Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Isolates
2.2. Antimicrobial Susceptibility Testing
2.3. Determination of Minimum Inhibitory Concentration
2.4. Molecular Detection of MAC Resistance Genes
2.4.1. Chromosomal DNA Extraction of the Tested Isolates
2.4.2. PCR Amplification of MAC Resistance Genes
2.4.3. Agarose Gel Electrophoresis
2.5. Phenotypic Analysis of the Recovered MRSA Isolates Using Heatmap Signature Analysis
2.6. Checkerboard Titration Method for Studying the Effect of Combinations between MACs and Different Antimicrobial Agents
2.7. Statistical Analysis
3. Results
3.1. The Antimicrobial Susceptibility Testing of the Collected Staphylococci (n = 72)
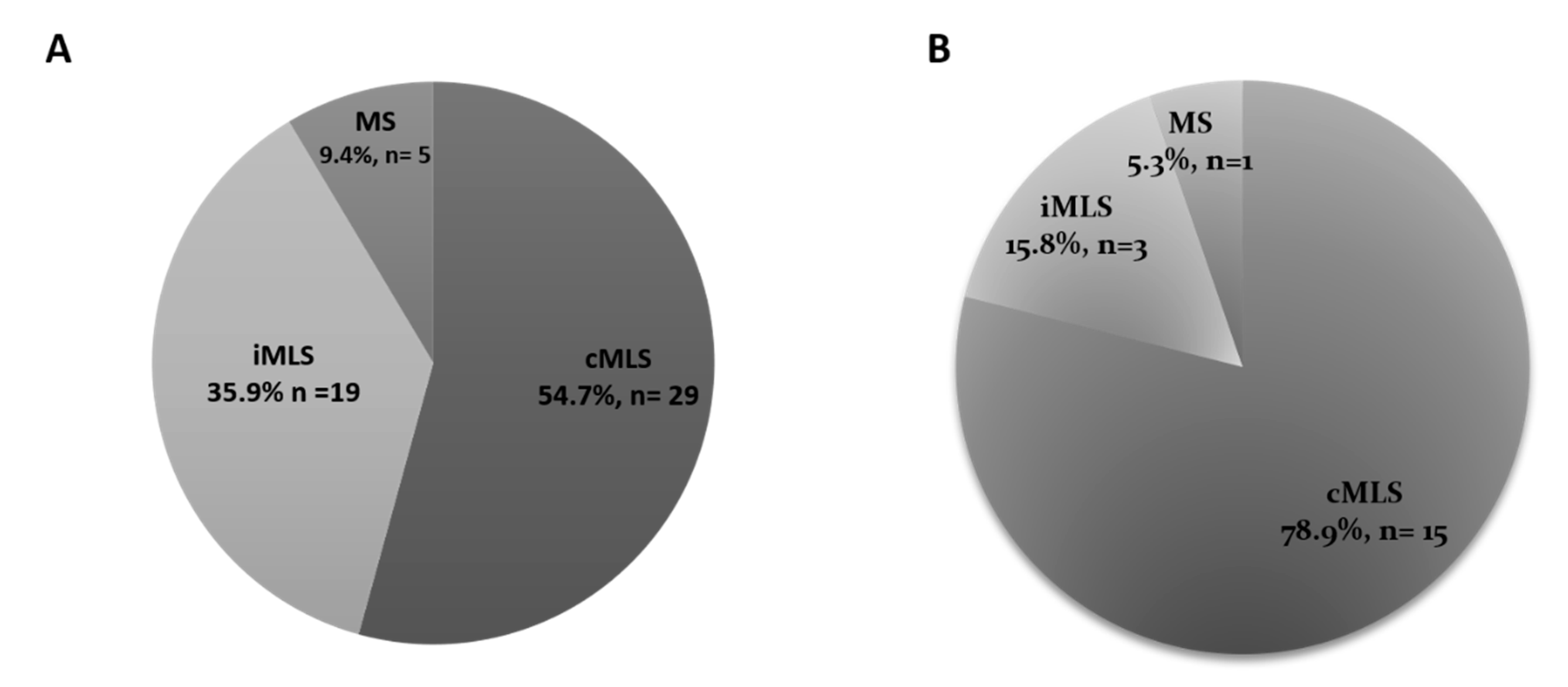
3.2. MAC-Resistance Phenotypes
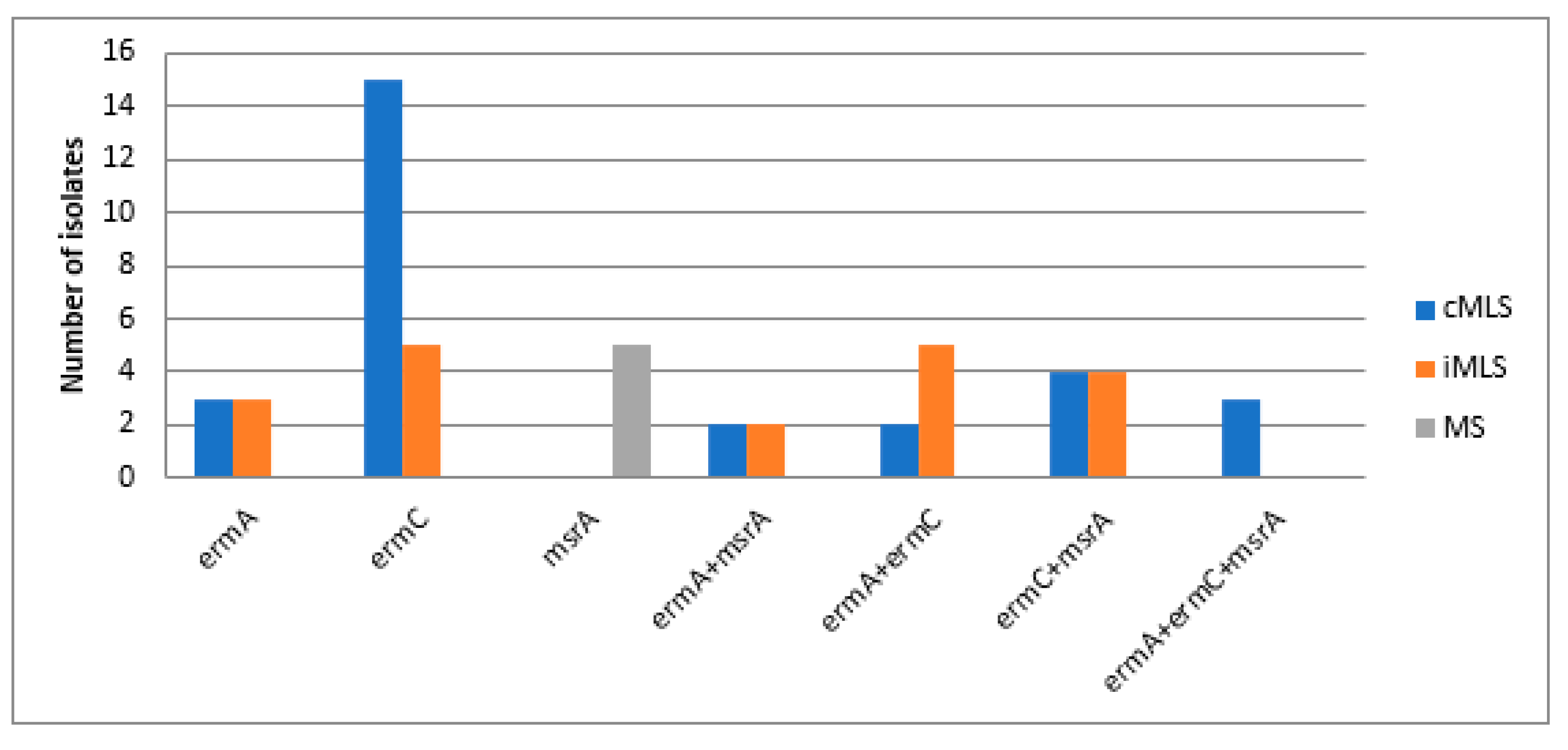
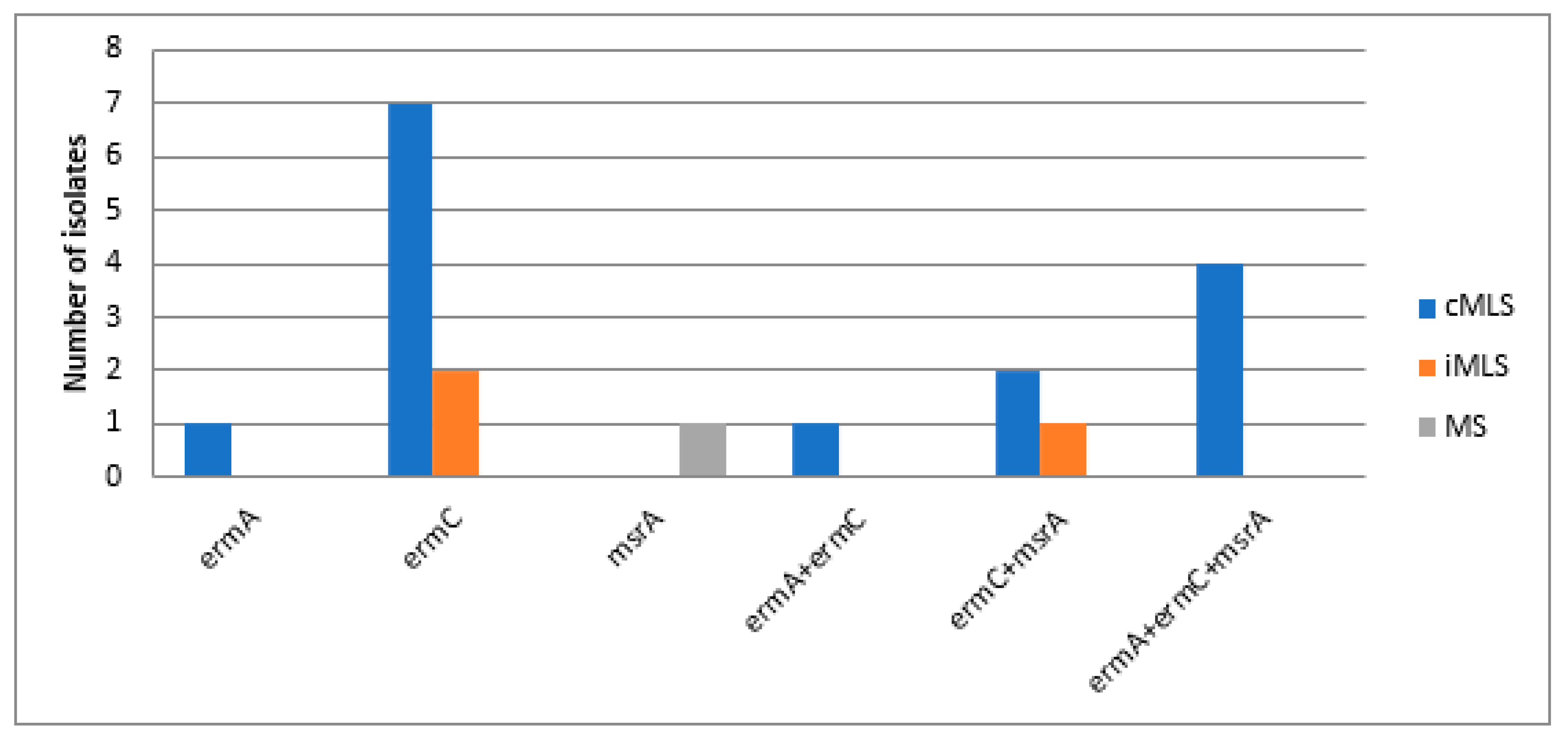
3.3. Molecular Detection of MAC Resistance Genes among MAC Resistant Staphylococci
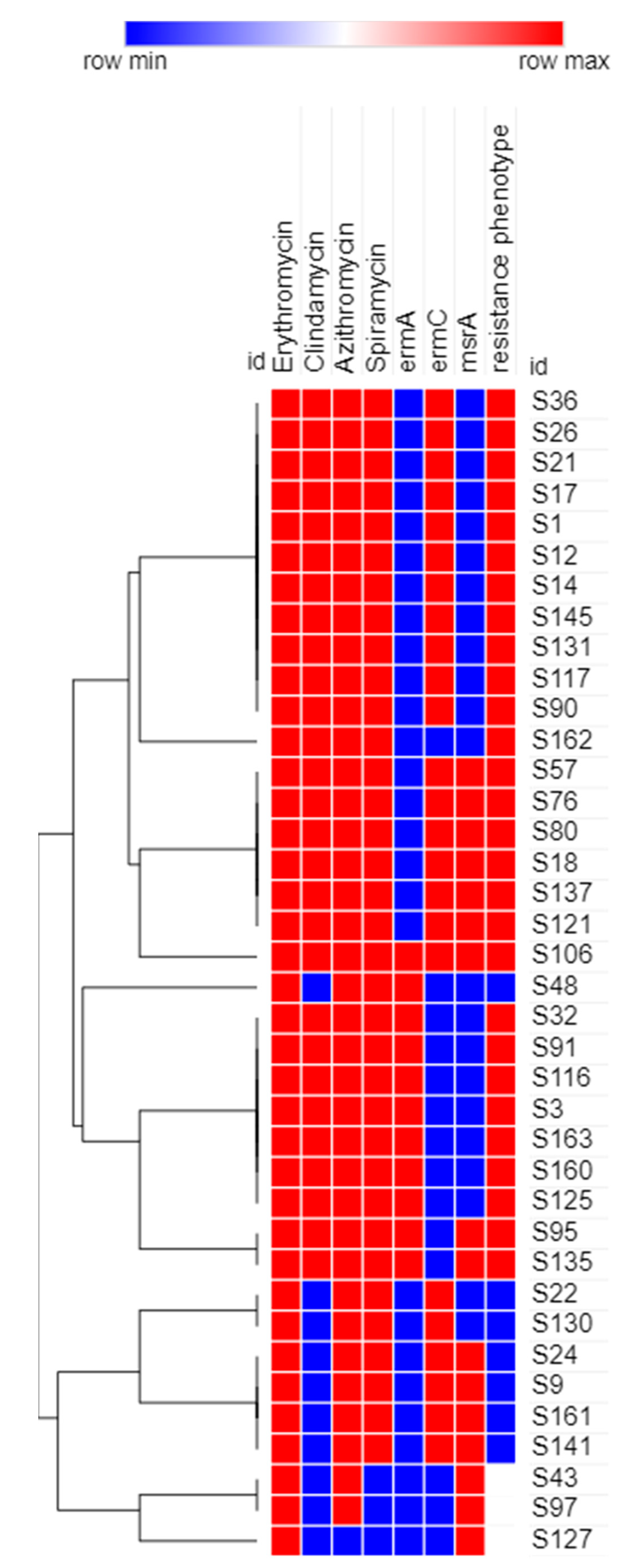
3.4. Phenotypic Analysis of the Recovered MRSA Isolates Using Heatmap Signature Analysis
3.5. Effect Azithromycin Combinations with Different Antimicrobial Agents on MAC MRSA Isolates (n = 38)
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossato, A.M.; Primon-Barros, M.; Rocha, L.D.L.; Reiter, K.C.; Dias, C.A.G.; D’Azevedo, P.A. Resistance profile to antimicrobials agents in methicillin-resistant Staphylococcus aureus isolated from hospitals in South Brazil between 2014–2019. Rev. Da Soc. Bras. Med. Trop. 2020, 53, e20200431. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Nelson, J.; Paterson, D.L.; Fowlerjr, V.G.; Howden, B.P.; Cheng, A.C.; Chatfield, M.; Lipman, J.; Van Hal, S.; O’Sullivan, M.; et al. CAMERA2–combination antibiotic therapy for methicillin-resistant Staphylococcus aureus infection: Study protocol for a randomised controlled trial. Trials 2016, 17, 170. [Google Scholar] [CrossRef]
- Ochoa, S.A.; Cruz-Córdova, A.; Mancilla-Rojano, J.; Escalona-Venegas, G.; Esteban-Kenel, V.; Franco-Hernández, I.; Parra-Ortega, I.; Arellano-Galindo, J.; Hernández-Castro, R.; Perez-López, C.F.; et al. Control of Methicillin-Resistant Staphylococcus aureus Strains Associated With a Hospital Outbreak Involving Contamination From Anesthesia Equipment Using UV-C. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Khodabandeh, M.; Mohammadi, M.; Abdolsalehi, M.R.; Alvandimanesh, A.; Gholami, M.; Bibalan, M.H.; Pournajaf, A.; Kafshgari, R.; Rajabnia, R. Analysis of Resistance to Macrolide–Lincosamide–Streptogramin B Among mecA-Positive Staphylococcus Aureus Isolates. Osong Public Health Res. Perspect. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef]
- Leclercq, R. Mechanisms of Resistance to Macrolides and Lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-Negative Staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Nayyar, C.; Tak, V.; Saigal, K. Mannitol-fermenting and Tube Coagulase-negative Staphylococcal Isolates: Unraveling the Diagnostic Dilemma. J. Lab. Physicians 2017, 9, 65–66. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Rodrigues, M.X.; Silva, N.C.C. Methicillin-resistant Staphylococcus aureus in food and the prevalence in Brazil: A review. Braz. J. Microbiol. 2019, 51, 347–356. [Google Scholar] [CrossRef]
