Urban Pit-Building Insects Are Attracted to Walls for Multiple Reasons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
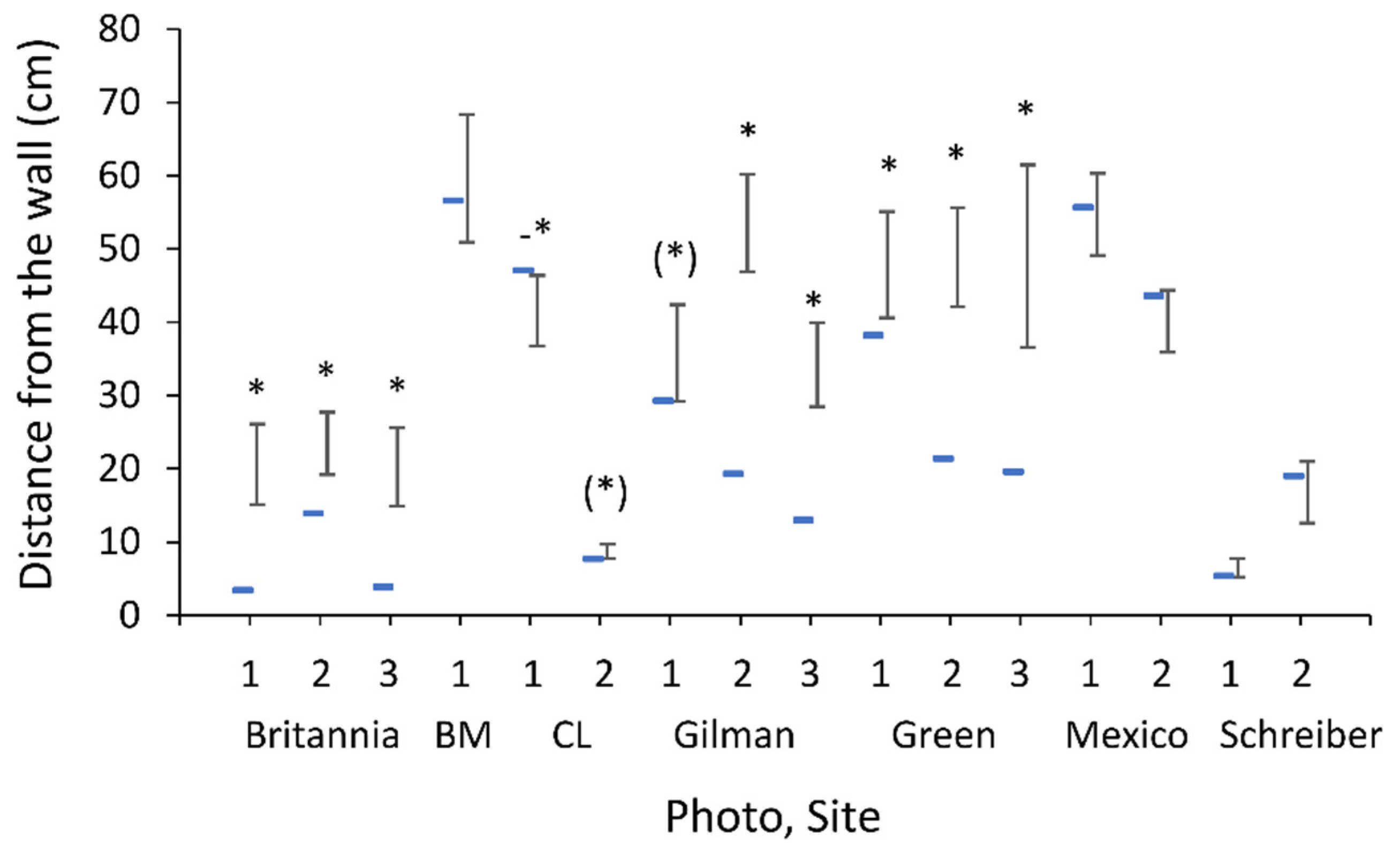
2.1. Study Sites and Wormlion Zones
2.2. The Link Between Soil Depth, Distance from the Wall, and Pit Area
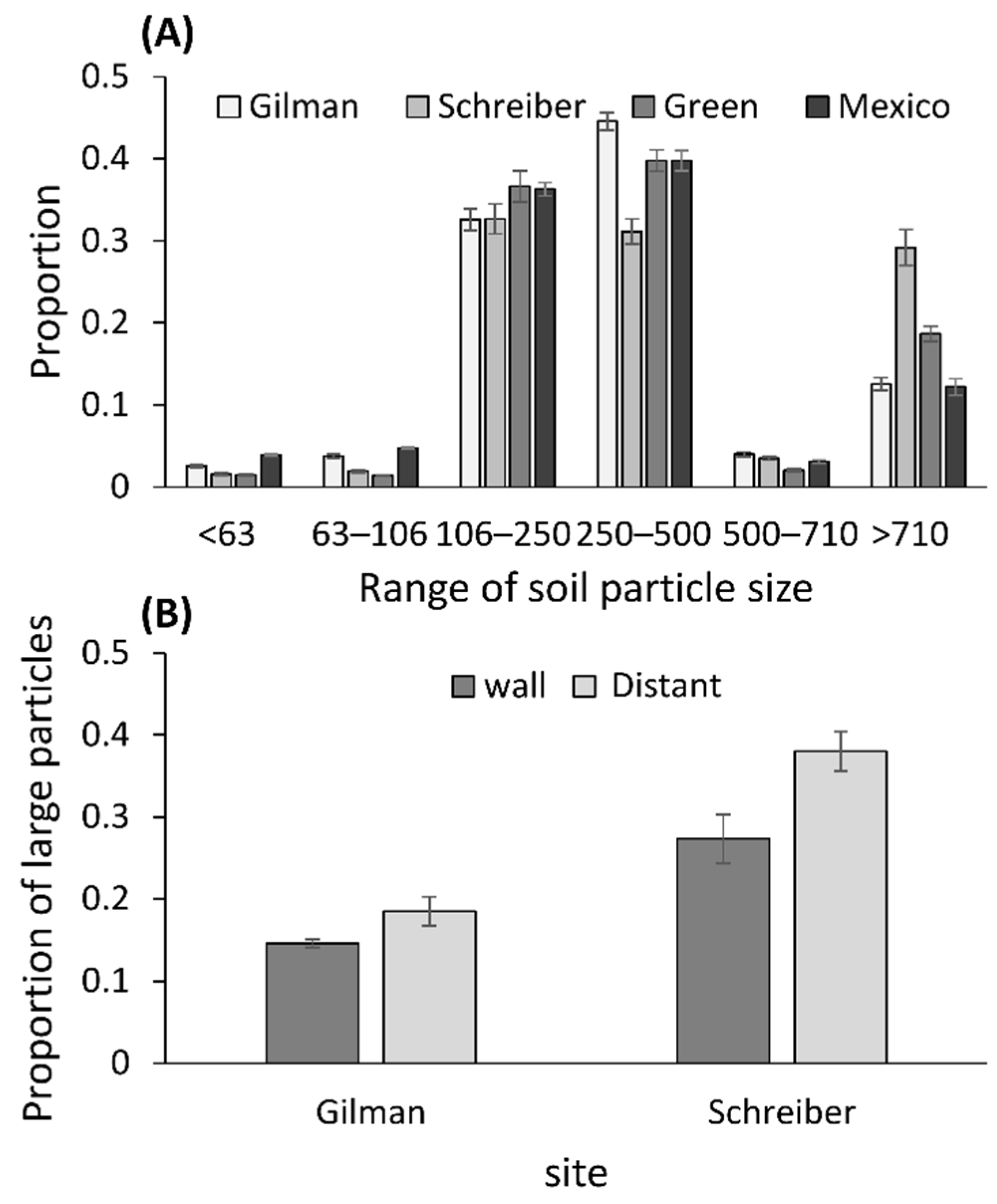
2.3. Measurements of the Soil Particle Sizes
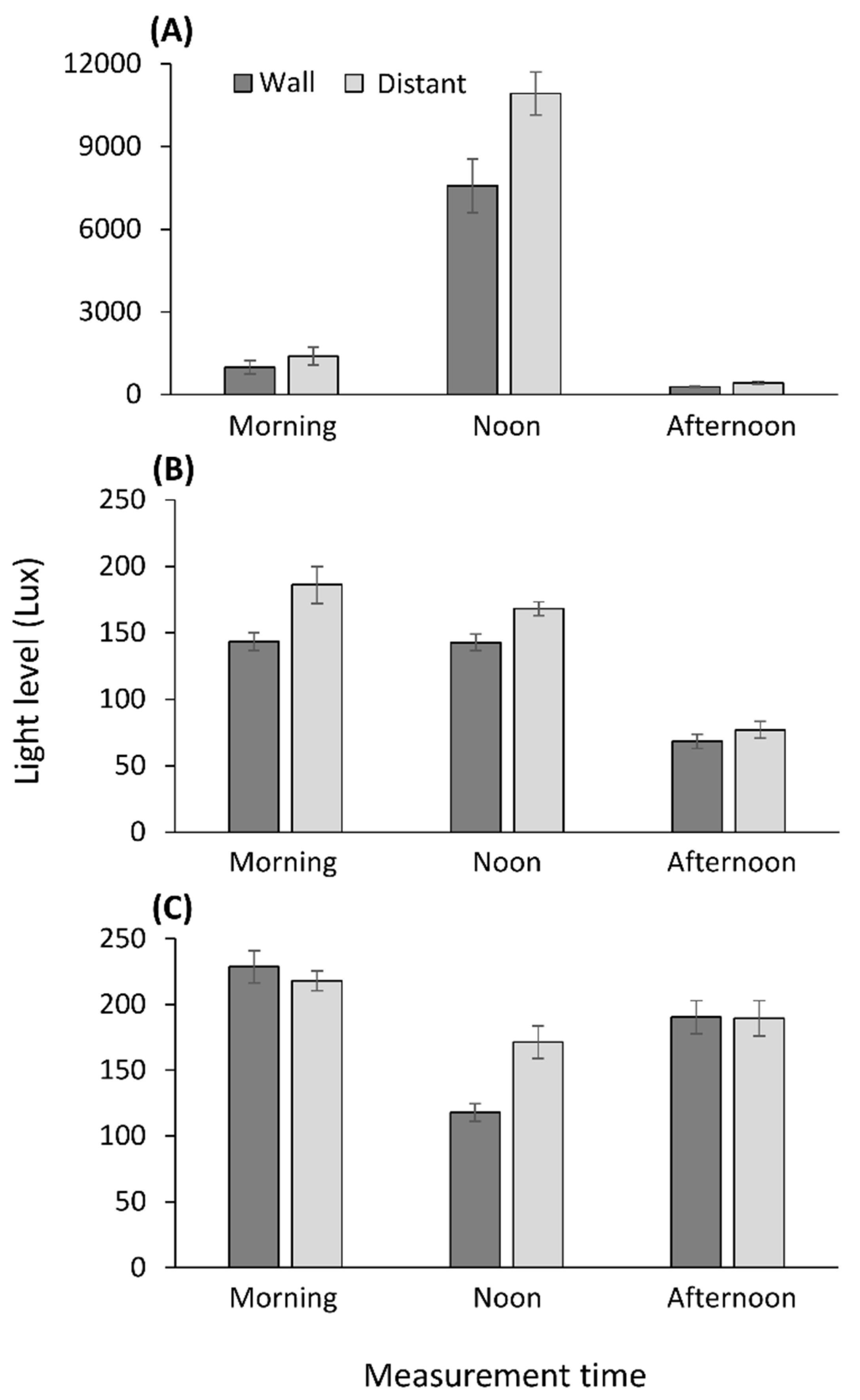
2.4. Light Measurements
2.5. Arthropod Abundance
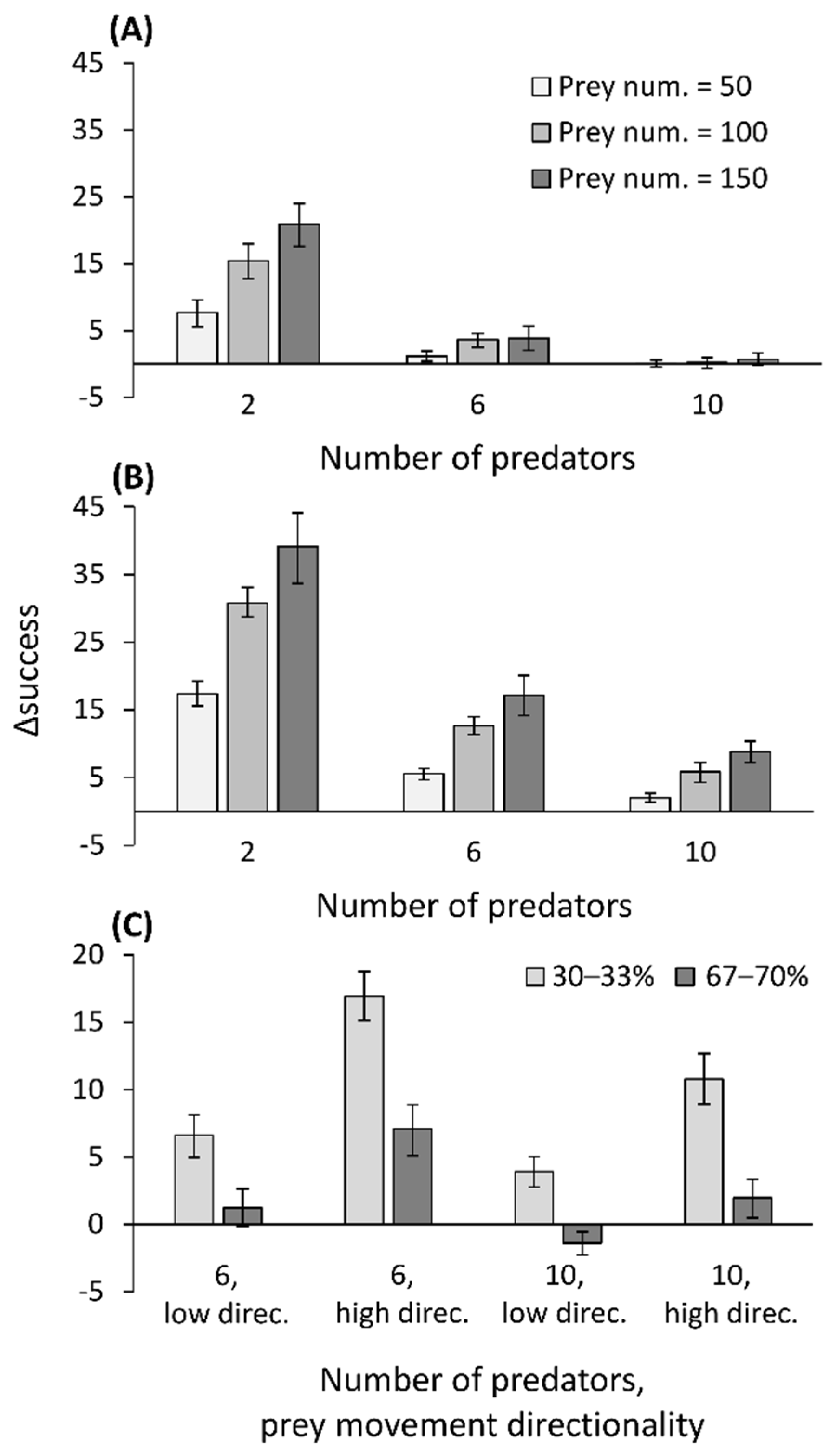
2.6. Simulation Model: Prey Movement Affects Capture Success
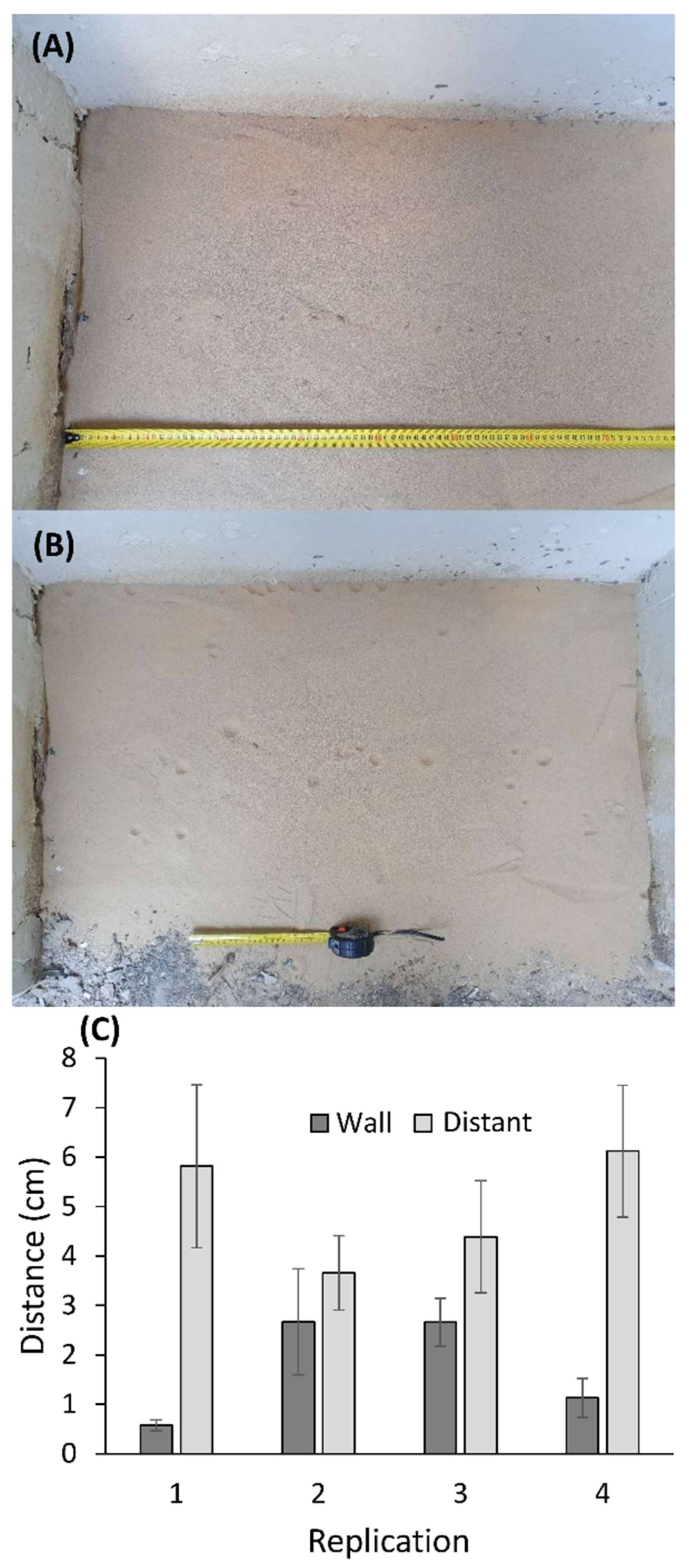
2.7. Experiment: Wormlion Relocation as a Function of Distance from the Wall
3. Results
3.1. Study Sites and Wormlion Zones
3.2. The Link between Soil Depth, Distance from the Wall, and Pit Area
3.3. Measurements of Soil Particle Sizes
3.4. Light Measurements
3.5. Arthropod Abundance
3.6. Simulation Model: Prey Movement Affects Capture Success
3.7. Experiment: Wormlion Relocation as a Function of Distance from the Wall
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Sauerwein, M. Urban soils–characterization, pollution, and relevance in urban ecosystems. In Urban Ecology: Patterns, Processes, and Applications; Niemelä, J., Breuste, J., Elmqvist, J.H., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 45–58. [Google Scholar]
- Vonshak, M.; Gordon, D.M. Intermediate disturbance promotes invasive ant abundance. Biol. Conserv. 2015, 186, 359–367. [Google Scholar] [CrossRef]
- McMahon, T.A.; Rohr, J.R.; Bernal, X.E. Light and noise pollution interact to disrupt interspecific interactions. Ecology 2017, 98, 1290–1299. [Google Scholar] [CrossRef]
- Kotze, J.; Venn, S.; Niemelä, J.; Spence, J. Effects of urbanization on the ecology and evolution of arthropods. In Urban Ecology; Oxford University Press (OUP): Oxford, UK, 2011; pp. 159–166. [Google Scholar]
- Buczkowski, G.; Richmond, D.S. The effect of urbanization on ant abundance and diversity: A temporal examination of factors affecting biodiversity. PLoS ONE 2012, 7, e41729. [Google Scholar] [CrossRef] [Green Version]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef]
