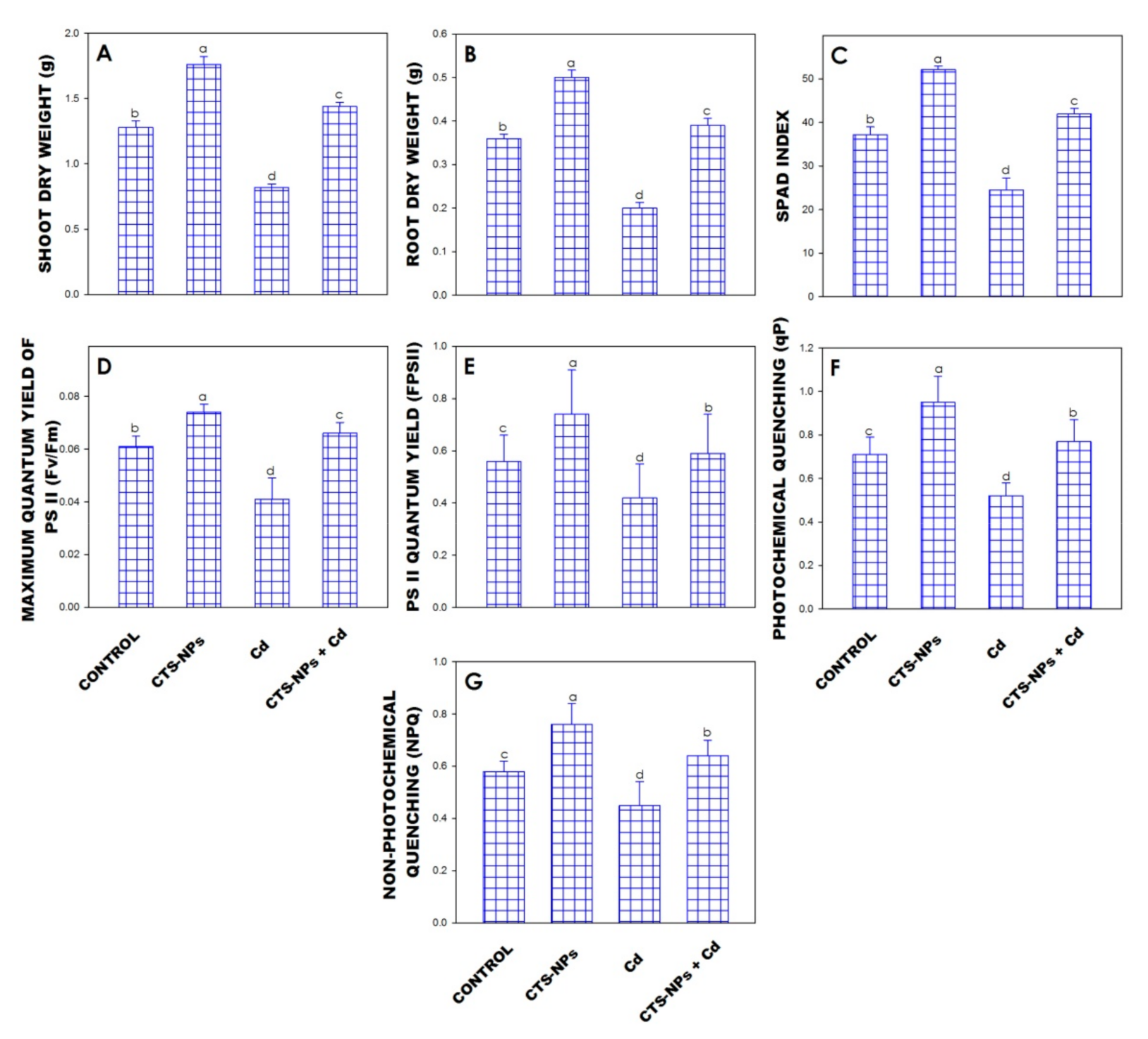
3.1. Dry Weight
3.2. SPAD Index
3.3. Chlorophyll Fluorescence
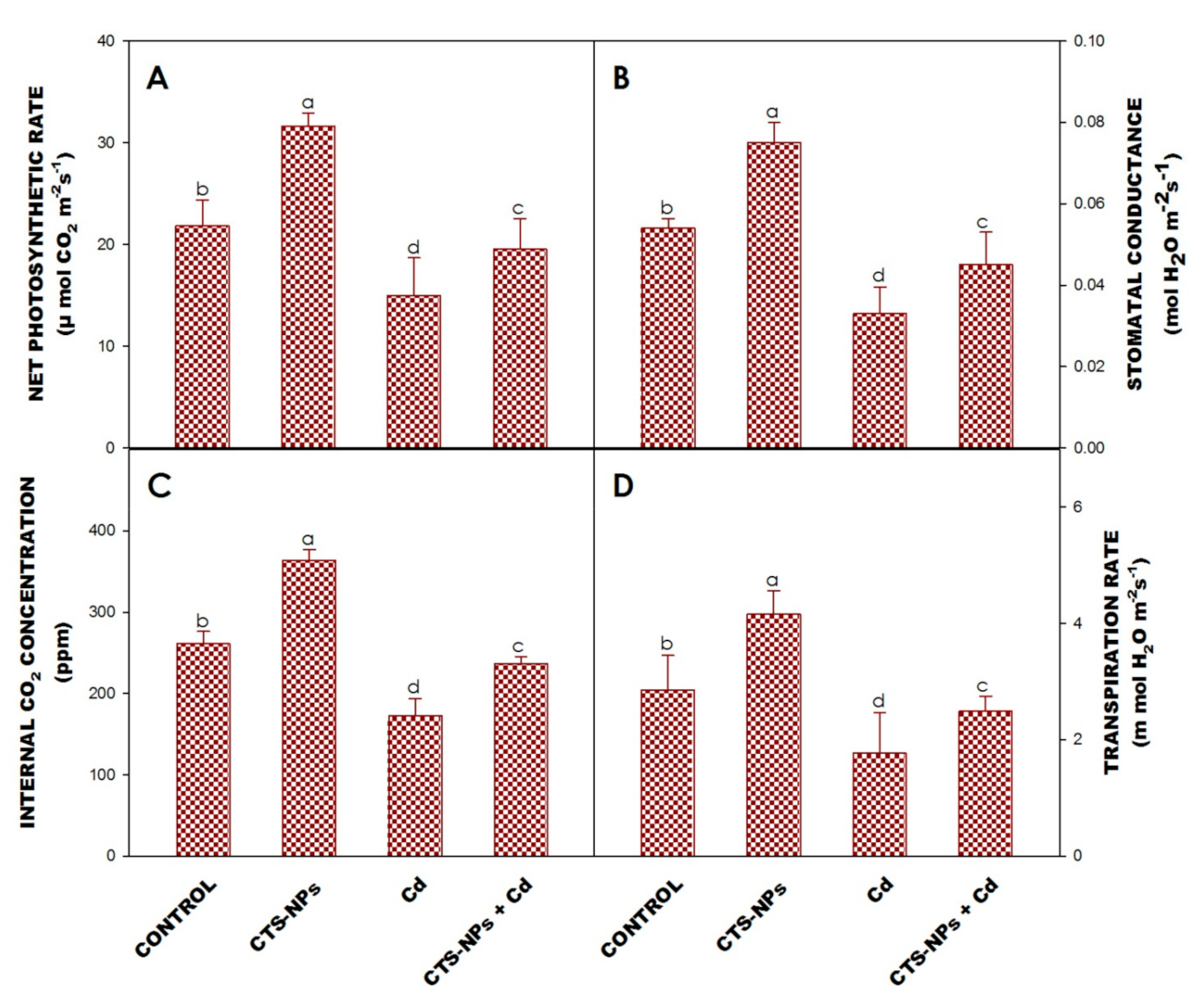
3.4. Leaf Gas Exchange Characteristics
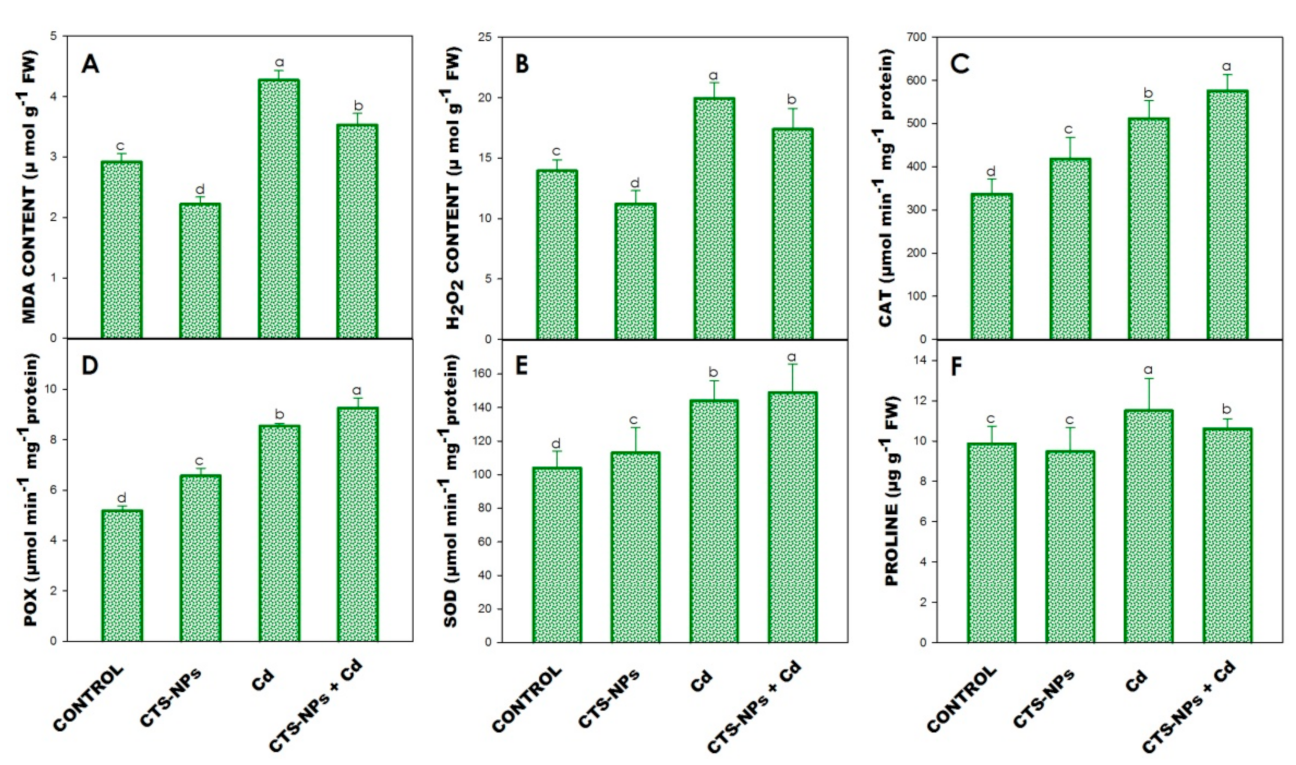
3.5. Lipid Peroxidation (MDA) and Hydrogen Peroxide (H2O2) Determination
3.6. Antioxidant Enzymes
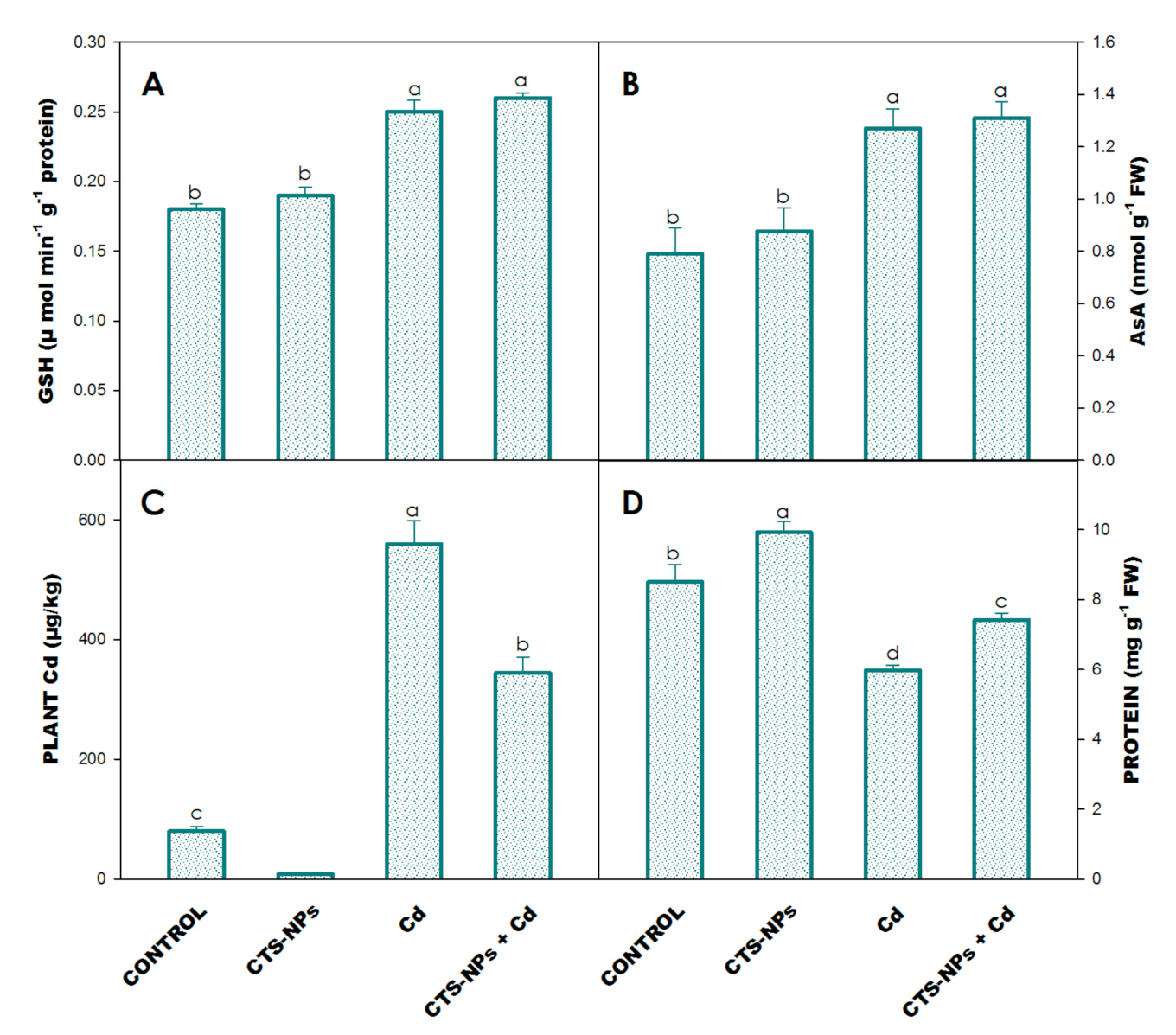
3.7. Glutathione and Ascorbic Acid
3.8. Leaf Protein and Proline
3.9. Plant Cd Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valdez, F.; Fernández-Luqueño, F. (Eds.) Agricultural Nanobiotechnology; Springer: Basingstoke, UK, 2018; ISBN 9783319967189. [Google Scholar]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc Oxide Nanoparticles Alleviate Drought-Induced Alterations in Sorghum Performance, Nutrient Acquisition, and Grain Fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Ahmed, B.; Sushkova, S.; Singh, R.; Soldatov, M.; Laratte, B.; Fedorenko, A.; Mandzhieva, S.; Blicharska, E.; et al. Interaction of Copper-Based Nanoparticles to Soil, Terrestrial, and Aquatic Systems: Critical Review of the State of the Science and Future Perspectives. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2020; pp. 51–96. [Google Scholar]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Santana, R.V.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on the Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO Nanoparticles: A Threat to Soil Organism, Plants, and Human Health. Environ. Geochem. Health 2020, 42, 147–158. [Google Scholar] [CrossRef]
- Marx, S.; Zaltsman, A.; Turyan, I.; Mandler, D. Parathion Sensor Based on Molecularly Imprinted Sol-Gel Films. Ann. Chem. 2004, 76, 120–126. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Fedorenko, A.; Sushkova, S.; Mandzhieva, S.; Lysenko, V.; Duplii, N.; Fedorenko, G.; Dvadnenko, K.; Ghazaryan, K. Toxicity of Copper Oxide Nano-Particles on Spring Barley (Hordeum sativum distichum). Sci. Total Environ. 2018, 645, 1103–1113. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of Nanoparticles on Crops and Soil Microbial Communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of Zinc-Oxide Nanoparticles on Soil, Plants, Animals and Soil Organisms: A Review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, M.; Ma, C.; White, J.C.; Hao, Y.; Wang, Y.; Tang, X.; Yang, J.; Jiang, F.; Ali, A.; Rui, Y.; et al. Metal Oxide Nanoparticles Alter Peanut (Arachis hypogaea L.) Physiological Response and Reduce Nutritional Quality: A Life Cycle Study. Environ. Sci. Nano 2018, 5, 2088–2102. [Google Scholar] [CrossRef]
