Simple Summary
Mounting evidence suggests a role for extracellular vesicles in cell-to-cell communication, in both physiological and pathological conditions. Moreover, the molecular content of vesicles can be exploited for diagnostic and therapeutic purposes. Inflamed tissues and tumors are often characterized by hypoxic areas, where oxygen levels drop dramatically. Several studies demonstrated that hypoxic stress affects the release of vesicles and their content. This review is intended to provide an exhaustive overview on the relationship between hypoxia and vesicles in inflammatory diseases and cancer.
Abstract
Hypoxia is a severe stress condition often observed in cancer and chronically inflamed cells and tissues. Extracellular vesicles play pivotal roles in these pathological processes and carry biomolecules that can be detected in many biofluids and may be exploited for diagnostic purposes. Several studies report the effects of hypoxia on extracellular vesicles’ release, molecular content, and biological functions in disease. This review summarizes the most recent findings in this field, highlighting the areas that warrant further investigation.
Keywords:
extracellular vesicles; exosomes; microvesicles; liquid biopsy; biomarker discovery; hypoxia; HIF; cancer; inflammation 1. Introduction
1.1. Extracellular Vesicles
In the last two decades, extracellular vesicles (EVs) have been the subject of extensive research. EVs were first discovered in the 1970s and, by this generic term, researchers referred to a heterogeneous group of membrane vesicles with different sizes (from 10 nm to 10 µm), biological origins, and molecular content [1,2,3,4].
EVs have been classified based on size or biogenesis. Exosomes are nanoparticles with a diameter ranging between 30–120 nm, microvesicles (MVs), microparticles, and ectosomes between 120–1000 nm and large EVs, such as oncosomes and apoptotic bodies, between 1–10 µm [5,6,7]. Recently, the Lyden lab discovered the exomeres, a novel type of small nanoparticles (35 nm) without an external membrane structure [8].
Two categories of EVs can be distinguished according to their biogenesis. The so-called MVs are generated following rearrangement of the cell cytoskeleton and budding of plasma membrane. Exosomes, instead, are produced after the inward budding of the endosomal membrane and the formation of multivesicular bodies (MVBs). These MVBs can fuse with the cell plasma membrane releasing their content, the exosomes, in the extracellular environment [2,3,5,9].
It has been extensively demonstrated that most cell types produce EVs (e.g., red blood cells; platelets; neurons; cells of the immune system, like dendritic cells, B cells, T cells; fibroblasts; epithelial cells; tumor cells, etc.), and different cell types release different EV repertoires [5,6,9,10,11,12,13]. EV content is highly heterogeneous (lipids, proteins, DNA, mRNA, miRNA, ncRNA species), and it is not simply a reflection of the donor cell composition, but the result of a well-regulated sorting mechanism that can be modulated in response to different stimuli or depending on the physiological or pathological state of the cell [5,6,14,15]. The release of EVs is an evolutionary-preserved mechanism of both unicellular and multicellular organisms [5,16,17]. EV secretion was initially believed to be a mechanism to discard cell waste [3,10,11]. Further research demonstrated that EVs are an effective means for autocrine and paracrine cell-to-cell signaling, and they influence the phenotype of recipient cells [1,5,6,18,19,20,21]. EVs exert a wide variety of biological functions. In cancer, EVs are involved in primary tumor formation and invasive processes like angiogenesis and metastasis [1,22,23,24,25,26]. EVs also promote the spread of infectious pathogens [10]. By transferring biomolecules from one cell to another, EVs from dendritic cells may mediate adaptive immune response to tumor cells or pathogen agents [27].
1.2. Diagnostic Value of EVs
EVs are considered potential candidates for early detection, diagnosis and monitoring of cancer, protecting biomarkers from degradation, and carrying them in most circulating body fluids, including plasma, serum, saliva, urine, cerebrospinal fluid, broncheo-alveolar lavage, breast milk, amniotic fluid, and semen ([2,9,10,19,28,29,30,31,32,33,34,35]; Figure 1). EVs are released at an early stage of pathogenesis, providing an ideal source of biomarkers for screening, early diagnosis and improving clinical decision-making that, currently, relies on risk factors (for example, tobacco and alcohol consumption), medical history, and clinical examination with imaging and biomarkers with limited sensitivity/specificity to detect early-stage disease (e.g., Prostate Specific Antigen) [36]. EVs are also emerging as a next-generation platform for liquid biopsy and provide, together with circulating tumor cells and cell-free DNA, diagnostic benefits that overcome the limits of tissue biopsies (Table 1). In fact, with the latter approach, which is still the reference method for cancer diagnosis, the information obtained may not be representative of the whole tumor, and this invasive technique cannot be used to monitor patient treatment. On the contrary, minimally invasive liquid biopsy approaches can provide real-time information about the nature and growth of the tumor, enabling detection of minimal residual disease and monitoring treatment response [37].
Figure 1.
A non-invasive blood sampling allows EV isolation for biomarkers studies (DNA, RNA species, proteins).

Table 1.
Features that make EVs potential diagnostic candidates.
