Life-On-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
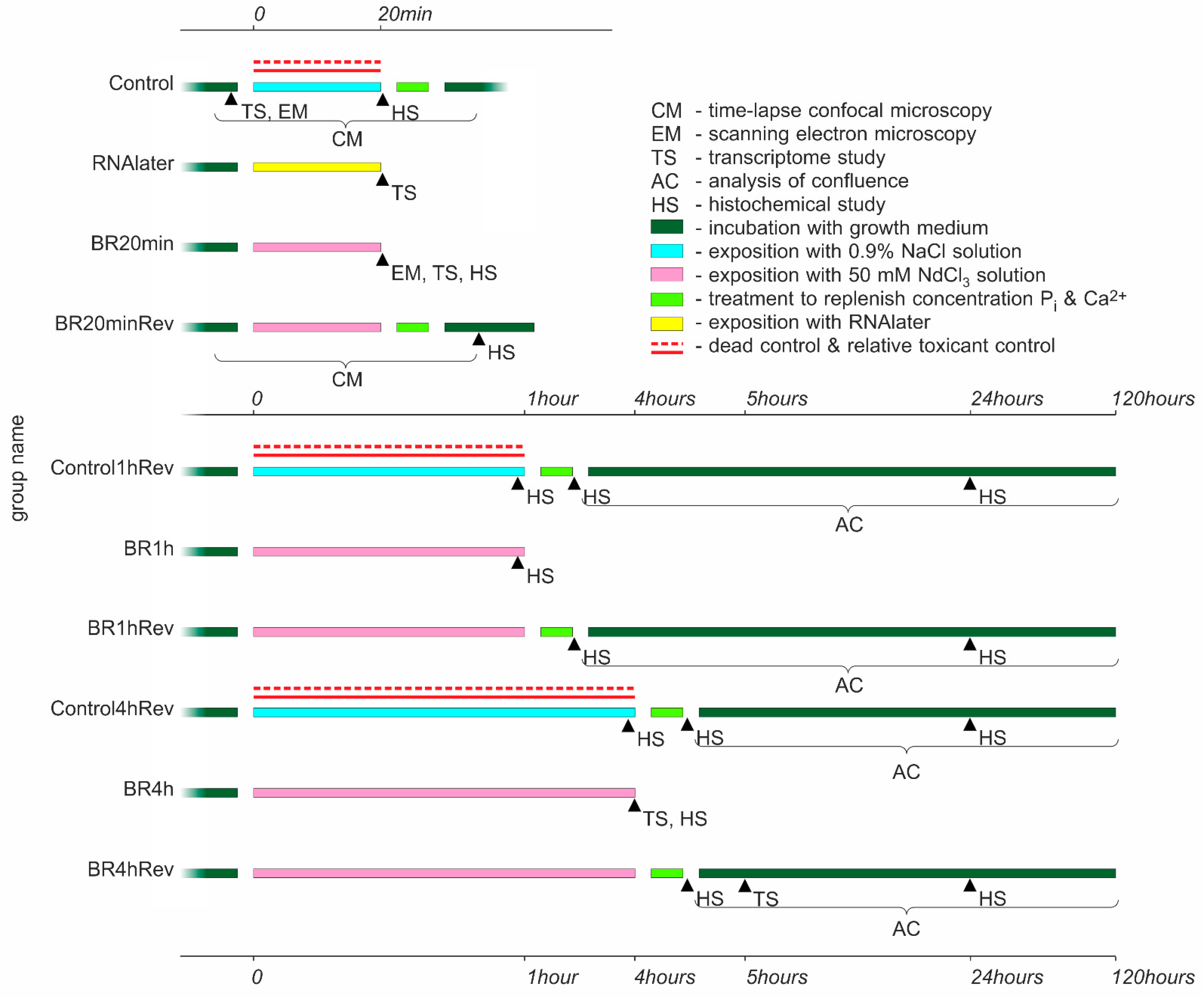
2.1. General Study Design
2.2. Cell Culture
- (1)
- For the transcriptome study: on 3.5 cm diameter Petri dishes (SPL Life Sciences, Pocheon-si, Korea) at a density of 60 × 103/cm2 and grown to a density of 300 × 103/cm2 for three days before harvesting;
- (2)
- For proliferation assay: on 12-well plate (SPL life sciences) at a density of 40 × 103/cm2 one day before the experiment;
- (3)
- For SEM: histochemical staining on the 3.5 cm diameter Petri dishes at a density of 40 × 103/cm2 one day before the experiment;
- (4)
- For confocal microscopy: on the POC-R2 (PeCon GmbH, Erbach, Germany) closed perfusion cell cultivation system at a density of 100 × 103/cm2 one day before the experiment.
