Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages
Abstract
:Simple Summary
Abstract
1. Introduction
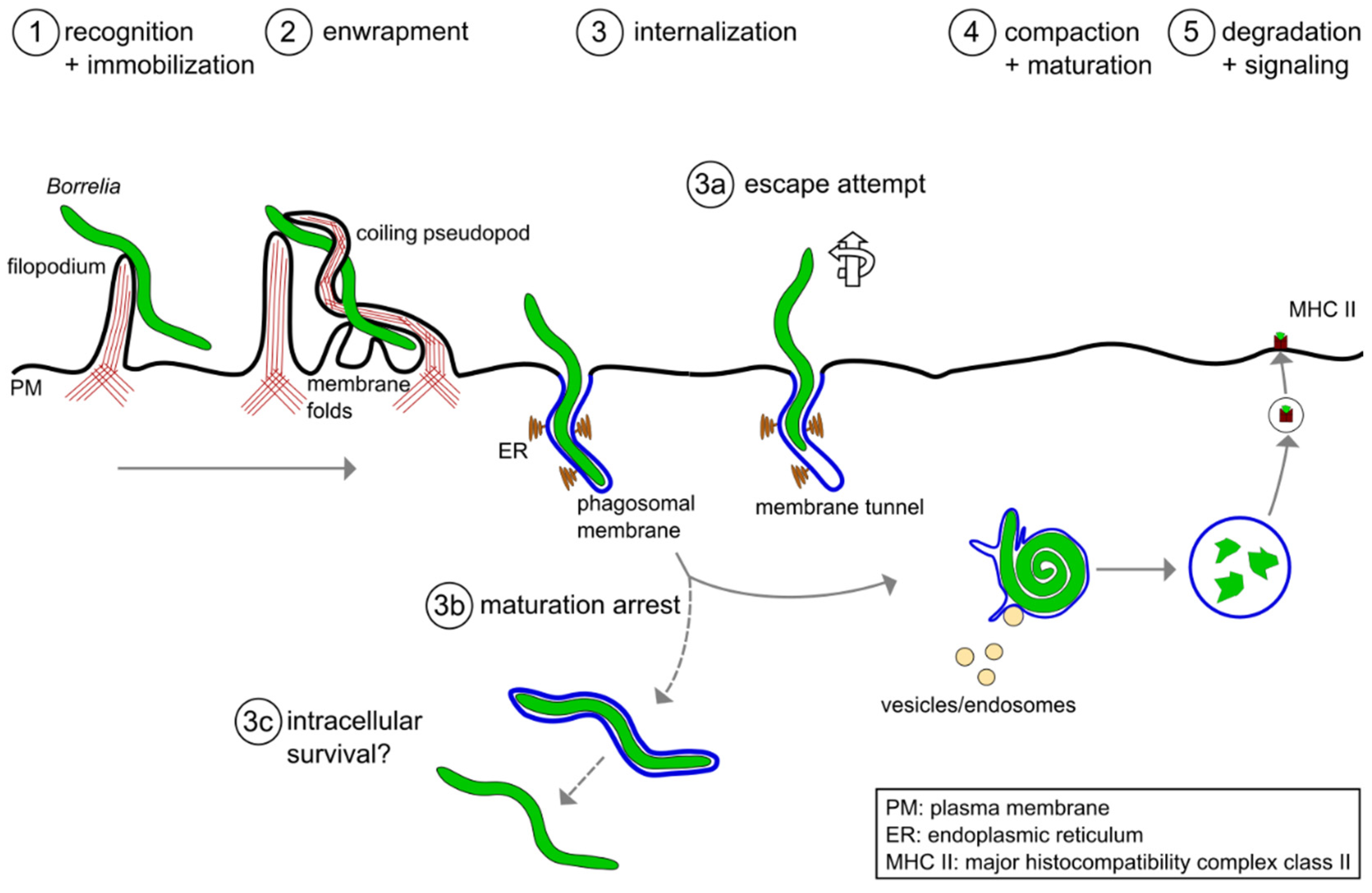
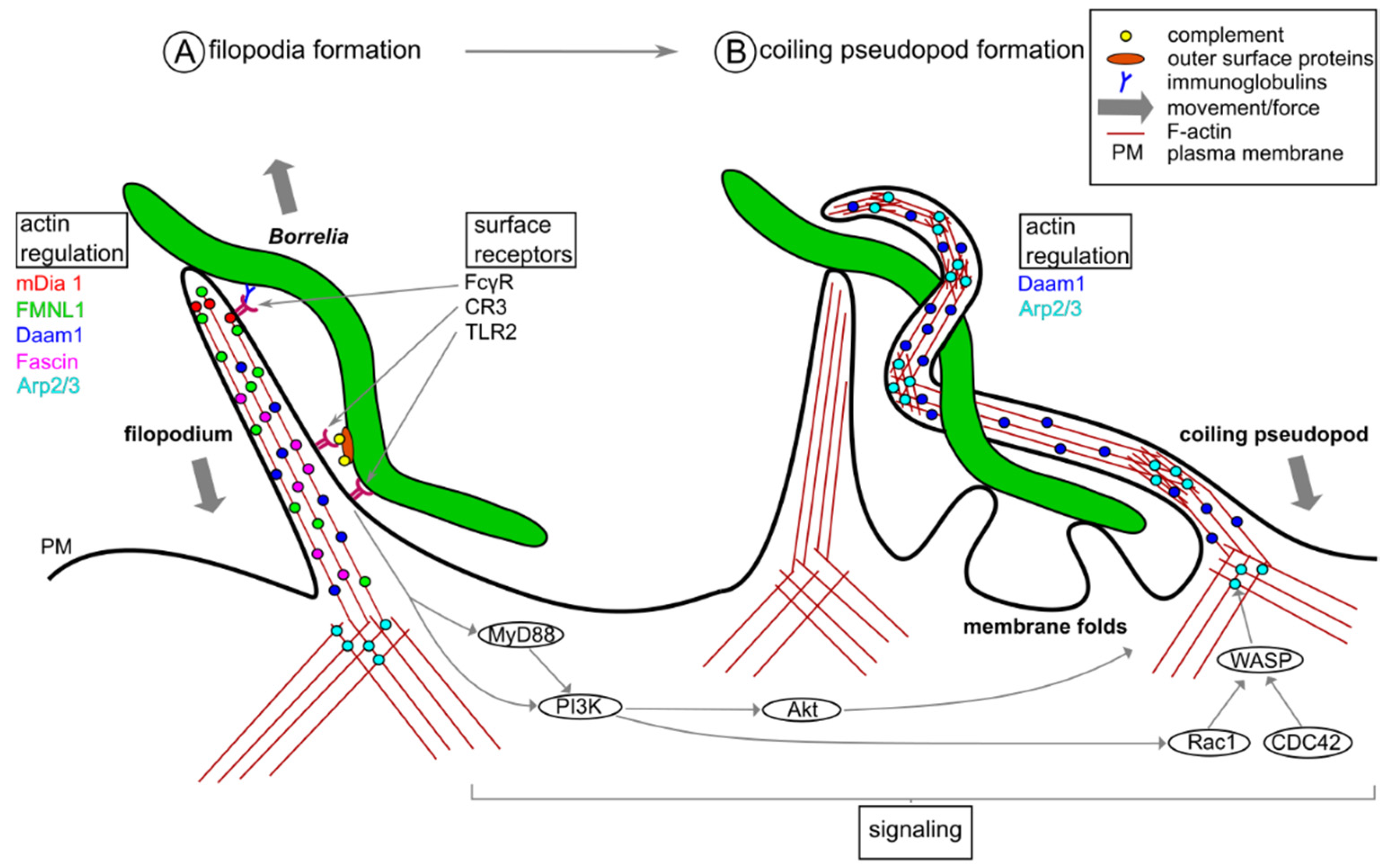
2. Phagocytic Uptake of Borrelia burgdorferi by Macrophages
3. Intracellular Processing of Borrelia burgdorferi in Macrophages
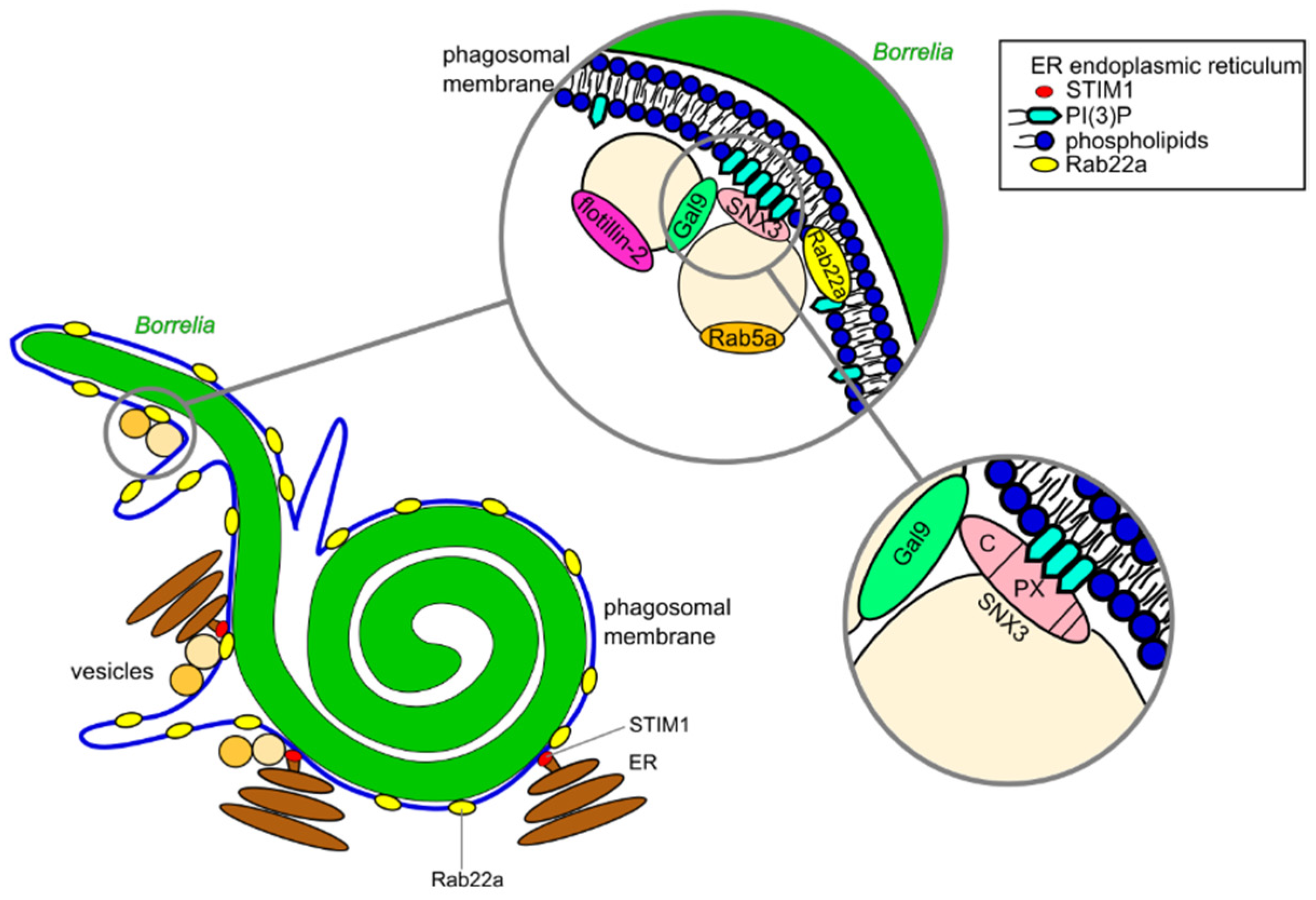
3.1. Compaction of Borrelia in the Phagosome
3.2. Phagosomal Maturation and Development of the Phagolysosome
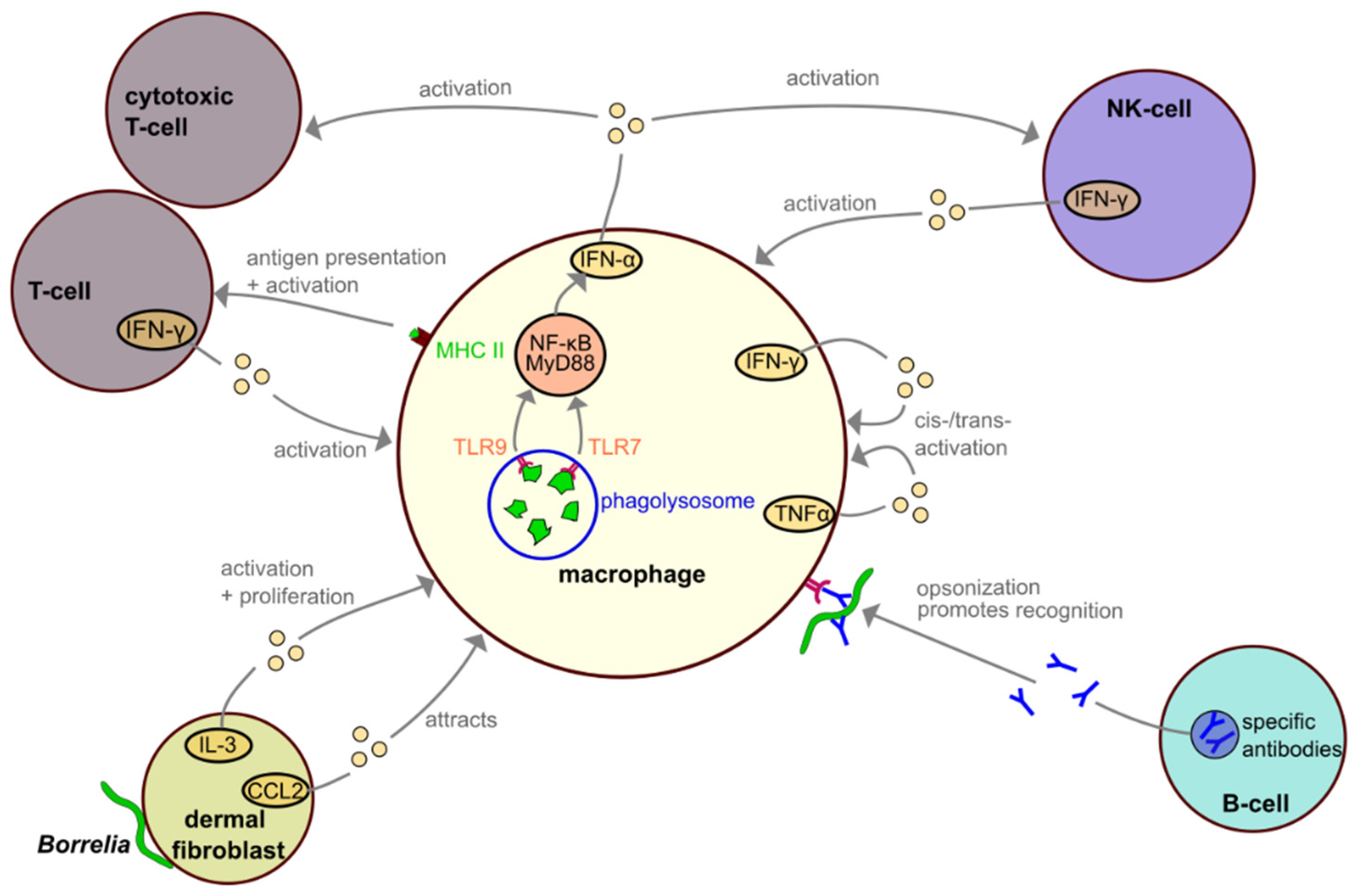
4. Interactions among Macrophages and Other Immune Cells during Borrelia Infection
5. Potential Immune Evasion Strategies of Borrelia
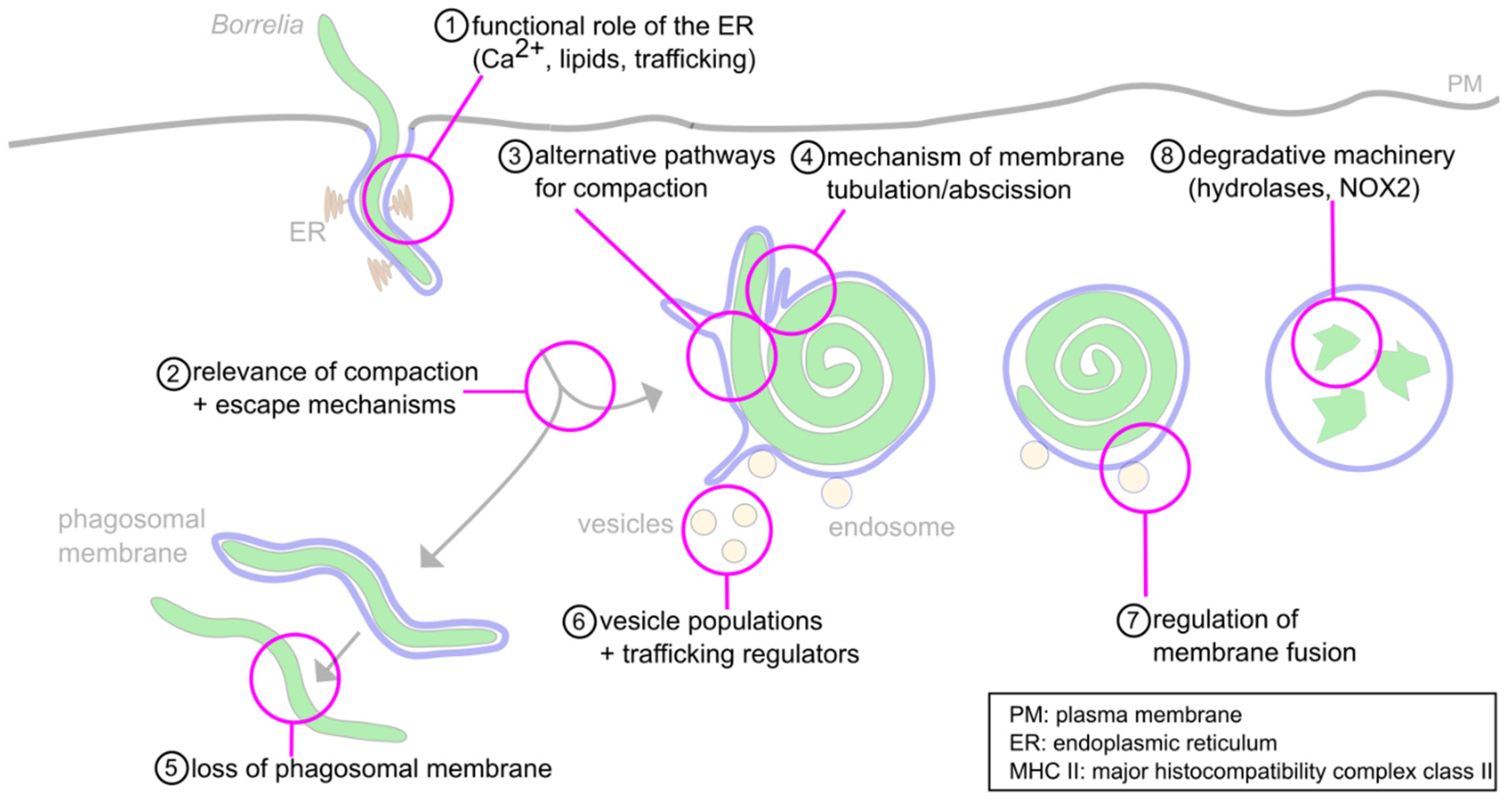
6. Concluding Remarks and Open Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMDM | bone-marrow derived macrophages |
| CR3 | complement-receptor 3 |
| Dbp | decorin binding protein |