1.3. Hypoxia
Tissue hypoxia occurs when the state of oxygen homeostasis is altered and oxygen demand exceeds supply ensues. Hypoxia can be a physiological condition, as it happens in intestinal mucosa, renal medulla, bone marrow and lymph nodes, or a pathological state [38,39]. Hypoxia as pathological stress arises when blood supply to a tissue is compromised, as in myocardial infarction, renal ischemic injury, or when a reduction of oxygen levels and nutrients occurs in the cellular microenvironment, as in inflammation and solid cancers. Inflammation and hypoxia are closely interconnected: increased oxygen metabolism occurs in acute inflammation, thus leading to hypoxia, and hypoxic tissues are chronically inflamed [38,40]. Hypoxia is a common microenvironmental feature in a range of inflammatory disorders including inflammatory bowel disease, rheumatoid arthritis, and chronic infection. In inflamed and infected tissues, hypoxia is often the result of disrupted blood flow and increased metabolic activity of both inflamed resident and infiltrating activated immune cells and oxygen consumption by some bacterial species [41,42,43].
Hypoxia in cancer is a consequence of both high oxygen demand from proliferating cells and low oxygen supply due to the irregularities in tumor vascularization [44,45,46]. Indeed, the inner part of the tumor mass is in a low perfusion state due to its distance from blood vessels [47]. Hypoxic areas in tumors can act as incubators of cells with malignant evolution, since only the more aggressive phenotypes survive [11,45,48]. Malignant cells adapt to hypoxic microenvironment modulating the transcription of several genes associated with metabolic reprogramming, angiogenesis, epithelial-to-mesenchymal transition (EMT), proliferation, migration, metastasis, and therapeutic resistance [11,49,50,51,52].
Cells have developed molecular mechanisms to sense oxygen levels and adapt their metabolism based on oxygen availability [53,54]. The family of hypoxia-inducible factors (HIFs) are considered the “master regulators” of oxygen homeostasis: they regulate the expression of thousands of genes involved in cell survival, metabolism, angiogenesis, and erythropoiesis [38,39,41,42,44,55,56,57,58,59,60]. Three types of HIFs are known: HIF1, HIF2, and HIF3. They consist of heterodimers of two subunits, α (HIF1α, HIF2α or HIF3α) and β, while the β subunits are constitutively expressed in the nucleus and are largely insensitive to changes in oxygen tension, the level of the α subunits is acutely oxygen sensitive and they are synthesized de novo at a high rate [61]. When oxygen is available, the α subunits are hydroxylated at proline and asparagine residues, which targets them for proteasomal degradation [50,57,62,63]. In low oxygen conditions, α subunits can dimerize with HIFβ, allowing them to bind to promoters of target genes [51,64].
1.4. Purpose of the Review
Many recent studies have demonstrated that hypoxia increases the release of EVs in multiple inflammatory diseases and types of cancer, causing various biological effects depending on disease and cell type [65,66,67,68]. With this mechanism of cell-to-cell communication, cancer cells alter the phenotype of stromal cells and other tumor cells [22,69]. Moreover, hypoxic EVs seem to play a role in angiogenesis, stemness, activation of cancer associated fibroblasts (CAFs), and EMT [51]. Hypoxia-induced EVs promote the pathogenesis of diseases, through the transfer of specific biomolecules which could be exploited as potential exosome-derived biomarkers or to develop potential therapeutic targets. The aim of this review is to provide a comprehensive overview on the interplay between EVs and hypoxia in inflammatory diseases and cancer. In this review, we summarize the current literature on the following topics: (a) the effect of hypoxia on the release of vesicles in terms of amount and content; (b) the biological roles of extracellular vesicles released under hypoxia in different types of disease; and (c) the role of HIF signaling pathways in modulating EV release and functions.
2. Effects of Hypoxia on EVs in Inflammatory Diseases
Increase of EV secretion under hypoxia is described in several inflammatory diseases, like pulmonary arterial hypertension (PAH), kidney fibrosis, kidney injury, obesity, and obstructive sleep apnea (OSA) [70,71,72,73,74]. A schematic overview of the functions of EVs released under hypoxia in different inflammatory diseases is provided in Table 2.

Table 2.
Effects of hypoxia on EVs in inflammatory diseases.
Hypoxia-induced EVs play a role in disease pathogenesis, as described in PAH, obesity, and OSA. In PAH, exosomes derived from pulmonary artery endothelial cells (PAECs) are involved in the overproliferation and apoptosis resistance of pulmonary artery smooth muscle cells (PASMC), remodeling the pulmonary vasculature toward hypertension. These exosomes carry microRNAs, such as miR-17 and miR-20a, which target the BMPR2 gene, or pro-inflammatory miR-21 and miR-145, that may exert an effect on the recipient cells through specific signaling pathways, which need to be fully elucidated. Plasma level of endothelium-derived exosomes could be used as a diagnostic marker of PAH [70]. In obesity, the hypoxic microenvironment influences the exosome protein content. In particular, hypoxic exosomes show increased levels of proteins associated with metabolic processes, such as G6PD, FASN, ACC enzymes, responsible for lipogenesis. Due to these lipogenic enzymes, hypoxic exosomes can promote the accumulation of lipids in normoxic cells. Exosomal proteins may become novel biomarkers for obesity-associated adipose dysfunction [72]. OSA is characterized by intermittent hypoxia (IH), and it is correlated with increased incidence of cancer and poor prognosis. Several studies have demonstrated that IH leads to changes in exosomal miRNA cargo [73,75,76]. Khalyfa et al. showed that hypoxic-exosomes released from endothelial cells, progenitor cells, monocytes, lymphocytes and platelets carry a differentially expressed group of miRNAs, which regulate genes involved in cardiovascular dysfunction, immune and atherosclerosis-related pathways [73]. Moreover, exosomes obtained from sleep fragmented mice (a treatment mimicking OSA) and from OSA patients contain a unique set of miRNAs involved in cancer-related pathways [75]. In the study of Almendros et al., OSA-induced IH was found to promote the release of tumor-derived EVs from tumor bearing mice [76]. These EVs promoted in vitro proliferation of tumor cells, migration of TC1 cells and the disruption of the endothelial monolayer barrier, facilitating metastasis in vivo [76].