2.3. Lanthanoid Treatment
- 20 min for confocal and electron microscopy
- 1 h and 4 h for proliferation assays
- 20 min and 4 h for transcriptome study
- 20 min, 1 h, and 4 h for histochemical study
2.4. Treatment to Replenish the Ions Concentration
- (1)
- 0.9% NaCl solution—wash out remaining REE ions;
- (2)
- PBS, pH 7.4—returning Pi;
- (3)
- HBSS with Ca2+ and Mg2+ (the concentration of Ca2+ in this solution was increased to 3.9 mM)—returning calcium ions;
- (4)
- 0.9% NaCl solution—wash out of excess calcium;
- (5)
- Growth medium (RPMI-1640 with 10% FBS).
2.5. Control Groups
2.6. Histochemical Staining
2.7. Electron Microscopy
2.8. Epifluorescence Microscopy
2.9. Confocal Microscopy
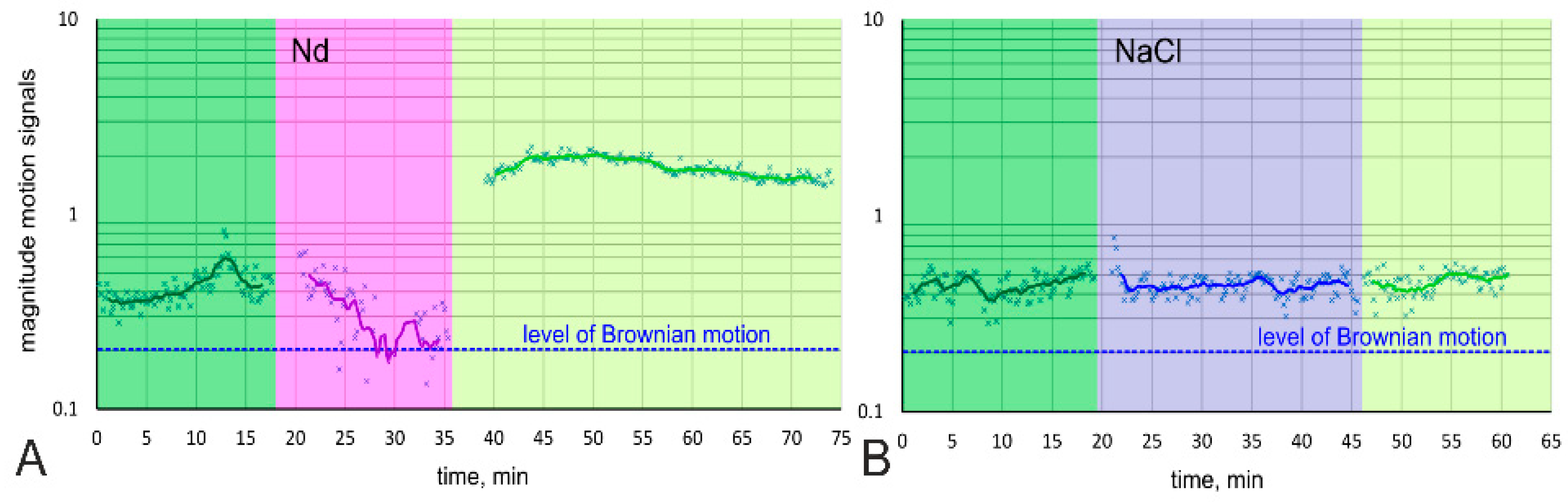
2.10. Analysis of Cell Motion
2.11. Proliferation Assays
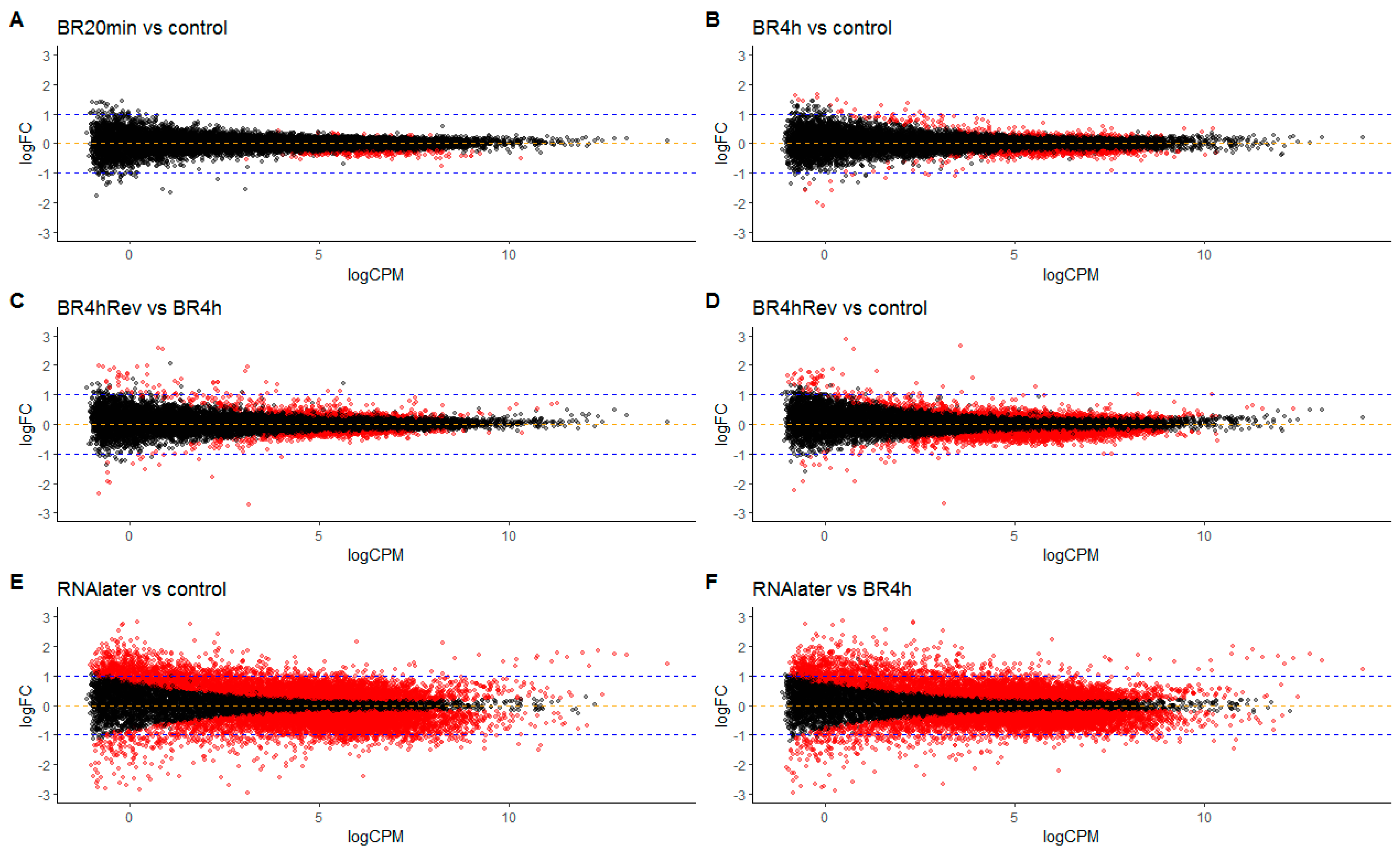
2.12. Transcriptome Experiments
2.12.1. RNA Extraction and Sequencing
2.12.2. Data Analysis
3. Results
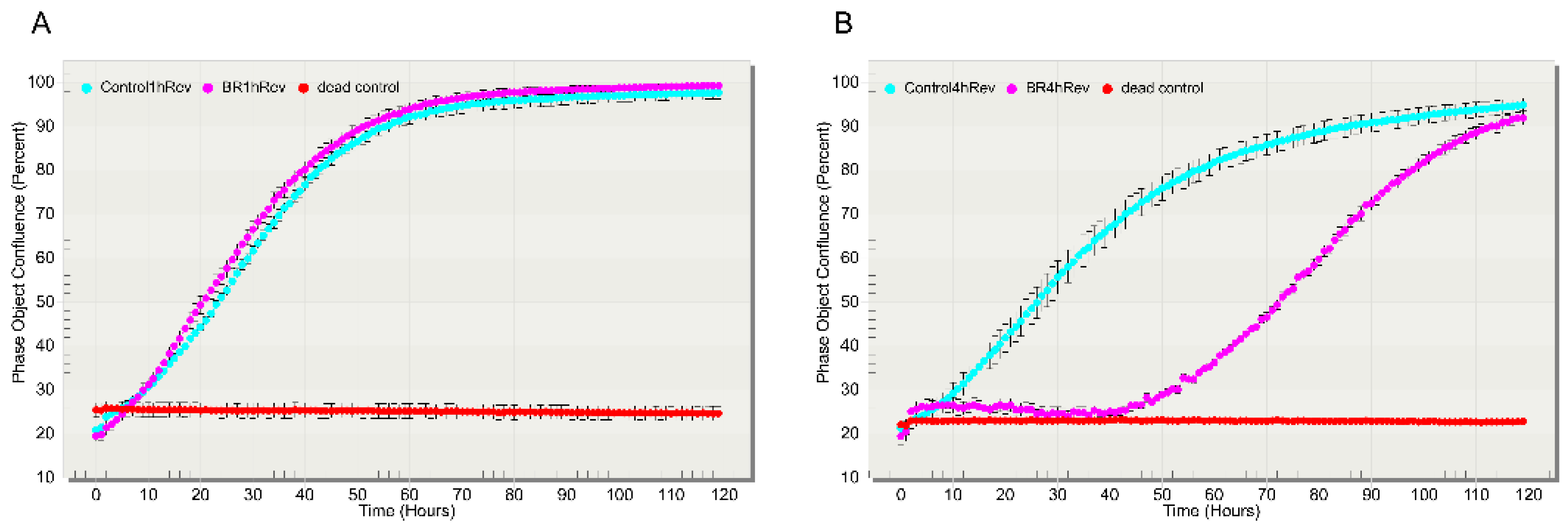
3.1. General Morphology of the Cell, Cell Movement Analysis
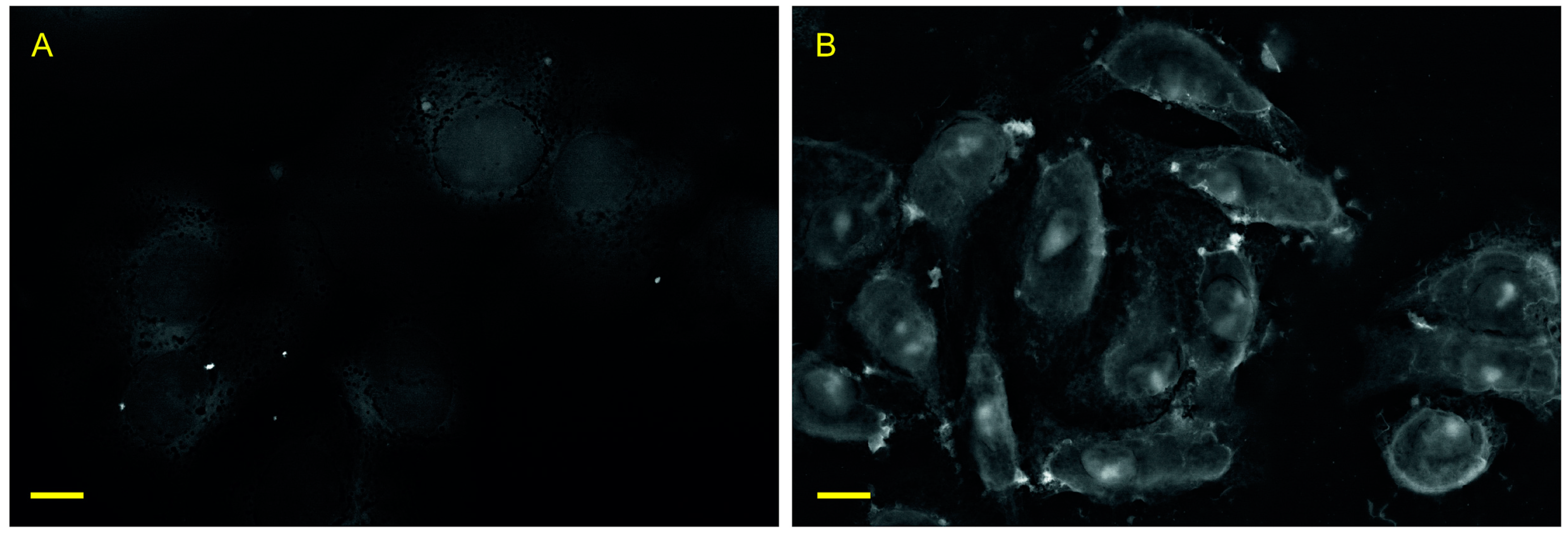
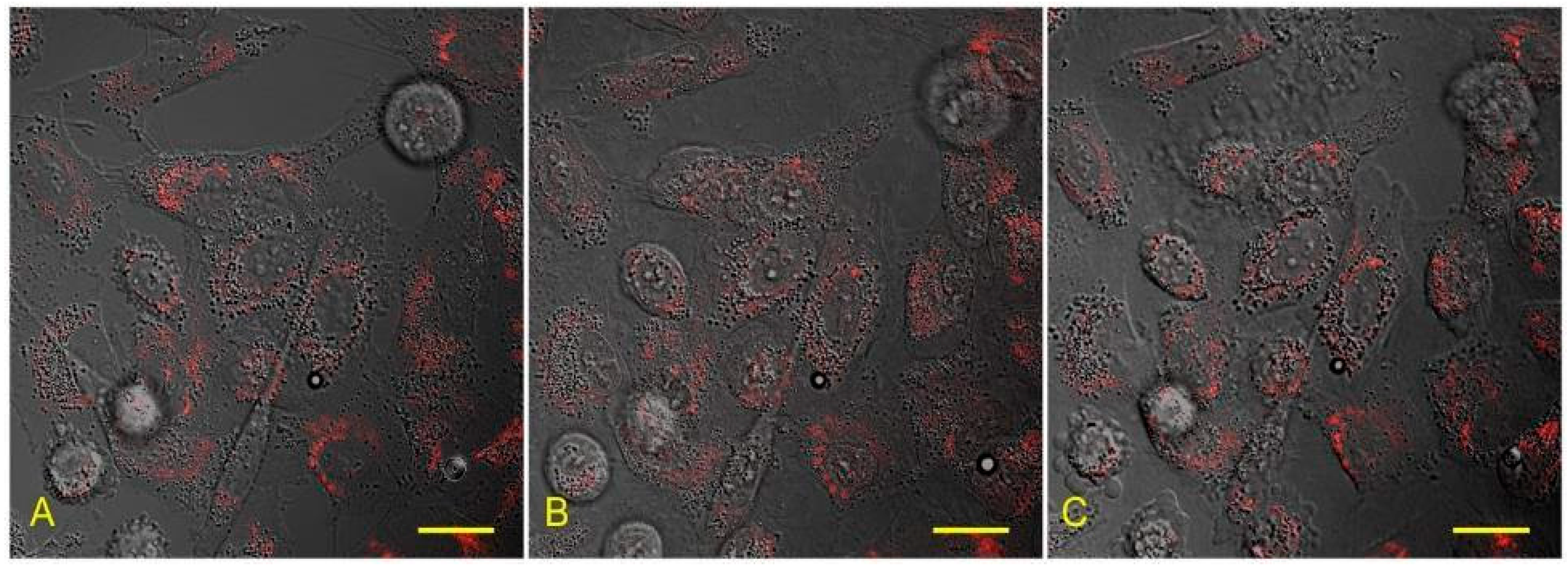
3.2. Preservation of Organelles in Cells after Recovery from Lanthanoid-Induced State
3.3. Proliferation of Cells after Lanthanoid Treatment
3.4. Differential Gene Expression in Cells in Return to Lanthanoid Treatment
4. Discussion
4.1. Nature of Freezing Processes
4.2. Presupposition