| DQ-BSA | dequenched bovine serum albumin |
| ER | endoplasmic reticulum |
| FcγR | Fcγ-receptor |
| LAMP | lysosome-associated membrane glycoprotein |
| MCS | membrane contact site |
| MHC | major histocompatibility complex |
| MyD88 | myeloid differentiation factor 88 |
| NOX | NADPH oxidase |
| Osp | outer surface protein |
| PAMP | pathogen associated molecular pattern |
| PIP | phosphatidylinositol-phosphate |
| PI3K | phosphoinositide 3-kinase |
| shRNA | small-hairpin RNA |
| siRNA | small-interfering RNA |
| SNAP | NSF-attachment protein |
| SNARE | soluble N-Ethylmaleimide-sensitive factor-attachment protein receptor |
| SNX | sorting nexin |
| SRBC | sheep red blood cells |
| STIM | stromal-interaction molecule |
| TLR | toll-like receptor |
| V-ATPase | vacuolar-type proton transporting ATPase |
| WASH | Wiskott–Aldrich syndrome protein and SCAR homologue |
| WASP | Wiskott–Aldrich syndrome protein |
References
- Russell, A.L.R.; Dryden, M.S.; Pinto, A.A.; Lovett, J.K. Lyme disease: Diagnosis and management. Pr. Neurol. 2018, 18, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S. Epidemiology of Lyme Disease. Infect. Dis. Clin. N. Am. 2015, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Malawista, S.E.; Snydman, D.R.; Shope, R.E.; Andiman, W.A.; Ross, M.R.; Steele, F.M. An epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977, 20, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease-a tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef] [Green Version]
- Aberer, E.; Duray, P.H. Morphology of Borrelia burgdorferi: Structural patterns of cultured borreliae in relation to staining methods. J. Clin. Microbiol. 1991, 29, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, S.F.; Charon, N.W.; Kreiling, J.A. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA 1994, 91, 3433–3437. [Google Scholar] [CrossRef] [Green Version]
- Cutler, S.J.; Ruzic-Sabljic, E.; Potkonjak, A. Emerging borreliae—Expanding beyond Lyme borreliosis. Mol. Cell. Probes 2017, 31, 22–27. [Google Scholar] [CrossRef]
- Lane, R.S.; Loye, J.E. Lyme Disease in California: Interrelationship of Ixodid Ticks (Acari), Rodents, and Borrelia burgdorferi. J. Med. Èntomol. 1991, 28, 719–725. [Google Scholar] [CrossRef]
- Gern, L. Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: Life in the wilds. Parasite 2008, 15, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect. Genet. Evol. 2011, 11, 1545–1563. [Google Scholar] [CrossRef] [Green Version]
- Bacon, R.M.; Kugeler, K.J.; Mead, P.S. Surveillance for Lyme Disease—United States, 1992–2006. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss5710a1.htm (accessed on 10 April 2021).