Several studies provide evidence for the therapeutic effects of hypoxia-induced EVs. In kidney fibrosis, hypoxic EVs promote repair of injured parenchyma, inducing fibroblasts’ activation and proliferation through the transfer of TGF-β1 mRNA [71]. Renal ischemia-reperfusion injury (I/R) is a condition where hypoxia induces tubular cell death, compromising renal function, and it can evolve into Acute Kidney Injury (AKI). In this context, EVs released from hypoxic renal proximal tubular cells (RPTC) have a protective role [62]. HIF1-induced exosomes from RPTCs seem to have a cytoprotective role, preventing apoptosis in the RPTC model [74]. Hypoxia preconditioning (HPC) in human kidney cells is a process that simulates ischemic preconditioning (IPC) in vitro. During HPC, renal tubular epithelial cells (RTECs) release functional EVs that have therapeutic effects in renal ischaemia-reperfusion (I/R) injury. A group of 16 differentially expressed miRNAs with protective properties was found in HPC-EVs, but the molecular mechanisms involved remain largely unknown [77]. Myocardial infarction (MI) leads to degenerative myocardial remodeling and cardiac dysfunction and, as a result, infarcted tissue is often hypoxic. Cardiac progenitor cells (CPCs) are a small population of stem-like cells residing in the heart. Exosomes isolated from CPCs under hypoxic conditions may have a therapeutic potential, since they are internalized by cardiac fibroblasts and endothelial cells and enhance tube formation in a dose-dependent manner. These hypoxic exosomes also reduce cardiac fibrosis in a rat model. A group of seven miRNAs, encapsulated by exosomes, are upregulated under hypoxia, and most of them are known to regulate cardiac functions [78]. Zhu et al. demonstrated that hypoxic exosomes released from mesenchymal stem cells (MSCs) facilitate ischemic myocardium repair after MI, through the transfer of miR-125b-5p which exhibits an anti-apoptotic effect in vivo and in vitro. Indeed, miR-125b-5p downregulates the expression of the apoptotic genes p53 and BAK1. For this reason, hypoxic-exosomes may be exploited for a therapeutic approach of ischemic disease [79]. Therapeutic effects of exosomes from overexpressing HIF1α-MSCs are reported in the study of Gonzalez-King et al. These MSC-exosomes carry microRNAs and Jagged1 protein, targeting Notch genes involved in angiogenesis in vitro and in vivo. Thus, MSC-derived exosomes may have a potential application for the treatment of ischemia [80].
Few studies described the correlation between HIFs and the release of EVs. During HPC, the production of EVs from RTECs is regulated by the HIF1α/Rab22 pathway [77]. Another study shows similar results in the rat RPTC model, testing the effects of an inhibitor and an inducer of HIF1, under normoxia and hypoxia. This study clearly demonstrates the involvement of HIF1 in the release of exosomes under hypoxic conditions [74]. Exosome release is enhanced in HIF1α-overexpressing MSCs and proteins are packaged into exosomes in an HIF1α-dependent manner [80]. In acute myocardial infarction (AMI), hypoxic cardiomyocytes secrete exosomes containing functionally active TNF-α, under the regulation of HIF1 [81].
3. Effects of Hypoxia on EVs in Cancer
It is now demonstrated that hypoxia affects the production, size, and molecular content of EVs during cancer. However, some studies have shown dissimilar observations using different cell models and hypoxic treatments [22,66,69,82,83,84,85,86,87,88,89]. Evidence that the number of EVs is enhanced under hypoxia is provided in several types of cancer. In three breast cancer cell models, the number of exosomes released is higher in both moderate and severe hypoxic conditions (1% and 0.1%, respectively) than normoxic controls [63]. The budding of MVs from breast cancer cells is enhanced under hypoxia, while it is impaired in HIF1- and HIF2-knockdown models [22]. Hypoxia-resistant multiple myeloma cells (HR-MM) secrete 2-fold more exosomes than normoxic cells, notwithstanding that the size and shape of EVs are identical [85]. Hypoxic CL1-5 lung cancer cells secrete a greater amount of exosomes, compared to normoxic cells [86]. Different types of hypoxic ovarian cancer cells show a 2–6-fold increase in exosome release, compared to normoxic cells [87]. Significant increased release of EVs is observed in pancreatic cancer (PC) cells under hypoxia [89]. Hepatocellular carcinoma (HCC) cells exposed to hypoxic conditions show increased exosomal production [88]. Contrarily, Ramteke et al. did not found differences in the amount of secreted EVs from hypoxic prostate cancer (PCA) cells compared to normoxic cells, although, under hypoxia, EVs have smaller size [69]. No significant differences were also found between hypoxic and normoxic exosomes from human leukemia K562 cells, in terms of count and size [84].