- The electrochemical activity of lanthanoid cations and their effective ionic radius allow them to affect all reactions involving calcium in the cell, as well as to replace calcium in organometallic compounds.
- It has a high reactivity with bases of weak and medium polybasic inorganic acids. The salts arising in the course of such reactions are insoluble and biologically inert in the intracellular environment.
- The lanthanoid cation and the paired chloride anion can exist in balanced solution under physicochemical conditions corresponding to the normal environment of the cell interior.
4.3. Blockage of Calcium Metabolism—“High Temperature Freezing”
- 2Ca2+ → Na+ + Nd3+
- 2Ca2+ → K+ + Nd3+
4.4. Conversion of Free Phosphate into an Insoluble form–“Discharging”
4.5. Reversibility of REEbernation State
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, L.; Beauchesne, P.R. Dimethyl sulfoxide-free cryopreservation for cell therapy: A review. Cryobiology 2020, 94, 9–17. [Google Scholar] [CrossRef]
- Crowe, J.H. Anhydrobiosis: An Unsolved Problem with Applications in Human Welfare. Membr. Hydr. 2015, 71, 263–280. [Google Scholar]
- Passow, C.N.; Kono, T.J.Y.; Stahl, B.A.; Jaggard, J.B.; Keene, A.C.; McGaugh, S.E. Nonrandom RNAseq gene expression associated with RNAlater and flash freezing storage methods. Mol. Ecol. Resour. 2019, 19, 456–464. [Google Scholar] [CrossRef]