- Ogden, N.H.; Feil, E.J.; Leighton, P.A.; Lindsay, L.R.; Margos, G.; Mechai, S.; Michel, P.; Moriarty, T.J. Evolutionary Aspects of Emerging Lyme Disease in Canada. Appl. Environ. Microbiol. 2015, 81, 7350–7359. [Google Scholar] [CrossRef] [Green Version]
- Schoen, R.T. Lyme disease: Diagnosis and treatment. Curr. Opin. Rheumatol. 2020, 32, 247–254. [Google Scholar] [CrossRef]
- Salazar, J.C.; Pope, C.D.; Sellati, T.J.; Feder, H.M.; Kiely, T.G.; Dardick, K.R.; Buckman, R.L.; Moore, M.W.; Caimano, M.J.; Pope, J.G.; et al. Coevolution of Markers of Innate and Adaptive Immunity in Skin and Peripheral Blood of Patients with Erythema Migrans. J. Immunol. 2003, 171, 2660–2670. [Google Scholar] [CrossRef] [Green Version]
- Moriarty, T.J.; Norman, M.U.; Colarusso, P.; Bankhead, T.; Kubes, P.; Chaconas, G. Real-Time High Resolution 3D Imaging of the Lyme Disease Spirochete Adhering to and Escaping from the Vasculature of a Living Host. PLoS Pathog. 2008, 4, e1000090. [Google Scholar] [CrossRef] [Green Version]
- Rebman, A.W.; Aucott, J.N. Post-treatment Lyme Disease as a Model for Persistent Symptoms in Lyme Disease. Front. Med. 2020, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Naj, X.; Linder, S. Actin-Dependent Regulation of Borrelia burgdorferi Phagocytosis by Macrophages. Curr. Top. Microbiol. Immunol. 2016, 399, 133–154. [Google Scholar] [CrossRef]
- Hoffmann, A.K.; Naj, X.; Linder, S. Daam1 is a regulator of filopodia formation and phagocytic uptake of Borrelia burgdorferi by primary human macrophages. FASEB J. 2014, 28, 3075–3089. [Google Scholar] [CrossRef]
- Naj, X.; Hoffmann, A.-K.; Himmel, M.; Linder, S. The Formins FMNL1 and mDia1 Regulate Coiling Phagocytosis of Borrelia burgdorferi by Primary Human Macrophages. Infect. Immun. 2013, 81, 1683–1695. [Google Scholar] [CrossRef] [Green Version]
- Naj, X.; Linder, S. ER-Coordinated Activities of Rab22a and Rab5a Drive Phagosomal Compaction and Intracellular Processing of Borrelia burgdorferi by Macrophages. Cell Rep. 2015, 12, 1816–1830. [Google Scholar] [CrossRef] [Green Version]
- Niedergang, F. Phagocytosis. In Encyclopedia of Cell Biology; Bradshaw, R.A., Stahl, P.D., Eds.; Academic Press: Waltham, MA, USA, 2016; pp. 751–757. [Google Scholar]
- Flannagan, R.; Jaumouillé, V.; Grinstein, S. The Cell Biology of Phagocytosis. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Kafatos, F.C.; Janeway, C.A.; Ezekowitz, R.A.B. Phylogenetic Perspectives in Innate Immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Rabinovitch, M. Professional and non-professional phagocytes: An introduction. Trends Cell Biol. 1995, 5, 85–87. [Google Scholar] [CrossRef]
- Benach, J.L.; Habicht, G.S.; Gocinski, B.L.; Coleman, J.L. Phagocytic cell responses to in vivo and in vitro expo-sure to the Lyme disease spirochete. Yale J. Biol. Med. 1984, 57, 599–605. [Google Scholar]
- Montgomery, R.R.; Nathanson, M.H.; Malawista, S.E. Fc- and Non-Fc-Mediated Phagocytosis of Borrelia Burgdorferi by Maerophages. J. Infect. Dis. 1994, 170, 890–893. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Cosío, G.; Grinstein, S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 2009, 7, 355–366. [Google Scholar] [CrossRef]
- Carreras-González, A.; Barriales, D.; Palacios, A.; Montesinos-Robledo, M.; Navasa, N.; Azkargorta, M.; Peña-Cearra, A.; Tomás-Cortázar, J.; Escobes, I.; Pascual-Itoiz, M.A.; et al. Regulation of macrophage activity by surface receptors contained within Borrelia burgdorferi-enriched phagosomal fractions. PLoS Pathog. 2019, 15, e1008163. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.C.; Murgia, R.; Cinco, M. Complement Receptor 3 Binds the Borrelia burgdorferi Outer Surface Proteins OspA and OspB in an iC3b-Independent Manner. Infect. Immun. 2005, 73, 6138–6142. [Google Scholar] [CrossRef] [Green Version]
- Cinco, M.; Murgia, R.; Presani, G.; Perticarari, S. Integrin CR3 mediates the binding of nonspecifically opsonized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect. Immun. 1997, 65, 4784–4789. [Google Scholar] [CrossRef] [Green Version]
- Hawley, K.L.; Olson, C.M.; Iglesias-Pedraz, J.M.; Navasa, N.; Cervantes, J.L.; Caimano, M.J.; Izadi, H.; Ingalls, R.; Pal, U.; Salazar, J.C.; et al. CD14 cooperates with complement receptor 3 to mediate MyD88-independent phagocytosis of Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2012, 109, 1228–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooten, R.M.; Ma, Y.; Yoder, R.A.; Brown, J.P.; Weis, J.H.; Zachary, J.F.; Kirschning, C.J.; Weis, J.J. Toll-Like Receptor 2 Is Required for Innate, But Not Acquired, Host Defense to Borrelia burgdorferi. J. Immunol. 2002, 168, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, J.C.; Duhnam-Ems, S.; La Vake, C.; Cruz, A.R.; Moore, M.W.; Caimano, M.J.; Velez-Climent, L.; Shupe, J.; Krueger, W.; Radolf, J.D. Activation of Human Monocytes by Live Borrelia burgdorferi Generates TLR2-Dependent and -Independent Responses Which Include Induction of IFN-β. PLoS Pathog. 2009, 5, e1000444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaegh, D.; Joosten, L.A.; Oosting, M. The role of host immune cells and Borrelia burgdorferi antigens in the etiology of Lyme disease. Eur. Cytokine Netw. 2017, 28, 70–84. [Google Scholar] [CrossRef]