- Li, M.; Adeel, M.; Peng, Z.; Yukui, R. Physiological Impacts of Zero Valent Iron, Fe3O4 and Fe2O3 Nanoparticles in Rice Plants and their Potential as Fe Fertilizers. Environ. Pollut. 2020, 269, 116134. [Google Scholar]
- Hao, Y.; Fang, P.; Ma, C.; White, J.C.; Xiang, Z.; Wang, H.; Zhang, Z.; Rui, Y.; Xing, B. Engineered Nanomaterials Inhibit Podosphaera pannosa Infection on Rose Leaves by Regulating Phytohormones. Environ. Res. 2019, 170, 1–6. [Google Scholar] [CrossRef]
- Adeel, M.; Farooq, T.; White, J.; Hao, Y.; He, Z.; Rui, Y. Carbon-Based Nanomaterials Suppress Tobacco Mosaic Virus (TMV) Infection and Induce Resistance in Nicotiana benthamian. J. Hazard. Mater. 2020, 404, 124167. [Google Scholar]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and Challenges for Nanotechnology in the Agri-Tech Revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in Agriculture: Current Status, Challenges and Future Opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-Enabled Improvements of Growth and Nutritional Quality in Food Plants Driven by Rhizosphere Processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A.; Mostafa, M.; Almoammar, H.; Abd-Elsalam, K.A. Chitosan-Based Nanostructures in Plant Protection Applications. In Nanobiotechnology Applications in Plant Protection; Springer: Cham, Switzerland, 2018; pp. 351–384. [Google Scholar]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Kumaraswamy, R.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered Chitosan Based Nanomaterials: Bioactivities, Mechanisms and Perspectives in Plant Protection and Growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-Based Nanomaterials: A State-of-the-Art Review. Int. J. Biol. Macro. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Malathi, B.; Mona, S.; Thiyagarajan, D.; Kaliraj, P. Immunopotentiatingnano-Chitosan as Potent Vaccine Carter for Efficacious Prophylaxis of Filarial Antigens. Int. J. Boil. Macromol. 2015, 73, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Abou Kana, M.T.H.; Hendy, A.A. Synthesis and Implementation of Nano-Chitosan and its Acetophenone Derivative for Enhanced Removal of Metals. Int. J. Boil. Macromol. 2015, 81, 672–680. [Google Scholar] [CrossRef]