- Shochat, E.; Lerman, S.B.; Katti, M.; Lewis, D.B. Linking optimal foraging behavior to bird community structure in an urban-desert landscape: Field experiments with artificial food patches. Am. Nat. 2004, 164, 232–243. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Palacio, F.X. Urban exploiters have broader dietary niches than urban avoiders. Ibis 2020, 162, 42–49. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Hahs, A.K. Adaptation and adaptedness of organisms to urban environments. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 261–280. [Google Scholar] [CrossRef] [Green Version]
- Møller, A.P. Successful city dwellers: A comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 2009, 159, 849–858. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Immler, S.; Gonzalez-Voyer, A.; Rönn, J.; Kolm, N. Brains and the city: Big-brained passerine birds succeed in urban environments. Biol. Lett. 2011, 7, 730–732. [Google Scholar] [CrossRef] [Green Version]
- Santini, L.; González-Suárez, M.; Russo, D.; Gonzalez-Voyer, A.; von Hardenberg, A.; Ancillotto, L. One strategy does not fit all: Determinants of urban adaptation in mammals. Ecol. Lett. 2019, 22, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winchell, K.M.; Battles, A.C.; Moore, T.Y. Terrestrial locomotor evolution in urban environments. In Urban Evolutionary Biology; Szulkin, M., Szulkin, J.M.S., Charmantier, A., Eds.; Oxford University Press: Oxford, UK, 2020; pp. 197–216. [Google Scholar]
- Martínez-Abraín, A.; Jiménez, J.F. Anthropogenic areas as incidental substitutes for original habitat. Conserv. Biol. 2016, 30, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelssohn, H.; Yom-Tov, Y. A report of birds and mammals which have increased their distribution and abundance in Israel due to human activity. Isr. J. Zool. 1999, 45, 35–47. [Google Scholar] [CrossRef]
- Corrales, L.; Bautista, L.M.; Santamaría, T.; Más, P. Hole selection by nesting swifts in medieval city-walls of central Spain. Ardeola 2013, 60, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.J.; Adams, R.; Bateman, A.; Bik, H.M.; Hawks, J.; Hird, S.; Hughes, D.; Kembel, S.W.; Kinney, K.; Kolokotronis, S.-O.; et al. Evolution of the indoor biome. Trends Ecol. Evol. 2015, 30, 223–232. [Google Scholar] [CrossRef]
- Francis, R.A. Wall ecology: A frontier for urban biodiversity and ecological engineering. Prog. Phys. Geogr. Earth Environ. 2010, 35, 43–63. [Google Scholar] [CrossRef]