- El-Baghdady, K.Z.; El-Borhamy, M.I.; El-Ghafar, H.A.A. Prevalence of resistance and toxin genes in community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus clinical isolates. Iran. J. Basic Med Sci. 2020, 23, 1251–1260. [Google Scholar] [CrossRef]
- Iordanskii, A.; Zhulkina, A.; Olkhov, A.; Fomin, S.; Burkov, A.; Stilman, M. Characterization and Evaluation of Controlled Antimicrobial Release from Petrochemical (PU) and Biodegradable (PHB) Packaging. Polymers 2018, 10, 817. [Google Scholar] [CrossRef]
- Coutinho, V.D.L.S.; Paiva, R.M.; Reiter, K.C.; De-Paris, F.; Barth, A.L.; Machado, A.B.M.P. Distribution of erm genes and low prevalence of inducible resistance to clindamycin among staphylococci isolates. Braz. J. Infect. Dis. 2010, 14, 564–568. [Google Scholar] [CrossRef][Green Version]
- Weisblum, B.; Graham, M.Y.; Gryczan, T.; Dubnau, D. Plasmid copy number control: Isolation and characterization of high-copy-number mutants of plasmid pE194. J. Bacteriol. 1979, 137, 635–643. [Google Scholar] [CrossRef]
- Rodríguez-Tudela, J.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, 1–8. [Google Scholar] [CrossRef]
- Zmantar, T.; Kouidhi, B.; Miladi, H.; Bakhrouf, A. Detection of macrolide and disinfectant resistance genes in clinical Staphylococcus aureus and coagulase-negative staphylococci. BMC Res. Notes 2011, 4, 453. [Google Scholar] [CrossRef]
- Duran, N.; Ozer, B.; Duran, G.G.; Onlen, Y.; Demir, C. Antibiotic resistance genes & susceptibility patterns in staphylococci. Indian J. Med Res. 2012, 135, 389–396. [Google Scholar]