- Safari, J.; Azizi, F.; Sadeghi, M. Chitosan Nanoparticles as a Green and Renewable Catalyst in the Synthesis of 1,4-Dihydropyridine under Solvent-Free Conditions. New J. Chem. 2015, 39, 1905–1909. [Google Scholar] [CrossRef]
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of Salinity Stress Response in Mung Bean through Solid Matrix Priming with Normal and Nanosized Chitosan. Int. J. Biol. Macromol. 2020, 145, 108–123. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Sun, X.; Zang, X.; Zhang, X.; Fang, T.; Xu, N. Comparative Transcriptome Analysis Reveals Chitooligosaccharides-Induced Stress Tolerance of Gracilariopsis lemaneiformis under High Temperature Stress. Aquaculture 2020, 519, 734876. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and in Vitro Characterization of Chitosan Nanoparticles and their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan Nanoparticle Based Delivery Systems for Sustainable Agriculture. Int. J. Biol. Macro. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawijorf, S.A.; Mallihipour, A.; Saremi, H.; Nikkhah, M. Application of Chitosan and Chitosan Nanoparticles for the Control of Fusarium Head Blight of Wheat (Fusarium graminearum) in Vitro and Green House. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V.; Kumarasamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-Chitosan Nanoparticle Mediated Sustainable Approach to Enhance Seedling Growth in Maize by Mobilizing Reserve Food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of Soil and Water Pollution on Food Safety and Health Risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of Multiple Heavy Metal-Contaminated Soil through the Combination of Soil Washing and in Situ Immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Sonia, A.; Muhammad, A.; Aqeel, A.; Waheed, U.K.; Nasim, A.Y.; Basharat, A.; Muhammad, R.; Shafaqat, A. Combined Effect of Bacillus fortis IAGS 223 and Zinc Oxide Nanoparticles to Alleviate Cadmium Phytotoxicity in Cucumis melo. Plant Physiol. Biochem. 