- Benjamin, S.J.; Hawley, K.L.; Vera-Licona, P.; La Vake, C.J.; Cervantes, J.L.; Ruan, Y.; Radolf, J.D.; Salazar, J.C. Macrophage mediated recognition and clearance of Borrelia burgdorferi elicits MyD88-dependent and -independent phagosomal signals that contribute to phagocytosis and inflammation. BMC Immunol. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Shin, O.S.; Miller, L.S.; Modlin, R.L.; Akira, S.; Uematsu, S.; Hu, L.T. Downstream Signals for MyD88-Mediated Phagocytosis of Borrelia burgdorferi can be Initiated by TRIF and Are Dependent on PI3K. J. Immunol. 2009, 183, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Ma, Y.; Buyuk, A.; McClain, S.; Weis, J.J.; Schwartz, I. Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolates in TLR2-deficient C3H/HeJ mice. FEMS Microbiol. Lett. 2004, 231, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Lawrenz, M.B.; Wooten, R.M.; Zachary, J.F.; Drouin, S.M.; Weis, J.J.; Wetsel, R.A.; Norris, S.J. Effect of Complement Component C3 Deficiency on Experimental Lyme Borreliosis in Mice. Infect. Immun. 2003, 71, 4432–4440. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, R.R.; Malawista, S.E. Borrelia burgdorferi and the macrophage: Routine annihilation but occasional haven? Parasitol. Today 1994, 10, 154–157. [Google Scholar] [CrossRef]
- Linder, S.; Heimerl, C.; Fingerle, V.; Aepfelbacher, M.; Wilske, B. Coiling Phagocytosis of Borrelia burgdorferi by Primary Human Macrophages Is Controlled by CDC42Hs and Rac1 and Involves Recruitment of Wiskott-Aldrich Syndrome Protein and Arp2/3 Complex. Infect. Immun. 2001, 69, 1739–1746. [Google Scholar] [CrossRef] [Green Version]
- Flannagan, R.S.; Harrison, R.E.; Yip, C.M.; Jaqaman, K.; Grinstein, S. Dynamic macrophage “probing” is required for the efficient capture of phagocytic targets. J. Cell Biol. 2010, 191, 1205–1218. [Google Scholar] [CrossRef] [Green Version]
- Mallavarapu, A.; Mitchison, T. Regulated Actin Cytoskeleton Assembly at Filopodium Tips Controls Their Extension and Retraction. J. Cell Biol. 1999, 146, 1097–1106. [Google Scholar] [CrossRef]
- Svitkina, T.M.; Bulanova, E.A.; Chaga, O.Y.; Vignjevic, D.M.; Kojima, S.-I.; Vasiliev, J.M.; Borisy, G.G. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 2003, 160, 409–421. [Google Scholar] [CrossRef]
- Bohnert, K.A.; Willet, A.H.; Kovar, D.R.; Gould, K.L. Formin-based control of the actin cytoskeleton during cytokinesis. Biochem. Soc. Trans. 2013, 41, 1750–1754. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Higgs, H.N. The Mouse Formin mDia1 Is a Potent Actin Nucleation Factor Regulated by Autoinhibition. Curr. Biol. 2003, 13, 1335–1340. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.S.; Li, F.; Higgs, H.N. The Mouse Formin, FRLα, Slows Actin Filament Barbed End Elongation, Competes with Capping Protein, Accelerates Polymerization from Monomers, and Severs Filaments. J. Biol. Chem. 2004, 279, 20076–20087. [Google Scholar] [CrossRef] [Green Version]
- Amann, K.J.; Pollard, T.D. The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat. Cell Biol. 2001, 3, 306–310. [Google Scholar] [CrossRef]
- Young, L.E.; Heimsath, E.G.; Higgs, H.N. Cell type–dependent mechanisms for formin-mediated assembly of filopodia. Mol. Biol. Cell 2015, 26, 4646–4659. [Google Scholar] [CrossRef]
- Kress, H.; Stelzer, E.H.K.; Holzer, D.; Buss, F.; Griffiths, G.; Rohrbach, A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. USA 2007, 104, 11633–11638. [Google Scholar] [CrossRef] [Green Version]
- Vonna, L.; Wiedemann, A.; Aepfelbacher, M.; Sackmann, E. Micromechanics of filopodia mediated capture of pathogens by macrophages. Eur. Biophys. J. 2007, 36, 145–151. [Google Scholar] [CrossRef]
- Heidemann, S.R.; Lamoureux, P.; Buxbaum, R.E. Growth cone behavior and production of traction force. J. Cell Biol. 1990, 111, 1949–1957. [Google Scholar] [CrossRef] [Green Version]
- Rittig, M.G.; Krause, A.; Häupl, T.; Schaible, U.E.; Modolell, M.; Kramer, M.D.; Lütjen-Drecoll, E.; Simon, M.M.; Burmester, G.R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect. Immun. 1992, 60, 4205–4212. [Google Scholar] [CrossRef] [Green Version]
- Rittig, M.G.; Wilske, B.; Krause, A. Phagocytosis of microorganisms by means of overshooting pseudopods: Where do we stand? Microbes Infect. 1999, 1, 727–735. [Google Scholar] [CrossRef]
- Klose, M.; Scheungrab, M.; Luckner, M.; Wanner, G.; Linder, S. FIB/SEM-based analysis of Borrelia intracellular processing by human macrophages. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef]
- Rittig, M.G.; Jagoda, J.C.; Wilske, B.; Murgia, R.; Cinco, M.; Repp, R.; Burmester, G.R.; Krause, A. Coiling Phagocytosis Discriminates between Different Spirochetes and Is Enhanced by Phorbol Myristate Acetate and Granulocyte- Macrophage Colony-Stimulating Factor. Infect. Immun. 1998, 66, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Mullins, R.D. How WASP-family proteins and the Arp2/3 complex convert intracellular signals into cytoskeletal structures. Curr. Opin. Cell Biol. 2000, 12, 91–96. [Google Scholar] [CrossRef]
- Vieira, O.V.; Botelho, R.J.; Grinstein, S. Phagosome maturation: Aging gracefully. Biochem. J. 2002, 366, 689–704. [Google Scholar] [CrossRef]