Through their cargo, hypoxic EVs mediate several processes that contribute to the development of cancer, like angiogenesis, proliferation, EMT, and metastasis. Some miRNAs packaged into EVs have been widely recognized to be responsible for specific effects, like miR-210 and miR-135b involved in angiogenesis and metastasis, and they may be considered as cancer biomarkers’ candidates or potential therapeutic targets. A schematic overview of the functions of EVs released under hypoxia is provided in Figure 2 and Table 3. Hypoxic exosomes from breast cancer cells contain high levels of miR-210, which is involved in endothelial cell tubulogenesis and mechanisms of repressing DNA repair [63]. Jung et al. reported that hypoxic exosomes transfer miR-210 to normoxic cells and endothelial cells, thus promoting angiogenic responses in breast cancer. Moreover, exosomes isolated from the serum of hypoxic tumor-bearing mice have high levels of miR-210, indicating it as a potential biomarker for hypoxic tumors [90]. Hypoxic MVs from breast cancer cells were incubated with naïve breast cancer cells, resulting in the increase of focal adhesion formation, invasion, and metastasis [22]. Tadokoro et al. found high levels of miR-210 in hypoxic K562 exosomes, involved in tube formation of HUVECs [84]. Exosomal miR-210 probably downregulates the expression of Ephrin-A3 and PTP1B genes, enhancing angiogenic responses [84,90]. In multiple myeloma cell models, hypoxia enhances exosomal miR-210 and miR-135b expression, which promote tube formation and local angiogenesis, respectively [85]. Hypoxic A549 cells release large amounts of exosomes enriched in miR-135b and miR-210 to enhance cancer cell survival, migration, and tube formation [91]. More than 50% of the proteins secreted by A431 squamous carcinoma cells, exposed to hypoxic stress, are associated with exosomes, and these proteins are involved in angiogenesis and metastasis [26]. Hypoxic glioblastoma multiforme (GBM) cells secrete MV bearing Tissue Factor (TF), involved in the coagulation cascade and also in angiogenesis [92]. Kucharzewska et al. showed that hypoxic exosomes from GBM cells have a pro-angiogenic effect through several exosome-associated proteins and mRNAs [93]. Moreover, CL1-5 hypoxic exosomes have been found to increase local and distant angiogenesis, compared to normoxic control, through the transfer of miR-23a. Exosomal miR-23a directly inhibits PHD1 and PHD2 expression, leading to HIF1 accumulation, and tight junction protein ZO-1, inducing increased vascular permeability and cancer transendothelial migration [86]. Ramteke et al. observed that hypoxic PCA exosomes support invasiveness, motility, and stemness of naïve PCA cells, having an higher metalloproteinase activity, targeting the expression of adherens junction molecules and inducing the CAF-type phenotype in prostate fibroblasts [69]. Another publication of the same group reported that hypoxic PCA exosomes possess specific lipid composition related to growth and invasiveness of hypoxic PCA cells [94]. In small cell lung cancer, NCI-H1688, and non-small cell lung cancer NCI-H2228, exosomes secreted under hypoxia are enriched in TGF-β and IL-10 and are able to promote the migration of endothelial and cancer cells and metastasis [95]. Hypoxic exosomes from ovarian cancer cells carry oncogenic proteins that can alter the surrounding cells, enhancing tumor progression, metastasis, and chemoresistance [87]. Two studies report the functional property of hypoxic-exosomes to induce M2 polarization of macrophages, a behavior associated with tumor proliferation [96]. Hypoxic exosomes from different cancer cells are enriched in chemokines and growth factors that mediate immunological effects, like monocyte/macrophage recruitment, host immunosuppression, and M2-like macrophage polarization [66]. Hypoxic PC exosomes carry miR-301a-3p, responsible for M2 polarization of macrophages, migration, invasion, and EMT of PC cells both ex vivo and in vivo. Since levels of exosomal miR-301a-3p are detectable also in the serum of PC patients, it can be considered a cancer biomarker of late TNM stage and poor survival in human PC [96]. Hypoxic HCC exosomes contain high levels of miR-1273f, responsible for activating the Wnt/β-catenin signaling and thus enhancing the proliferation, migration, invasiveness, and EMT in normoxic HCC cells [88]. Exosomes released by oral squamous cell carcinoma (OSCC) cells under hypoxia contain high levels of miR-21 which promotes EMT, migration, and invasion of target normoxic cells, both ex vivo and in vivo. Thus, hypoxic mir-21-containing exosomes drive normoxic cells toward a pre-metastatic phenotype [11].