- Voss, S.C.; Main, B.Y.; Dadour, I.R. Habitat preferences of the urban wall spider Oecobius navus (Araneae, Oecobiidae). Aust. J. Entomol. 2007, 46, 261–268. [Google Scholar] [CrossRef]
- Mammola, S.; Isaia, M.; Demonte, D.; Triolo, P.; Nervo, M. Artificial lighting triggers the presence of urban spiders and their webs on historical buildings. Landsc. Urban Plan. 2018, 180, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Heiling, A.M. Why do nocturnal orb-web spiders (Araneidae) search for light? Behav. Ecol. Sociobiol. 1999, 46, 43–49. [Google Scholar] [CrossRef]
- Adams, M.R. Choosing hunting sites: Web site preferences of the orb weaver spider, Neoscona Crucifera, relative to light cues. J. Insect Behav. 2000, 13, 299–305. [Google Scholar] [CrossRef]
- Lundholm, J.T.; Marlin, A. Habitat origins and microhabitat preferences of urban plant species. Urban. Ecosyst. 2006, 9, 139–159. [Google Scholar] [CrossRef]
- Petren, K.; Case, T.J. Habitat structure determines competition intensity and invasion success in gecko lizards. Proc. Natl. Acad. Sci. USA 1998, 95, 11739–11744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruxton, G.D.; Hansell, M.H. Why are pitfall traps so rare in the natural world? Evol. Ecol. 2007, 23, 181–186. [Google Scholar] [CrossRef]
- Blamires, S.J. Biomechanical costs and benefits of sit-and-wait foraging traps. Isr. J. Ecol. Evol. 2020, 66, 5–14. [Google Scholar] [CrossRef]
- Scharf, I.; Lubin, Y.; Ovadia, O. Foraging decisions and behavioural flexibility in trap-building predators: A review. Biol. Rev. 2011, 86, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.R. Metabolic Rates and pit-construction costs of two antlion species. J. Anim. Ecol. 1985, 54, 295. [Google Scholar] [CrossRef]
- Elimelech, E.; Pinshow, B. Variation in food availability influences prey-capture method in antlion larvae. Ecol. Entomol. 2008, 33, 652–662. [Google Scholar] [CrossRef]
- Lubin, Y.; Ellner, S.; Kotzman, M. Web relocation and habitat selection in desert widow spider. Ecology 1993, 74, 1915–1928. [Google Scholar] [CrossRef]
- Enders, F. Web-site selection by orb-web spiders, particularly Argiope aurantia lucas. Anim. Behav. 1977, 25, 694–712. [Google Scholar] [CrossRef]
- Hodge, M.A. Factors influencing web site residence time of the orb weaving spider, Micrathena Gracilis. Psyche 1987, 94, 363–371. [Google Scholar] [CrossRef]