- Klose, M.; Salloum, J.E.; Gonschior, H.; Linder, S. SNX3 drives maturation of Borrelia phagosomes by forming a hub for PI(3)P, Rab5a, and galectin-9. J. Cell Biol. 2019, 218, 3039–3059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Dixon, J.E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002, 3, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, 208884. [Google Scholar] [CrossRef] [Green Version]
- Hubner, S.; Couvillon, A.D.; Kas, J.A.; Bankaitis, V.A.; Vegners, R.; Carpenter, C.L.; Janmey, P.A. Enhancement of phosphoinositide 3-kinase (PI 3-kinase) activity by membrane curvature and inositol-phospholipid-binding peptides. Eur. J. Biochem. 1998, 258, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Meister, M.; Tikkanen, R. Endocytic Trafficking of Membrane-Bound Cargo: A Flotillin Point of View. Membranes 2014, 4, 356–371. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.R.; DiBenedetto, J.R.; West, M.; Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum–endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 2013, 24, 1030–1040. [Google Scholar] [CrossRef]
- Desjardins, M.; Huber, L.A.; Parton, R.G.; Griffiths, G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 1994, 124, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Mayorga, L.S.; Cebrian, I. Rab22a: A novel regulator of immune functions. Mol. Immunol. 2019, 113, 87–92. [Google Scholar] [CrossRef]
- Bucci, C.; Parton, R.; Mather, I.H.; Stunnenberg, H.; Simons, K.; Hoflack, B.; Zerial, M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992, 70, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Press, B.; Wandinger-Ness, A. Rab 7: An important regulator of late endocytic membrane traffic. J. Cell Biol. 1995, 131, 1435–1452. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.G. Functional role(s) of phagosomal Rab GTPases. Small GTPases 2013, 4, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Via, L.E.; Deretic, D.; Ulmer, R.J.; Hibler, N.S.; Huber, L.A.; Deretic, V. Arrest of Mycobacterial Phagosome Maturation Is Caused by a Block in Vesicle Fusion between Stages Controlled by rab5 and rab7. J. Biol. Chem. 1997, 272, 13326–13331. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, M. Biogenesis of phagolysosomes: The ‘kiss and run’ hypothesis. Trends Cell Biol. 1995, 5, 183–186. [Google Scholar] [CrossRef]
- Pauwels, A.-M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, Receptors, and Signals: Regulation of Phagosome Maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef] [Green Version]
- Rothman, J.E.; Warren, G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 1994, 4, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Christoforidis, S.; McBride, H.M.; Burgoyne, R.D.; Zerial, M. The Rab5 effector EEA1 is a core component of endosome docking. Nat. Cell Biol. 1999, 397, 621–625. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Rybin, V.; Murphy, C.; Giner, A.; Teasdale, R.; Zerial, M. Oligomeric Complexes Link Rab5 Effectors with NSF and Drive Membrane Fusion via Interactions between EEA1 and Syntaxin 13. Cell 1999, 98, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Linardopoulou, E.V.; Parghi, S.S.; Friedman, C.; Osborn, G.E.; Parkhurst, S.M.; Trask, B.J. Human Subtelomeric WASH Genes Encode a New Subclass of the WASP Family. PLoS Genet. 2007, 3, e237. [Google Scholar] [CrossRef] [Green Version]
- King, J.S.; Gueho, A.; Hagedorn, M.; Gopaldass, N.; Leuba, F.; Soldati, T.; Insall, R.H. WASH is required for lysosomal recycling and efficient autophagic and phagocytic digestion. Mol. Biol. Cell 2013, 24, 2714–2726. [Google Scholar] [CrossRef]
- Bozzaro, S.; Bucci, C.; Steinert, M. Phagocytosis and Host–Pathogen Interactions in Dictyostelium with a Look at Macrophages. Int. Rev. Cell Mol. Biol. 2008, 271, 253–300. [Google Scholar] [CrossRef] [PubMed]
- Kjeken, R.; Egeberg, M.; Habermann, A.; Kuehnel, M.; Peyron, P.; Floetenmeyer, M.; Walther, P.; Jahraus, A.; Defacque, H.; Kuznetsov, S.A.; et al. Fusion between Phagosomes, Early and Late Endosomes: A Role for Actin in Fusion between Late, but Not Early Endocytic Organelles. Mol. Biol. Cell 2004, 15, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Sun-Wada, G.-H.; Tabata, H.; Kawamura, N.; Aoyama, M.; Wada, Y. Direct recruitment of H+-ATPase from lysosomes for phagosomal acidification. J. Cell Sci. 2009, 122, 2504–2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, C.S.; Elliott, E.; Dennison, C. Endolysosomal proteolysis and its regulation. Biochem. J. 2002, 363, 417–429. [Google Scholar] [CrossRef]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab Conversion as a Mechanism of Progression from Early to Late Endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef] [Green Version]
- Henry, R.M.; Hoppe, A.D.; Joshi, N.; Swanson, J.A. The uniformity of phagosome maturation in macrophages. J. Cell Biol. 2004, 164, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, R.R.; Nathanson, M.H.; Malawista, S.E. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery. J. Immunol. 1993, 150, 909–915. [Google Scholar]
- Eskelinen, E.-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Balla, T. Ca2+ and lipid signals hold hands at endoplasmic reticulum-plasma membrane contact sites. J. Physiol. 2018, 596, 2709–2716. [Google Scholar] [CrossRef] [Green Version]