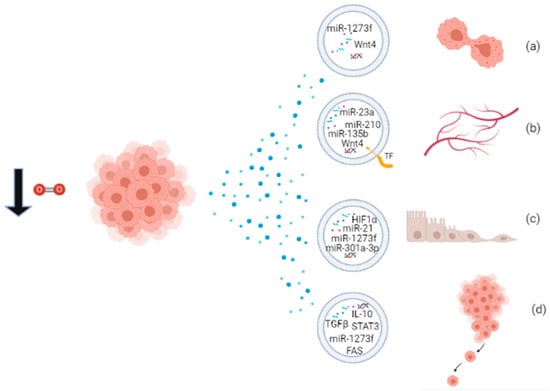
Figure 2.
Proliferating cancer cells in hypoxic condition release EVs packaged with biomolecules involved in (a) proliferation; (b) angiogenesis; (c) EMT; and (d) metastatic behavior.

Table 3.
Effects of hypoxia on EVs in cancer.
Recent studies have also shed some light on the role of HIF1 in the release of EVs under hypoxia. In a breast cancer cell model, the knockdown of HIF1α completely abrogated the enhanced release of EVs caused by hypoxia [63]. Moreover, breast cancer cells, exposed to hypoxic stress, release a large amount of MVs in an HIF-regulated mechanism, which mediates the expression of the small GTPase RAB22A. RAB22A has a critical role in vesicle formation, trafficking, and membrane fusion; it colocalizes with budding MVs and its expression is required for MVs production under hypoxia [22]. The functional property of hypoxia-induced exosomes from OSCC cells and exosomal miR-21 expression is related to HIF1 and HIF2 signaling pathways. In fact, hypoxic exosomes released by knockdown HIF1 and HIF2 OSCC cells failed to increase cell migration and invasion [11]. The publication of Aga et al. is the first to provide evidence for detectable HIF1α in exosomes secreted by nasopharyngeal carcinoma (NPC) cells. Exosomal HIF1α maintains DNA-binding activity and is transcriptionally active in recipient cells after exosome uptake. In the NPC model, exosomal cell-to-cell transmission of transcriptionally active HIF1α promotes cancer progression and invasive potential, through induction of EMT [57]. Exosomes from hypoxic colorectal cancer (CRC) cells contain a high level of Wnt4 protein, and exosomal Wnt4 upregulation is HIF1-dependent. Wnt4-bearing exosomes are able to promote endothelial cells’ proliferation and migration, tumor growth, and angiogenesis [97]. In PC cell models, stabilization of HIF-1α promotes the enhanced release of EVs [89].
4. Conclusions
Several studies have been recently published to describe the effects of hypoxic stress on EV release in different types of disease. It has been widely demonstrated that hypoxia enhances the secretion of EVs and changes their content and functions. However, the molecular mechanisms involved in EV release under hypoxia have not been fully elucidated. More studies are warranted to establish the role of HIFs in the regulation of EV release under different hypoxic conditions. Moreover, it remains to be proved if functionally active HIFs or HIF-related proteins can be carried by EVs as suggested by Aga and colleagues [57]. Finally, understanding the transcriptomic and proteomic profiles of hypoxia-induced EVs in pathological conditions may provide new diagnostic markers and can lead to potential therapeutic approaches.
Author Contributions
Writing—original draft preparation, M.V.; writing—review and editing, M.C., F.C., A.N. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures are created with BioRender.com (accessed on 15 March 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging Roles of Exosomes in Normal and Pathological Conditions: New Insights for Diagnosis and Therapeutic Applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Gould, S.J.; Raposo, G. As We Wait: Coping with an Imperfect Nomenclature for Extracellular Vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of Distinct Nanoparticles and Subsets of Extracellular Vesicles by Asymmetric Flow Field-Flow Fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of Extra-cellular Communication during Cancer Progression. J. Cell Sci. 2010, 123, 1603. [Google Scholar] [CrossRef] [Green Version]
- Schorey, J.S.; Bhatnagar, S. Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver MiR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016, 76, 1770. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. ExoCarta as a Resource for Exosomal Research. J. Extracell. Vesicle 2012, 1, 18374. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Jia, S.; Zocco, D.; Samuels, M.L.; Chou, M.F.; Chammas, R.; Skog, J.; Zarovni, N.; Momen-Heravi, F.; Kuo, W.P. Emerging Technologies in Extracellular Vesicle-Based Molecular Diagnostics. Expert Rev. Mol. Diagn. 2014, 14, 307–321. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting It out: Regulation of Exosome Loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An Integrated Database of High-Throughput Data for Systemic Analyses of Extracellular Vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The Secreted Factors Responsible for Pre-Metastatic Niche Formation: Old Sayings and New Thoughts. Semin. Cancer Biol. 2011, 21, 139–146. [Google Scholar] [CrossRef]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A Common Pathway for a Specialized Func-tion. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome Mediated Communication within the Tumor Microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypoxia-Inducible Factors and RAB22A Mediate Formation of Microvesicles That Stimulate Breast Cancer Invasion and Metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, E3234. [Google Scholar] [CrossRef] [Green Version]