- Pournajaf, A.; Ardebili, A.; Goudarzi, L.; Khodabandeh, M.; Narimani, T.; Abbaszadeh, H. PCR-based identification of methicillin–resistant Staphylococcus aureus strains and their antibiotic resistance profiles. Asian Pac. J. Trop. Biomed. 2014, 4, S293–S297. [Google Scholar] [CrossRef]
- Elemam, A.; Rahimian, J.; Doymaz, M. In Vitro Evaluation of Antibiotic Synergy for Polymyxin B-Resistant Carbapenemase-Producing Klebsiella pneumoniae. J. Clin. Microbiol. 2010, 48, 3558–3562. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, C.; Chun, H.-S.; Lee, D.-G.; Choi, J.-K.; Lee, H.-J.; Cho, S.-Y.; Park, S.H.; Choi, S.-M.; Choi, J.-H.; et al. Pilot Screening to Determine Antimicrobial Synergies in a Multidrug-Resistant Bacterial Strain Library. Microb. Drug Resist. 2016, 22, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Golkar, Z.; Bagasra, O.; Pace, D.G. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef]
- Viswanathan, V.K. Off-label abuse of antibiotics by bacteria. Gut Microbes 2014, 5, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, G.; Li, D.; Bai, B.; Wang, H.; Cheng, H.; Zheng, J.; Sun, X.; Lin, Z.; Deng, Q.; et al. Staphylococcus aureus with an erm-mediated constitutive macrolide-lincosamide-streptogramin B resistance phenotype has reduced susceptibility to the new ketolide, solithromycin. BMC Infect. Dis. 2019, 19, 175. [Google Scholar] [CrossRef]
- Chika, E.; Joseph, N.F.; Chijioke, E.; Peter, E. Detection of Constitutive- and Inducible-Clindamycin-Resistance in Clinical Isolates of Staphylococcus aureus from a Federal Teaching Hospital in Abakaliki, Nigeria. J. Bacteriol. Infect. Dis. 2018, 2, 31–34. [Google Scholar]