- Yang, J.; Diaz, N.; Adelsberger, J.; Zhou, X.; Stevens, R.; Rupert, A.; Metcalf, J.A.; Baseler, M.; Barbon, C.; Imamichi, T.; et al. The effects of storage temperature on PBMC gene expression. BMC Immunol. 2016, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Nesmelov, A.; Cornette, R.; Gusev, O.; Kikawada, T. The antioxidant system in the anhydrobiotic midge as an essential, adaptive mechanism for desiccation survival. Adv. Exp. Med. Biol. 2018, 1081, 259–270. [Google Scholar]
- McSkimming, A.; Cheisson, T.; Carroll, P.J.; Schelter, E.J. Functional Synthetic Model for the Lanthanide-Dependent Quinoid Alcohol Dehydrogenase Active Site. J. Am. Chem. Soc. 2018, 140, 1223–1226. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Yuan, L.; Yang, X.; Wang, K.; Ke, Y.; Qian, Z.M. La3+-promoted proliferation is interconnected with apoptosis in NIH 3T3 cells. J. Cell. Biochem. 2005, 94, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G.; Wengler, G.; Koschinski, A. A short treatment of cells with the lanthanide ions La3+, Ce3+, Pr3+ or Nd3+ changes the cellular chemistry into a state in which RNA replication of flaviviruses is specifically blocked without interference with host-cell multiplication. J. Gen. Virol. 2007, 88, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Novikov, I.; Subbot, A.; Turenok, A.; Mayanskiy, N.; Chebotar, I. A rapid method of whole cell sample preparation for scanning electron microscopy using neodymium chloride. Micron 2019, 124, 102687. [Google Scholar] [CrossRef] [PubMed]