- Kahlert, C.; Kalluri, R. Exosomes in Tumor Microenvironment Influence Cancer Progression and Metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Zocco, D.; Ferruzzi, P.; Cappello, F.; Kuo, W.P.; Fais, S. Extracellular Vesicles as Shuttles of Tumor Biomarkers and Anti-Tumor Drugs. Front. Oncol. 2014, 4, 267. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic Tumor Cell Modu-lates Its Microenvironment to Enhance Angiogenic and Metastatic Potential by Secretion of Proteins and Ex-osomes *. Mol. Cell. Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-B.; Zhang, Z.-R.; Schluesener, H.J.; Xu, S.-Q. Role of Exosomes in Immune Regulation. J. Cell. Mol. Med. 2006, 10, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of Exosomal Proteins in Cancer Diagnosis. Mol. Cancer 2017, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Torrano, V.; Royo, F.; Peinado, H.; Loizaga-Iriarte, A.; Unda, M.; Falcón-Perez, J.M.; Carracedo, A. Vesi-cle-MaNiA: Extracellular Vesicles in Liquid Biopsy and Cancer. Curr. Opin. Pharmacol. 2016, 29, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturella, M.; Carpi, F.M.; Zocco, D. Standardization of Blood Collection and Processing for the Diagnostic Use of Extracellular Vesicles. Curr. Pathobiol. Rep. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Pant, S.; Hilton, H.; Burczynski, M.E. The Multifaceted Exosome: Biogenesis, Role in Normal and Aberrant Cellular Function, and Frontiers for Pharmacological and Biomarker Opportunities. Biochem. Pharmacol. 2012, 83, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Hannafon, B.N.; Ding, W.-Q. Intercellular Communication by Exosome-Derived MicroRNAs in Cancer. Int. J. Mol. Sci. 2013, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clancy, J.; D’Souza-Schorey, C. Extracellular Vesicles in Cancer: Purpose and Promise. Cancer J. 2018, 24, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal MiRNAs as Biomarkers for Prostate Cancer. Front. Genet. 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosaka, N.; Kogure, A.; Yamamoto, T.; Urabe, F.; Usuba, W.; Prieto-Vila, M.; Ochiya, T. Exploiting the Message from Cancer: The Diagnostic Value of Extracellular Vesicles for Clinical Applications. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, T.; Wong, D.T.W. Liquid Biopsy in Head and Neck Cancer: Promises and Challenges. J. Dent. Res. 2018, 97, 701–708. [Google Scholar] [CrossRef]
- Taylor, C.T.; Colgan, S.P. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef]
- Vanderkooi, J.M.; Erecinska, M.; Silver, I.A. Oxygen in Mammalian Tissue: Methods of Measurement and Af-finities of Various Reactions. Am. J. Physiol. Cell Physiol. 1991, 260, C1131–C1150. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Cummins, E.P.; Keogh, C.E.; Crean, D.; Taylor, C.T. The Role of HIF in Immunity and Inflammation. Mol. Asp. Med. 2016, 47–48, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Monaci, S.; Aldinucci, C.; Rossi, D.; Giuntini, G.; Filippi, I.; Ulivieri, C.; Marotta, G.; Sozzani, S.; Carraro, F.; Naldini, A. Hypoxia Shapes Autophagy in LPS-Activated Dendritic Cells. Front. Immunol. 2020, 11, 3071. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Morena, E.; Pucci, A.; Pellegrini, M.; Baldari, C.T.; Pelicci, P.G.; Presta, M.; Ribatti, D.; Carraro, F. The Adaptor Protein P66shc Is a Positive Regulator in the Angiogenic Response Induced by Hypoxic T Cells. J. Leukoc. Biol. 2010, 87, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Bernards, R.; Weinberg, R.A. Metastasis Genes: A Progression Puzzle. Nature 2002, 418, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, A.L. Hypoxia—A Key Regulatory Factor in Tumour Growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in Cancer: Significance and Impact on Clinical Outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Meehan, J.; Ward, C.; Turnbull, A.; Bukowski-Wills, J.; Finch, A.J.; Jarman, E.J.; Xintaropoulou, C.; Mar-tinez-Perez, C.; Gray, M.; Pearson, M.; et al. Inhibition of PH Regulation as a Therapeutic Strategy in Hypoxic Human Breast Cancer Cells. Oncotarget 2017, 8, 42857–42875. [Google Scholar] [CrossRef]
- Finger, E.C.; Giaccia, A.J. Hypoxia, Inflammation, and the Tumor Microenvironment in Metastatic Disease. Cancer Metastasis Rev. 2010, 29, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y.; Shen, H. Role of Hypoxia-Induced Exosomes in Tumor Biology. Mol. Cancer 2018, 17, 120. [Google Scholar] [CrossRef]