2021, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Tanveer, A.K.; Samavia, M.; Iqra, S.; Waheed, A.; Taiba, S.; Zoobia, B.; Rui, W.; Mufid, A.; Shakeel, A.; et al. Metabolic and Proteomic Perspectives of Augmentation of Nutritional Contents and Plant Defense in Vigna unguiculata. Biomolecules 2020, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of Zinc Oxide Nanoparticles in Countering Negative Effects Generated by Cadmium in Lycopersicon esculentum. J. Plant Grow. Regul. 2020, 40, 101–115. [Google Scholar] [CrossRef]
- Ahmad, A.; Iqra, S.; Samavia, M.; Nasim, A.Y.; Waheed, A.; Waheed, U.K.; Tingquan, W. Karrikinolide Alleviates BDE-28, Heat and Cd Stressors in Brassica alboglabra by Correlating and Modulating Biochemical Attributes, Antioxidative Machinery and Osmoregulators. Ecotoxicol. Environ. Saf. 2021, 213, 112047. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An Explanation of Soil Amendments to Reduce Cadmium Phytoavailability and Transfer to Food Chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Angadi, V.; Rai, P.K.; Bara, B.M. Effect of Organic Manures and Biofertilizers on Plant Growth, Seed Yield and Seedling Characteristics in Tomato (Lycopersicon esculentum Mill.). J. Pharmacogn. Phytochem. 2017, 6, 807–810. [Google Scholar]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Tan, J.; Kerr, W.L. Rheological Properties and Microstructure of Tomato Puree Subject to Continuous High-Pressure Homogenization. J. Food Eng. 2015, 166, 45–54. [Google Scholar] [CrossRef]
- Marti, R.; Leiva-Brondo, M.; Lahoz, I.; Campillo, C.; Cebolla Cornejo, J.; Rosello, S. Polyphenol and L-ascorbic Acid Content in Tomato as Influenced by High Lycopene Genotypes and Organic Farming at Different Environments. Food Chem. 2018, 239, 148–156. [Google Scholar] [CrossRef]
- Colak, N.G.; Eken, N.T.; Ulger, M.; Frary, A.; Doganlar, S. Mapping of Quantitative Trait Loci for Antioxidant Molecules in Tomato Fruit: Carotenoids, Vitamins C and E, Glutathione, and Phenolic Acids. Plant Sci. 2020, 292, 110393. [Google Scholar]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles and 24-Epibrassinolide Alleviates Cu Toxicity in Tomato by Regulating ROS Scavenging, Stomatal Movement and Photosynthesis. Ecotox. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of Catalase and Peroxidases. Methods Enzymol. 1956, 2, 764–775. [Google Scholar]