- Avetisov, S.J.; Novikov, I.A.; Mahotin, S.S.; Surnina, Z.V. Calculation of anisotropy and symmetry coefficients of corneal nerve orientation based on automated recognition of digital confocal images. Biomed. Eng. 2015, 49, 155–159. [Google Scholar] [CrossRef]
- Horn, B.K.P.; Schunck, B.G. Determining optical flow. Artif. Intell. 1981, 17, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Mutter, G.L.; Zahrieh, D.; Liu, C.; Neuberg, D.; Finkelstein, D.; Baker, H.E.; Warrington, J.A. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics 2004, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Marotta, R.; Leasi, F.; Uggetti, A.; Ricci, C.; Melone, G. Dry and survive: Morphological changes during anhydrobiosis in a bdelloid rotifer. J. Struct. Biol. 2010, 171, 11–17. [Google Scholar] [CrossRef]
- Gusev, O.; Nakahara, Y.; Vanyagina, V.; Malutina, L.; Cornette, R.; Sakashita, T.; Hamada, N.; Kikawada, T.; Kobayashi, Y.; Okuda, T. Anhydrobiosis-associated nuclear DNA damage and repair in the sleeping chironomid: Linkage with radioresistance. PLoS ONE 2010, 5, e14008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoport, A.; Golovina, E.A.; Gervais, P.; Dupont, S.; Beney, L. Anhydrobiosis: Inside yeast cells. Biotechnol. Adv. 2019, 37, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation tolerance: Avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef] [Green Version]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Smith, R.S.; Kay, B.D. The existence of supercooled liquid water at 150K. Nature 1999, 398, 788–791. [Google Scholar] [CrossRef]