- Deep, G.; Panigrahi, G.K. Hypoxia-Induced Signaling Promotes Prostate Cancer Progression: Exosomes Role as Messenger of Hypoxic Response in Tumor Microenvironment. Crit. Rev. Oncog. 2015, 20, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Shi, R.; Zhang, Q. Hypoxia and Oxygen-Sensing Signaling in Gene Regulation and Cancer Progres-sion. Int. J. Mol. Sci. 2020, 21, 8162. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Shakir, D.; Batie, M.; Frost, M.; Rocha, S. Oxygen-Sensing Mechanisms in Cells. FEBS J. 2020, 287, 3888–3906. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, B.; Druker, J.; Rocha, S. Cell Cycle Progression in Response to Oxygen Levels. Cell. Mol. Life Sci. 2014, 71, 3569–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Kang, Y. Hypoxia and Hypoxia-Inducible Factors: Master Regulators of Metastasis. Clin. Cancer Res. 2010, 16, 5928. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Aga, M.; Bentz, G.L.; Raffa, S.; Torrisi, M.R.; Kondo, S.; Wakisaka, N.; Yoshizaki, T.; Pagano, J.S.; Shackelford, J. Exosomal HIF1α Supports Invasive Potential of Nasopharyngeal Carcinoma-Associated LMP1-Positive Exo-somes. Oncogene 2014, 33, 4613–4622. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Life with Oxygen. Science 2007, 318, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Path-way. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Smith, T.G.; Robbins, P.A.; Ratcliffe, P.J. The Human Side of Hypoxia-Inducible Factor. Br. J. Haematol. 2008, 141, 325–334. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Y.; Huang, Y.; Zhang, L.; Ling, Z.; Zhu, Y.; Wang, F.; Zou, X.; Chen, M. Hypoxia-Induced Ex-tracellular Vesicles Mediate Protection of Remote Ischemic Preconditioning for Renal Ischemia-Reperfusion Injury. Biomed. Pharmacother. 2017, 90, 473–478. [Google Scholar] [CrossRef] [PubMed]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic Enhancement of Exosome Release by Breast Cancer Cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional Regulation by Hypoxia Inducible Factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Chen, M.; Jiang, R.; Guo, Y.; Wu, M.; Zhang, X. Exosome-Related Tumor Microenvironment. J. Cancer 2018, 9, 3084–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.E.; Dutta, B.; Tse, S.W.; Gupta, N.; Tan, C.F.; Low, J.K.; Yeoh, K.W.; Kon, O.L.; Tam, J.P.; Sze, S.K. Hypox-ia-Induced Tumor Exosomes Promote M2-like Macrophage Polarization of Infiltrating Myeloid Cells and Mi-croRNA-Mediated Metabolic Shift. Oncogene 2019, 38, 5158–5173. [Google Scholar] [CrossRef]
- Belting, M.; Christianson, H.C. Role of Exosomes and Microvesicles in Hypoxia-Associated Tumour Develop-ment and Cardiovascular Disease. J. Intern. Med. 2015, 278, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Yaghoubi, S.; Najminejad, H.; Dabaghian, M.; Karimi, M.H.; Abdollahpour-Alitappeh, M.; Rad, F.; Mahi-Birjand, M.; Mohammadi, S.; Mohseni, F.; Sobhani Lari, M.; et al. How Hypoxia Regulate Exosomes in Ischemic Diseases and Cancer Microenvironment? IUBMB Life 2020, 72, 1286–1305. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes Secreted under Hypoxia Enhance Invasiveness and Stemness of Prostate Cancer Cells by Targeting Adherens Junction Molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Luo, H.; Li, X.; Li, T.; He, J.; Qi, Q.; Liu, Y.; Yu, Z. Exosomes Derived from Human Pulmonary Artery Endothelial Cells Shift the Balance between Proliferation and Apoptosis of Smooth Muscle Cells. Cardiology 2017, 137, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.; Melo, S.; Özdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Ii, V.H.G.; LeBleu, V.S.; Kalluri, R. TGF-β1–Containing Exosomes from Injured Epithelial Cells Activate Fibroblasts to Initiate Tissue Regenerative Responses and Fibrosis. J. Am. Soc. Nephrol. 2012, 24, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H.; et al. Lipid Synthesis Is Promoted by Hypoxic Adipocyte-Derived Exosomes in 3T3-L1 Cells. Biochem. Biophys. Res. Commun. 2014, 445, 327–333. [Google Scholar] [CrossRef]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Hanly, P.J.; Poulin, M.J.; Gozal, D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, X.; Yao, Q.; Liu, Y.; Zhang, H.; Dong, Z. HIF-1-Mediated Production of Exosomes during Hy-poxia Is Protective in Renal Tubular Cells. Am. J. Physiol. Ren. Physiol. 2017, 313, F906–F913. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Almendros, I.; Gileles-Hillel, A.; Akbarpour, M.; Trzepizur, W.; Mokhlesi, B.; Huang, L.; Andrade, J.; Farré, R.; Gozal, D. Circulating Exosomes Potentiate Tumor Malignant Properties in a Mouse Model of Chronic Sleep Fragmentation. Oncotarget 2016, 7, 54676–54690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almendros, I.; Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Huang, L.; Akbarpour, M.; Andrade, J.; Farré, R.; Gozal, D. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. CHEST 2016, 150, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, H.; Xu, K.; Ling, Z.; Huang, Y.; Hu, Q.; Lu, K.; Liu, C.; Wang, Y.; Liu, N.; et al. Hypoxia Precondi-tioned Renal Tubular Epithelial Cell-Derived Extracellular Vesicles Alleviate Renal Ischaemia-Reperfusion Injury Mediated by the HIF-1α/Rab22 Pathway and Potentially Affected by MicroRNAs. Int. J. Biol. Sci. 2019, 15, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.D.; French, K.M.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Platt, M.O.; Searles, C.D.; Davis, M.E. Identification of Therapeutic Covariant MicroRNA Clusters in Hypoxia-Treated Cardiac Progenitor Cell Exosomes Using Systems Biology. Circ. Res. 2015, 116, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.; Qiu, X.-T.; Li, C.-C.; et al. Hypox-ia-Elicited Mesenchymal Stem Cell-Derived Exosomes Facilitates Cardiac Repair through MiR-125b-Mediated Prevention of Cell Death in Myocardial Infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Deng, L.; Wang, D.; Li, N.; Chen, X.; Cheng, X.; Yuan, J.; Gao, X.; Liao, M.; Wang, M.; et al. Mechanism of TNF-α Autocrine Effects in Hypoxic Cardiomyocytes: Initiated by Hypoxia Inducible Factor 1α, Presented by Exosomes. J. Mol. Cellular Cardiol. 2012, 53, 848–857. [Google Scholar] [CrossRef]
- Duan, P.; Tan, J.; Miao, Y.; Zhang, Q. Potential Role of Exosomes in the Pathophysiology, Diagnosis, and Treatment of Hypoxic Diseases. Am. J. Transl. Res. 2019, 11, 1184–1201. [Google Scholar] [PubMed]
- Wysoczynski, M.; Ratajczak, M.Z. Lung Cancer Secreted Microvesicles: Underappreciated Modulators of Mi-croenvironment in Expanding Tumors. Int. J. Cancer 2009, 125, 1595–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes Derived from Hypoxic Leukemia Cells Enhance Tube Formation in Endothelial Cells *. J. Biol. Chem. 2013, 288, 34343–34351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal MiR-135b Shed from Hypoxic Multiple Myeloma Cells Enhances Angiogenesis by Targeting Factor-Inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung Cancer-Secreted Exosomal MiR-23a Increased Angiogenesis and Vascular Permeability by Targeting Prolyl Hydroxylase and Tight Junction Protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef] [PubMed]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-Induced Exosomes Contribute to a More Aggressive and Chemoresistant Ovarian Cancer Pheno-type: A Novel Mechanism Linking STAT3/Rab Proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Yu, Y.; Min, Z.; Zhou, Z.; Linhong, M.; Tao, R.; Yan, L.; Song, H. Hypoxia-Induced Exosomes Promote Hepatocellular Carcinoma Proliferation and Metastasis via MiR-1273f Transfer. Experimental Cell Res. 2019, 385, 111649. [Google Scholar] [CrossRef] [PubMed]
- Patton, M.C.; Zubair, H.; Khan, M.A.; Singh, S.; Singh, A.P. Hypoxia Alters the Release and Size Distribution of Extracellular Vesicles in Pancreatic Cancer Cells to Support Their Adaptive Survival. J. Cell Biochem. 2020, 121, 828–839. [Google Scholar] [CrossRef]
- Jung, K.O.; Youn, H.; Lee, C.-H.; Kang, K.W.; Chung, J.-K. Visualization of Exosome-Mediated MiR-210 Trans-fer from Hypoxic Tumor Cells. Oncotarget 2016, 8, 9899–9910. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xu, R.; Xia, J.; Huang, J.; Su, B.; Wang, S. Aspirin Inhibits Hypoxia-Mediated Lung Cancer Cell Stem-ness and Exosome Function. Pathol. Res. Pract. 2019, 215, 152379. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Sköld, S.; Löfstedt, T.; Johansson, M.C.; Mörgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia Triggers a Proangiogenic Pathway Involving Cancer Cell Microvesi-cles and PAR-2–Mediated Heparin-Binding EGF Signaling in Endothelial Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes Reflect the Hypoxic Status of Glioma Cells and Me-diate Hypoxia-Dependent Activation of Vascular Cells during Tumor Development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaepfer, I.R.; Nambiar, D.K.; Ramteke, A.; Kumar, R.; Dhar, D.; Agarwal, C.; Bergman, B.; Graner, M.; Maro-ni, P.; Singh, R.P.; et al. Hypoxia Induces Triglycerides Accumulation in Prostate Cancer Cells and Extracellu-lar Vesicles Supporting Growth and Invasiveness Following Reoxygenation. Oncotarget 2015, 6, 22836–22856. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Chen, X.; Zhang, Y.; Xu, M.; Yang, Z. The Regulation of Cancer Cell Migration by Lung Cancer Cell-Derived Exosomes through TGF-β and IL-10. Oncol. Lett. 2016, 11, 1527–1530. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Luo, G.; Zhang, K.; Cao, J.; Huang, C.; Jiang, T.; Liu, B.; Su, L.; Qiu, Z. Correction: Hypoxic Tu-mor-Derived Exosomal MiR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pan-creatic Cancer Metastasis. Cancer Res. 2020, 80, 922. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Feng, Y. Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced β-Catenin Signaling in Endothelial Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 651–661. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).