- Scharf, I.; Ovadia, O. Factors influencing site abandonment and site selection in a sit-and-wait predator: A review of pit-building antlion larvae. J. Insect Behav. 2006, 19, 197–218. [Google Scholar] [CrossRef] [Green Version]
- Blamires, S.J.; Thompson, M.B.; Hochuli, D.F. Habitat selection and web plasticity by the orb spider Argiope keyserlingi (Argiopidae): Do they compromise foraging success for predator avoidance? Austral. Ecol. 2007, 32, 551–563. [Google Scholar] [CrossRef]
- Lowe, E.C.; Wilder, S.; Hochuli, D.F. Urbanisation at multiple scales is associated with larger size and higher fecundity of an orb-weaving spider. PLoS ONE 2014, 9, e105480. [Google Scholar] [CrossRef]
- Ripp, J.; Eldakar, O.T.; Gallup, A.C.; Arena, P.T. The successful exploitation of urban environments by the golden silk spider, Nephila clavipes (Araneae, Nephilidae). J. Urban Ecol. 2018, 4, juy005. [Google Scholar] [CrossRef] [Green Version]
- Dor, R.; Rosenstein, S.; Scharf, I. Foraging behaviour of a neglected pit-building predator: The wormlion. Anim. Behav. 2014, 93, 69–76. [Google Scholar] [CrossRef]
- Miler, K.; Yahya, B.E.; Czarnoleski, M. Different predation efficiencies of trap-building larvae of sympatric antlions and wormlions from the rainforest of Borneo. Ecol. Entomol. 2018, 43, 255–262. [Google Scholar] [CrossRef]
- Devetak, D. Wormlion Vermileo vermileo (L.) (Diptera: Vermileonidae) in Slovenia and Croatia. Ann. Ser. Hist. Nat. 2008, 18, 283–286. [Google Scholar]
- Devetak, D.; Arnett, A.E. Preference of antlion and wormlion larvae (Neuroptera: Myrmeleontidae; Diptera: Vermileonidae) for substrates according to substrate particle sizes. Eur. J. Entomol. 2015, 112, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Adar, S.; Dor, R.; Scharf, I. Habitat choice and complex decision making in a trap-building predator. Behav. Ecol. 2016, 27, 1491–1498. [Google Scholar] [CrossRef] [Green Version]
- Scharf, I.; Gilad, T.; Bar-Ziv, M.A.; Katz, N.; Gregorian, E.; Pruitt, J.; Subach, A. The contribution of shelter from rain to the success of pit-building predators in urban habitats. Anim. Behav. 2018, 142, 139–145. [Google Scholar] [CrossRef]
- Bar-Ziv, M.A.; Subach, A.; Hirsch-Ionescu, A.; Belmaker, J.; Zweifler, A.; Scharf, I. Comparison of wormlions and their immediate habitat under man-made and natural shelters: Suggesting factors making wormlions successful in cities. Zoology 2018, 130, 38–46. [Google Scholar] [CrossRef]