- Debnath, A.; Ghosh, R.; Ghosh, D. Detection of Inducible Clindamycin Resistance (IMLSB) among the Erythromycin Resistant CONS Isolates in a Rural Tertiary Care Hospital-Need of Time. Int. J. Health Sci. Res. 2020, 7, 31–34. [Google Scholar]
- Adhikari, R.P.; Shrestha, S.; Barakoti, A.; Amatya, R. Inducible clindamycin and methicillin resistant Staphylococcus aureus in a tertiary care hospital, Kathmandu, Nepal. BMC Infect. Dis. 2017, 17, 1–5. [Google Scholar] [CrossRef]
- Kaur, D.C.; Chate, S.S. Study of antibiotic resistance pattern in methicillin resistant staphylococcus aureus with special reference to newer antibiotic. J. Glob. Infect. Dis. 2015, 7, 78–84. [Google Scholar] [CrossRef]
- Dash, M.; Majhi, S.; Mohapatra, D.; Mohapatra, A.; Chayani, N. Detection of inducible and constitutive clindamycin resistance among Staphylococcus aureus isolates in a tertiary care hospital, Eastern India. Avicenna J. Med. 2016, 6, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.; Rao, S.; Rao, V. Inducible Clindamycin Resistance in Staphylococcus aureus Isolated from Clinical Samples. J. Lab. Physicians 2011, 3, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Sah, P.; Khanal, R.; Lamichhane, P.; Upadhaya, S.; Lamsal, A.; Pahwa, V.K. Inducible and constitutive clindamycin resistance in Staphylococcus aureus: An experience from Western Nepal. Int. J. Biomed. Res. 2015, 6, 316–319. [Google Scholar] [CrossRef]
- Fluit, A.C.; Visser, M.R.; Schmitz, F.-J. Molecular Detection of Antimicrobial Resistance. Clin. Microbiol. Rev. 2001, 14, 836–871. [Google Scholar] [CrossRef]