- McGann, L.E.; Farrant, J. Survival of tissue culture cells frozen by a two-step procedure to -196 degrees C. II. Warming rate and concentration of dimethyl sulphoxide. Cryobiology 1976, 13, 269–273. [Google Scholar] [CrossRef]
- Horovitz, C.T. Biochemistry of Scandium and Yttrium, Part 2: Biochemistry and Applications 1–38; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Sigel, H. Metal Ions in Biological Systems: Volume 40: The Lanthanides and Their Interrelations with Biosystems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Miller, T.W.; Tormey, J.M. Calcium displacement by lanthanum in subcellular compartments of rat ventricular myocytes: Characterisation by electron probe microanalysis. Cardiovasc. Res. 1993, 27, 2106–2112. [Google Scholar] [CrossRef]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Briner, W. The toxic effect of lanthanum on planaria is mediated by a variety of ion channels. Toxics 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, G.A.; Kohmoto, O.; Barry, W.H. Detection of La3+ influx in ventricular cells by indo-1 fluorescence. Am. J. Physiol. 1989, 256, C351-7. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Bikle, D.D. Lanthanum influx into cultured human keratinocytes: Effect on calcium flux and terminal differentiation. J. Cell. Physiol. 1992, 151, 623–629. [Google Scholar] [CrossRef]