- Scharf, I.; Silberklang, A.; Avidov, B.; Subach, A. Do pit-building predators prefer or avoid barriers? Wormlions’ preference for walls depends on light conditions. Sci. Rep. 2020, 10, 10928. [Google Scholar] [CrossRef]
- Tsoar, H.; Pye, K. Dust transport and the question of desert loess formation. Sedimentology 1987, 34, 139–153. [Google Scholar] [CrossRef]
- Grafals-Soto, R.; Nordstrom, K. Sand fences in the coastal zone: Intended and unintended effects. Environ. Manag. 2009, 44, 420–429. [Google Scholar] [CrossRef]
- Vandenberghe, J. Grain size of fine-grained windblown sediment: A powerful proxy for process identification. Earth Sci. Rev. 2013, 121, 18–30. [Google Scholar] [CrossRef]
- Samocha, Y.; Scharf, I. Comparison of wormlion behavior under man-made and natural shelters: Urban worm-lions more strongly prefer shaded, fine-sand microhabitats, construct larger pits and respond faster to prey. Curr. Zool. 2020, 66, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, S.C.; Brooks, S.E.; Franks, N.R. The use of edges in visual navigation by the ant Leptothorax albipennis. Ethology 2001, 107, 1125–1136. [Google Scholar] [CrossRef]
- Dussutour, A.; Deneubourg, J.-L.; Fourcassié, V. Amplification of individual preferences in a social context: The case of wall-following in ants. Proc. R. Soc. B Boil. Sci. 2005, 272, 705–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, N.J.; Lee, J.; Full, R.J. Task-level control of rapid wall following in the American cockroach. J. Exp. Biol. 2006, 209, 1617–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soibam, B.; Mann, M.; Liu, L.; Tran, J.; Lobaina, M.; Yuan Kang, Y.; Gunaratne, G.H.; Pletcher, S.; Roman, G. Open-field arena boundary is a primary object of exploration for Drosophila. Brain Behav. 2012, 2, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Scharf, I.; Hershkovitz Reshef, M.; Avidov, B.; Ovadia, O. Evidence for competition and cannibalism in wormlions. Sci. Rep. 2021, 11, 12733. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics. Int. 2004, 11, 36–42. [Google Scholar]
- Bar-Ziv, M.A.; Bega, D.; Subach, A.; Scharf, I. Wormlions prefer both fine and deep sand but only deep sand leads to better performance. Curr. Zool. 2019, 65, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Skevington, J.H.; Dang, P.T. Exploring the diversity of flies (Diptera). Biodiversity 2002, 3, 3–27. [Google Scholar] [CrossRef]