- Mišić, M.; Čukić, J.; Vidanovic, D.; Šekler, M.; Matić, S.; Vukašinović, M.; Baskić, D. Prevalence of Genotypes That Determine Resistance of Staphylococci to Macrolides and Lincosamides in Serbia. Front. Public Health 2017, 5, 200. [Google Scholar] [CrossRef]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; De Biasi, M.G.; De Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef]
- Pero, R.; Angrisano, T.; Brancaccio, M.; Falanga, A.; Lombardi, L.; Natale, F.; Laneri, S.; Lombardo, B.; Galdiero, S.; Scudiero, O. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS ONE 2019, 14, e0222295. [Google Scholar] [CrossRef]
- Singh, V.; Bala, M.; Bhargava, A.; Kakran, M.; Bhatnagar, R. In vitro efficacy of 21 dual antimicrobial combinations comprising novel and currently recommended combinations for treatment of drug resistant gonorrhoea in future era. PLoS ONE 2018, 13, e0193678. [Google Scholar] [CrossRef] [PubMed]

| Step | Temperature °C | Time | No. of cycles |
|---|---|---|---|
| Initial denaturation | 95 | 5 min | 1 |
| Denaturation | 95 | 30 s | 30 |
| Annealing | X | 30 s | |
| Extension | 72 | 1 min | |
| Final extension | 72 | 5 min | 1 |
| Primer | Target Gene | Primer Sequence (5’⟶3’) | Ta (°C) | PCR Product (Kb) | Reference |
|---|---|---|---|---|---|
| ErmA-f | ermA | TATCTTATCGTTGAGAAGGGATT | 50 | 139 | [18] |
| ErmA-r | CTACACTTGGCTTAGGATGAAA | ||||
| ErmC-f | ermC | AATCGTCAATTCCTGCATGT | 51 | 299 | [19] |
| ErmC-r | TAATCGTGGAATACGGGTTTG | ||||
| Msr-f | msrA | TCCAATCATAGCACAAAATC | 47 | 163 | [18] |
| Msr-r | AATTCCCTCTATTTGGTGGT | ||||
| MecA-f | mecA | AAAATCGATGGTAAAGGTTGGC | 50 | 533 | [20] |
| MecA-r | AGTTCTGGAGTACCGGATTTGC |
| Isolates | Resistance Phenotype, n (%) | p Value | ||