- Beauchamp, C.O.; Fridovich, I. Superoxide Dismutase: Improved Assays and Assays Applicable to Acrylamide Gel. Ann. Clin. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inze, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water Stress Studies. Plant Sci. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging Investigator Series: Polymeric Nanocarriers for Agricultural Applications: Synthesis, Characterization, and Environmental and Biological Interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Li, K.; Liu, S.; Xing, R.; Qin, Y.; Yu, H.; Zhou, M.; Li, P. Effect of Chitooligosaccharides with Different Degrees of Acetylation on Wheat Seedlings under Salt Stress. Carbohydr. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Bernstein, N.; Anusuya, S. Chitosan Elicitation for Increased Curcumin Production and Stimulation of Defence Response in Turmeric (Curcuma longa L.). Ind. Crops Prod. 2016, 89, 87–94. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of Cadmium Toxicity and Enhancing Cadmium-Tolerance in Wheat Seedlings by Exogenous Application of Polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic Acid-Induced Protection Against Cadmium Toxicity in Wheat Plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Perez-de-Luque, A. Interaction of Nanomaterials with Plants: What do We Need for Real Applications in Agriculture? Front. Plant Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; Jassby, D. Delivery, Uptake, Fate and Transport of Engineered Nanoparticles in Plants: A Critical Review and Data Analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Sakthikumar, D. Nanoparticulate Material Delivery to Plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Wang, W.N.; Tarafdar, J.C.; Biswas, P. Nanoparticle Synthesis and Delivery by an Aerosol Route for Watermelon Plant Foliar Uptake. J. Nanopart. Res. 2013, 15, 1417. [Google Scholar] [CrossRef]
- Gu, L.; Li, C.; Gao, F.; Dong, Y.; Hu, B. Effect of Chitosan under the Cadmium Stress on the Physiological and Biochemical Index of Flamingo anthurium. J. Anhui Agric. Sci. 2010, 38, 8934–8935. [Google Scholar]