- Scharf, I. Factors that can affect the spatial positioning of large and small individuals in clusters of sit-and-wait predators. Am. Nat. 2020, 195, 649–663. [Google Scholar] [CrossRef]
- Zuñiga-Palacios, J.; Zuria, I.; Castellanos, I.; Lara, C.; Sánchez-Rojas, G. What do we know (and need to know) about the role of urban habitats as ecological traps? Systematic review and meta-analysis. Sci. Total. Environ. 2021, 780, 146559. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F. The Mediterranean gecko, Hemidactylus turcicus: Life in an urban landscape. Fla. Sci. 2001, 64, 56–66. [Google Scholar]
- Parris, K.M. Urban amphibian assemblages as metacommunities. J. Anim. Ecol. 2006, 75, 757–764. [Google Scholar] [CrossRef]
- Scharf, I.; Samocha, Y.; Katz, N. Limited perceptual range and presence of conspecifics both affect the ability of pit-building wormlions to choose favorable microhabitats. Isr. J. Ecol. Evol. 2019, 66, 48–56. [Google Scholar] [CrossRef]
- Devetak, D. Substrate particle size-preference of wormlion Vermileo vermileo (Diptera: Vermileonidae) larvae and their interaction with antlions. Eur. J. Entomol. 2008, 105, 631–635. [Google Scholar] [CrossRef] [Green Version]
- Miler, K.; Yahya, B.E.; Czarnoleski, M. Substrate moisture, particle size and temperature preferences of trap-building larvae of sympatric antlions and wormlions from the rainforest of Borneo. Ecol. Entomol. 2019, 44, 488–493. [Google Scholar] [CrossRef]
- Frouz, J. Use of soil dwelling Diptera (Insecta, Diptera) as bioindicators: A review of ecological requirements and response to disturbance. Agric. Ecosyst. Environ. 1999, 74, 167–186. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil health and arthropods: From complex system to worthwhile investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, L.; Thys, S.; Scheirs, J.; Verhagen, R. Effects of vegetation and soil on species diversity of soil dwelling diptera in a heathland ecosystem. J. Insect Conserv. 2001, 5, 87–97. [Google Scholar] [CrossRef]
- Jones, K.B.; Whitford, W.G. Feeding behavior of free-roaming Masticophis flagellum: An efficient ambush predator. Southwest. Nat. 1989, 34, 460. [Google Scholar] [CrossRef]