|---|---|---|---|---|
| cMLS | iMLS | MS | ||
| MRSA (n = 38) | 28 (73.7%) | 7 (18.4%) | 3 (7.9%) | 0.009 (Significant) |
| MSSA (n = 15) | 4 (26.7%) | 10 (66.6%) | 1 (6.7%) | |
| Resistance Genotype | No. of Isolates | Pearson Chi square (p) | ||||
|---|---|---|---|---|---|---|
| AZM + LIN | AZM + CEF | AZM + GEN | AZM + AMI | AZM + CTX | ||
| ermA | 8 | 0.189 | 0.050 | 0.034 | NA | 0.351 |
| ermC | 14 | 0.452 | 0.393 | 0.08 | NA | 0.168 |
| msrA | 3 | 0.369 | 0.045 | 0.173 | NA | 0.597 |
| ermA, msrA | 2 | 0.215 | 0.191 | 0.324 | NA | 0.023 |
| ermC, msrA | 10 | 0.099 | 0.275 | 0.9 | NA | 0.098 |
| ermA, ermC, msrA | 1 | 0.387 | 0.362 | 0.491 | NA | 0.767 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bishr, A.S.; Abdelaziz, S.M.; Yahia, I.S.; Yassien, M.A.; Hassouna, N.A.; Aboshanab, K.M. Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients. Biology 2021, 10, 624. https://doi.org/10.3390/biology10070624
Bishr AS, Abdelaziz SM, Yahia IS, Yassien MA, Hassouna NA, Aboshanab KM. Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients. Biology. 2021; 10(7):624. https://doi.org/10.3390/biology10070624
Chicago/Turabian StyleBishr, Amr S., Salma M. Abdelaziz, Ibrahim S. Yahia, Mahmoud A. Yassien, Nadia A. Hassouna, and Khaled M. Aboshanab. 2021. "Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients" Biology 10, no. 7: 624. https://doi.org/10.3390/biology10070624
APA StyleBishr, A. S., Abdelaziz, S. M., Yahia, I. S., Yassien, M. A., Hassouna, N. A., & Aboshanab, K. M. (2021). Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients. Biology, 10(7), 624. https://doi.org/10.3390/biology10070624