- Behera, S.; Long, Y.; Schmitz-Thom, I.; Wang, X.; Zhang, C.; Li, H.; Steinhorst, L.; Manishankar, P.; Ren, X.; Offenborn, J.N.; et al. Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol. 2017, 213, 739–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Merino, F.C.; Ramírez-Martínez, M.; Castillo-González, A.M.; Trejo-Téllez, L.I. Lanthanum prolongs vase life of cut tulip flowers by increasing water consumption and concentrations of sugars, proteins and chlorophylls. Sci. Rep. 2020, 10, 4209. [Google Scholar] [CrossRef]
- Barman, S.A.; Saari, J.T.; Olson, M.D. Calcium Antagonists: Mechanism of Action on Cardiac Muscle and Vascular Smooth Muscle; Sperelakis, N., Caulfield, J.B., Eds.; Springer: New York, NY, USA, 1984; Volume 39, pp. 257–273. [Google Scholar]
- Shimizu, H.; Borin, M.L.; Blaustein, M.P. Use of La3+ to distinguish activity of the plasmalemmal Ca2+ pump from Na+/Ca2+ exchange in arterial myocytes. Cell Calcium 1997, 21, 31–41. [Google Scholar] [CrossRef]
- Rai, D.; Felmy, A.R.; Yui, M. Thermodynamic model for the solubility of NdPO 4 (c) in the aqueous Na+-H+-H2PO4–HPO42–OH–Cl–H2O system. J. Radioanal. Nucl. Chem. 2003, 256, 37–43. [Google Scholar] [CrossRef]
- Levi, M.; Gratton, E.; Forster, I.C.; Hernando, N.; Wagner, C.A.; Biber, J.; Sorribas, V.; Murer, H. Mechanisms of phosphate transport. Nat. Rev. Nephrol. 2019, 15, 482–500. [Google Scholar] [CrossRef]
- Yajima, H.; Sumaoka, J.; Miyama, S.; Komiyama, M. Lanthanide ions for the first non-enzymatic formation of adenosine 3′,5′-cyclic monophosphate from adenosine triphosphate under physiological conditions. J. Biochem. 1994, 115, 1038–1039. [Google Scholar] [CrossRef]