- Shaheen, S.M.; Rinklebe, J. Impact of Emerging and Low Cost Alternative Amendments on the (im)Mobilization and Phytoavailability of Cd and Pb in a Contaminated Floodplain Soil. Ecol. Eng. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Dzung, P.D.; Phu, D.V.; Du, B.D.; Ngoc, L.S.; Duy, N.N.; Hiet, H.D.; Nghia, D.H.; Thang, N.T.; Le, V.B.; Hien, N.Q. Effect of Foliar Application of Oligochitosan with Different Molecular Weight on Growth Promotion and Fruit Yield Enhancement of Chili Plant. Plant Prod. Sci. 2017, 20, 389–395. [Google Scholar] [CrossRef]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchanwan, S. Effect of Chitosan on Floral Production, Gene Expression and Anatomical Changes in the Dendrobium orchid. Sci. Hortic-Amst. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous Application of Chitosan on Biochemical and Physiological Characteristics, Phenolic Content and Antioxidant Activity of Two Species of Basil (Ocimum ciliatum and Ocimum basilicum) under Reduced Irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Farouk, S.; Ghoneem, K.M.; Ali Abeer, A. Induction and Expression of Systematic Resistance to Downy Mildew Disease in Cucumber Plant by Elicitors. Egypt J. Phytopathol. 2008, 2, 95–111. [Google Scholar]
- Farouk, S.; Mosa, A.A.; Taha, A.A.; Ibrahim Heba, M.; EL-Gahmery, A.M. Protective Effect of Humic Acid and Chitosan on Radish (Raphanus sativus L. var. sativus) Plants Subjected to Cadmium Stress. J. Stress Physiol. Biochem. 2011, 7, 99–116. [Google Scholar]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Ali, S.; Rafiq, M.T.; Zhou, W. Hydrogen Sulfide Alleviates Cadmium-Induced Morpho-Physiological and Ultrastructural Changes in Brassica napus. Ecotoxicol. Environ. Saf. 2014, 110, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective Effect of Chitosan on Photosynthesis and Antioxidative Defense System in Edible Rape (Brassica rape L.) in the Presence of Cadmium. Ecotox. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A Vital Role of Chitosan Nanoparticles in Improvisation the Drought Stress Tolerance in Catharanthus roseus (L.) through Biochemical and Gene Expression Modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Liu, J.; Gai, L.; Zong, H. Foliage Application of Chitosan Alleviates the Adverse Effects of Cadmium Stress in Wheat Seedlings (Triticum aestivum L.). Plant Physiol. Biochem. 