- Tsairi, H.; Bouskila, A. Ambush site selection of a desert snake (echis coloratus) at an oasis. Herpetologica 2004, 60, 13–23. [Google Scholar] [CrossRef]
- Katz, N.; Scharf, I. Habitat geometry and limited perceptual range affect habitat choice of a trap-building predator. Behav. Ecol. 2018, 29, 958–964. [Google Scholar] [CrossRef]
- Alyokhin, A.V.; Mille, C.; Messing, R.H.; Duan, J.J. Selection of pupation habitats by oriental fruit fly larvae in the laboratory. J. Insect Behav. 2001, 14, 57–67. [Google Scholar] [CrossRef]
- Cornelisse, T.M.; Hafernik, J.E. Effects of soil characteristics and human disturbance on tiger beetle oviposition. Ecol. Entomol. 2009, 34, 495–503. [Google Scholar] [CrossRef]
- Rao, D. Experimental evidence for the amelioration of shadow competition in an orb-web spider through the ‘ricochet’ effect. Ethology 2009, 115, 691–697. [Google Scholar] [CrossRef]
- Reinert, H.K.; Cundall, D.; Bushar, L.M. Foraging behavior of the timber rattlesnake, Crotalus horridus. Copeia 1984, 1984, 976. [Google Scholar] [CrossRef]
- Douglass, N.J.; Reinert, H.K. The utilization of fallen logs as runways by small mammals. In Proceedings of the Pennsylvania Academy of Science; Penn State University Press: University Park, PA, USA, 1982; Volume 56, pp. 162–164. [Google Scholar]
- Barkae, E.D.; Abramsky, Z.; Ovadia, O. Can models of density-dependent habitat selection be applied for trap-building predators? Popul. Ecol. 2014, 56, 175–184. [Google Scholar] [CrossRef]
- Matsura, T.; Yamaga, Y.; Itoh, M. Substrate selection for pit making and oviposition in an antlion, Myrmeleon bore Tjeder, in terms of sand particle size. Entomol. Sci. 2005, 8, 347–353. [Google Scholar] [CrossRef]
- Tavares, R.I.S.; Pestana, G.C.; Rocha, A.D.; Schiavone, D.C.; Guillermo-Ferreira, R. Come to the dark side: Habitat selection of larval odonates depends on background visual patterns. Ecol. Entomol. 2018, 43, 640–646. [Google Scholar] [CrossRef]

| Site, Photo, Orientation | Area (cm), Pit # | Soil Depth (mm), [Range] | Slope, p Value |
|---|---|---|---|
| Britannia, 1, S | 65 × 50, 24 | 9.3 ± 4.2, [3.3, 17.8] | −0.070, 0.069 |
| Britannia, 2, S | 95 × 60, 49 | 12.4 ± 4.7, [6.2, 27.2] | −0.066, 0.077 |
| Britannia, 3, S | 70 × 55, 25 | 7.5 ± 6.4, [2.9, 22.1] | −0.137, <0.001 |