- Matsumura, K.; Komiyama, M. Enormously fast RNA hydrolysis by lanthanide(III) ions under physiological conditions: Eminent candidates for novel tools of biotechnology. J. Biochem. 1997, 122, 387–394. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subbot, A.; Kondratieva, S.; Novikov, I.; Gogoleva, N.; Kozlova, O.; Chebotar, I.; Gazizova, G.; Ryabova, A.; Vorontsova, M.; Kikawada, T.; et al. Life-On-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells. Biology 2021, 10, 607. https://doi.org/10.3390/biology10070607
Subbot A, Kondratieva S, Novikov I, Gogoleva N, Kozlova O, Chebotar I, Gazizova G, Ryabova A, Vorontsova M, Kikawada T, et al. Life-On-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells. Biology. 2021; 10(7):607. https://doi.org/10.3390/biology10070607
Chicago/Turabian StyleSubbot, Anastasia, Sabina Kondratieva, Ivan Novikov, Natalia Gogoleva, Olga Kozlova, Igor Chebotar, Guzel Gazizova, Anastasia Ryabova, Maria Vorontsova, Takahiro Kikawada, and et al. 2021. "Life-On-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells" Biology 10, no. 7: 607. https://doi.org/10.3390/biology10070607
APA StyleSubbot, A., Kondratieva, S., Novikov, I., Gogoleva, N., Kozlova, O., Chebotar, I., Gazizova, G., Ryabova, A., Vorontsova, M., Kikawada, T., Shagimardanova, E., & Gusev, O. (2021). Life-On-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells. Biology, 10(7), 607. https://doi.org/10.3390/biology10070607