2021, 164, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemic, M. Interactive Effects of Drought Stress and Chitosan Application on Physiological Characteristics and Essential Oil Yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Improving Seed Germination of Salicornia rubra. (Chenopodiaceae) under Saline Conditions using Germination Regulating Chemicals. West. N. Am. Nat. 2002, 62, 101–105. [Google Scholar]
- Yang, F.; Hu, J.; Li, J.; Wu, X.; Qian, Y. Chitosan Enhances Leaf Membrane Stability and Antioxidant Enzyme Activities in Apple Seedlings under Drought Stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- Cho, U.H.; Seo, N.H. Oxidative stress in Arabidopsis thaliana Exposed to Cadmium is due to Hydrogen Peroxide Accumulation. Plant Sci. 2005, 168, 113–120. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the Challenges of Abiotic Stress in Plants: New Dimensions in the Field Application of Nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.A.; Asada, K. Superoxide Anion Permeability of Phospholipid Membranes and Chloroplast Thylakoids. Arch. Biochem. Biophys. 1983, 226, 558–566. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous Treatment with Indole-3-acetic Acid and Salicylic Acid Alleviates Cadmium Toxicity in Wheat Seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Ravnskov, S.; Rubæk, G.H.; Sun, Z.; Andersen, M.N. Impact of Wood Biochar and its Interactions with Mycorrhizal Fungi, Phosphorus Fertilization and Irrigation Strategies on Potato Growth. J. Agron. Crop Sci. 2017, 203, 131–145. [Google Scholar] [CrossRef]
- Pompeiano, A.; Landi, M.; Meloni, G.; Vita, F.; Guglielminetti, L.; Guidi, L. Allocation Pattern, Ion Partitioning, and Chlorophyll a Fluorescence in Arundo donax L. in Responses to Salinity Stress. Plant Biosyst. 2017, 151, 613–622. [Google Scholar] [CrossRef]
- Sotiras, M.; Papadakis, I.; Landi, M.; Tsaniklidis, G.; Tsiantas, P.; Psychoyou, M. Allocation Pattern, Photosynthetic Performance and Sugar Metabolism in Hydroponically Grown Seedlings of Loquat (Eriobotrya japonica Lindl.) Subjected to Salinity. Photosynthetica 2019, 57, 258–267. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-evaluastion of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Sharmila, P.; Pardha Saradhi, P. Proline Accumulation in Heavy Metal Stressed Plants: An Adaptive Strategy. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Prasad, M.N.V., Strzalka, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 179–199. [Google Scholar]