| Buchmann-Mehta, 1, W | 120 × 90, 61 | 51.2 ± 29.6, [12.9, 114.5] | 0.655, <0.001 |
| Cent. library, 1, N | 85 × 75, 86 | 36.8 ± 13.6, [18.3, 72.7] | −0.111, 0.239 |
| Cent. library, 2, E | 60 × 45, 90 | 90.6 ± 42.8, [22.0, 150.0] | −1.189, 0.011 |
| Gilman, 1, N | 105 × 75, 41 | 54.4 ± 31.5, [10.6, 144.8] | 0.156, 0.575 |
| Gilman, 2, N | 105 × 75, 85 | 57.6 ± 18.9, [31.8, 100.8] | −0.477, 0.001 |
| Gilman, 3, N | 105 × 70, 44 | 82.1 ± 35.1, [28.4, 150.0] | −0.078, 0.827 |
| Green, 1, S | 100 × 100, 50 | 31.1 ± 20.3, [8.2, 84.8] | −0.350, 0.008 |
| Green, 2, S | 100 × 100, 59 | 35.0 ± 26.2, [12.8, 105.8] | −0.095, 0.606 |
| Green, 3, S | 100 × 100, 21 | 38.3 ± 36.9, [12.4, 150.0] | 0.183, 0.479 |
| Mexico, 1, N | 110 × 110, 112 | 33.2 ± 15.9, [10.3, 70.8] | −0.359, <0.001 |
| Mexico, 2, N | 110 × 80, 121 | 37.1 ± 14.8, [7.9, 68.7] | −0.126, 0.328 |
| Schreiber, 1, W | 75 × 30, 27 | 75.2 ± 30.7, [28.5, 140.0] | 0.019, 0.974 |
| Schreiber, 2, E | 55 × 35, 21 | 127.5 ± 32.2, [53.8, 150.0] | 0.491, 0.420 |
| Site | F | p |
|---|---|---|
| Britannia | 246.867 9.788 0.043 | <0.001 N, M, A 0.005 D, W 0.958 |
| Buchmann- Mehta | 3.887 8.065 1.246 | 0.035 N, A, M 0.009 W, D 0.306 |
| Central library | 10.921 0.037 0.028 | <0.001 N, A, M 0.848 0.972 |
| Gilman | 91.806 0.037 0.507 | <0.001 M, N, A 0.849 0.609 |
| Green | 27.562 4.475 6.801 | <0.001 M, A, N 0.045 D, W 0.005 |
| Mexico | 76.352 11.599 0.310 | <0.001 M, N, A 0.002 D, W 0.736 |
| Schreiber | 92.211 10.456 0.498 | <0.001 M, N, A 0.004 D, W 0.614 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scharf, I.; Gilad, T.; Taichman, Y.; Subach, A. Urban Pit-Building Insects Are Attracted to Walls for Multiple Reasons. Biology 2021, 10, 635. https://doi.org/10.3390/biology10070635
Scharf I, Gilad T, Taichman Y, Subach A. Urban Pit-Building Insects Are Attracted to Walls for Multiple Reasons. Biology. 2021; 10(7):635. https://doi.org/10.3390/biology10070635
Chicago/Turabian StyleScharf, Inon, Tomer Gilad, Yuval Taichman, and Aziz Subach. 2021. "Urban Pit-Building Insects Are Attracted to Walls for Multiple Reasons" Biology 10, no. 7: 635. https://doi.org/10.3390/biology10070635
APA StyleScharf, I., Gilad, T., Taichman, Y., & Subach, A. (2021). Urban Pit-Building Insects Are Attracted to Walls for Multiple Reasons. Biology, 10(7), 635. https://doi.org/10.3390/biology10070635