- Balestrasse, K.B.; Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Effect of Cadmium Stress on Nitrogen Metabolism in Nodules and Roots of Soybean Plants. Funct. Plant Biol. 2003, 30, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Chitin and Chitosan Based Derivatives in Plant Protection against Biotic and Abiotic Stresses and in Recovery of Contaminated Soil and Water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- Sathiyanarayanan, A.; Sathiyabama, M. Effect of Chitosan on Growth, Yield and Curcumin Content in Turmeric under Field Condition. Biocatal. Agric. Biotechnol. 2016, 6, 102–106. [Google Scholar]
- Xu, D.; Li, H.; Lin, L.; Liao, M.; Deng, Q.; Wang, J.; Lv, X.; Deng, H.; Liang, D.; Xia, H. Effect of Carboxymethyl Chitosan on the Growth and Nutrient Uptake in Prunus davidiana Seedlings. Physiol. Mol. Biol. Plants 2020, 26, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, G.S.; Mahawar, L.; Rajput, P.; Rajput, V.D.; Minkina, T.; Singh, R.K. Role of Engineered Carbon Nanoparticles (CNPs) in Promoting Growth and Metabolism of Vigna radiata (L.) Wilczek: Insights into the Biochemical and Physiological Responses. Plants 2021, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Rajput, V.D.; Al-Khuraif, A.A.; Arshad, M.; Minkina, T.; Sushkova, S.; Yu, F. Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum. Biology 2021, 10, 666. https://doi.org/10.3390/biology10070666
Faizan M, Rajput VD, Al-Khuraif AA, Arshad M, Minkina T, Sushkova S, Yu F. Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum. Biology. 2021; 10(7):666. https://doi.org/10.3390/biology10070666
Chicago/Turabian StyleFaizan, Mohammad, Vishnu D. Rajput, Abdulaziz Abdullah Al-Khuraif, Mohammed Arshad, Tatiana Minkina, Svetlana Sushkova, and Fangyuan Yu. 2021. "Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum" Biology 10, no. 7: 666. https://doi.org/10.3390/biology10070666
APA StyleFaizan, M., Rajput, V. D., Al-Khuraif, A. A., Arshad, M., Minkina, T., Sushkova, S., & Yu, F. (2021). Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum. Biology, 10(7), 666. https://doi.org/10.3390/biology10070666