- Helle, S.C.; Kanfer, G.; Kolar, K.; Lang, A.; Michel, A.H.; Kornmann, B. Organization and function of membrane contact sites. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 2526–2541. [Google Scholar] [CrossRef] [Green Version]
- Nunes-Hasler, P.; Demaurex, N. The ER phagosome connection in the era of membrane contact sites. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1513–1524. [Google Scholar] [CrossRef]
- Rowland, A.A.; Chitwood, P.J.; Phillips, M.J.; Voeltz, G.K. ER Contact Sites Define the Position and Timing of Endosome Fission. Cell 2014, 159, 1027–1041. [Google Scholar] [CrossRef] [Green Version]
- Nunes, P.; Demaurex, N. The role of calcium signaling in phagocytosis. J. Leukoc. Biol. 2010, 88, 57–68. [Google Scholar] [CrossRef]
- Malik, Z.A.; Denning, G.M.; Kusner, D.J. Inhibition of Ca2+ Signaling by Mycobacterium tuberculosis is Associated with Reduced Phagosome–Lysosome Fusion and Increased Survival within Human Macrophages. J. Exp. Med. 2000, 191, 287–302. [Google Scholar] [CrossRef]
- Stockinger, W.; Zhang, S.C.; Trivedi, V.; Jarzylo, L.A.; Shieh, E.C.; Lane, W.S.; Castoreno, A.B.; Nohturfft, A. Differential Requirements for Actin Polymerization, Calmodulin, and Ca2+ Define Distinct Stages of Lysosome/Phagosome Targeting. Mol. Biol. Cell 2006, 17, 1697–1710. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.G.; Booth, J.W.; Stambolic, V.; Mak, T.; Balla, T.; Schreiber, A.D.; Meyer, T.; Grinstein, S. Restricted Accumulation of Phosphatidylinositol 3-Kinase Products in a Plasmalemmal Subdomain during Fcγ Receptor-Mediated Phagocytosis. J. Cell Biol. 2001, 153, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Vieira, O.V.; Botelho, R.J.; Rameh, L.; Brachmann, S.M.; Matsuo, T.; Davidson, H.W.; Schreiber, A.; Backer, J.M.; Cantley, L.C.; Grinstein, S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 2001, 155, 19–26. [Google Scholar] [CrossRef]
- Levin, R.; Grinstein, S.; Schlam, D. Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 805–823. [Google Scholar] [CrossRef]
- Ramachandra, L.; Song, R.; Harding, C.V. Phagosomes are fully competent antigen-processing organelles that mediate the formation of peptide: Class II MHC complexes. J. Immunol. 1999, 162, 3263–3272. [Google Scholar]
- Altenschmidt, U.; Ricciardi-Castagnoli, P.; Modolell, M.; Otto, H.; Wiesmüller, K.-H.; Jung, G.; Simon, M.M. Bone marrow-derived macrophage lines and immortalized cloned macrophage and dendritic cells support priming of Borrelia burgdorferi—specific T cell responses in vitro and/or in vivo. Immunol. Lett. 1996, 50, 41–49. [Google Scholar] [CrossRef]
- Brouwer, M.A.; Jones-Warner, W.; Rahman, S.; Kerstholt, M.; Ferreira, A.V.; Oosting, M.; Hooiveld, G.J.; Netea, M.G.; Joosten, L.A. B. burgdorferi sensu lato-induced inhibition of antigen presentation is mediated by RIP1 signaling resulting in impaired functional T cell responses towards Candida albicans. Ticks Tick-Borne Dis. 2021, 12, 101611. [Google Scholar] [CrossRef]
- Schramm, F.; Kern, A.; Barthel, C.; Nadaud, S.; Meyer, N.; Jaulhac, B.; Boulanger, N. Microarray Analyses of Inflammation Response of Human Dermal Fibroblasts to Different Strains of Borrelia burgdorferi Sensu Stricto. PLoS ONE 2012, 7, e40046. [Google Scholar] [CrossRef] [Green Version]
- Müllegger, R.R.; Means, T.K.; Shin, J.J.; Lee, M.; Jones, K.L.; Glickstein, L.J.; Luster, A.D.; Steere, A.C. Chemokine Signatures in the Skin Disorders of Lyme Borreliosis in Europe: Predominance of CXCL9 and CXCL10 in Erythema Migrans and Acrodermatitis and CXCL13 in Lymphocytoma. Infect. Immun. 2007, 75, 4621–4628. [Google Scholar] [CrossRef] [Green Version]
- Katchar, K.; Drouin, E.E.; Steere, A.C. Natural killer cells and natural killer T cells in Lyme arthritis. Arthritis Res. Ther. 2013, 15, R183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, C.M.; Bates, T.C.; Izadi, H.; Radolf, J.D.; Huber, S.A.; Boyson, J.E.; Anguita, J. Local Production of IFN-γ by Invariant NKT Cells Modulates Acute Lyme Carditis. J. Immunol. 2009, 182, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, F.L.; Ma, Y.; Maylor-Hagan, H.; Brewster, J.; Huang, X.; Spangrude, G.J.; Zachary, J.F.; Weis, J.H.; Weis, J.J. Localized Production of IL-10 Suppresses Early Inflammatory Cell Infiltration and Subsequent Development of IFN-γ–Mediated Lyme Arthritis. J. Immunol. 2011, 188, 1381–1393. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Petzke, M.M.; Brooks, A.; Krupna, M.A.; Mordue, D.; Schwartz, I. Recognition of Borrelia burgdorferi, the Lyme Disease Spirochete, by TLR7 and TLR9 Induces a Type I IFN Response by Human Immune Cells. J. Immunol. 2009, 183, 5279–5292. [Google Scholar] [CrossRef] [Green Version]
- Barthold, S.W.; De Souza, M.S.; Janotka, J.L.; Smith, A.L.; Persing, D.H. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 1993, 143, 959–971. [Google Scholar]
- Rumianek, A.N.; Greaves, D.R. How Have Leukocyte In Vitro Chemotaxis Assays Shaped Our Ideas about Macrophage Migration? Biology 2020, 9, 439. [Google Scholar] [CrossRef]
- Barros-Becker, F.; Lam, P.-Y.; Fisher, R.; Huttenlocher, A. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. J. Cell Sci. 2017, 130, 3801–3808. [Google Scholar] [CrossRef] [Green Version]
- Grabher, C.; Cliffe, A.; Miura, K.; Hayflick, J.; Pepperkok, R.; Rørth, P.; Wittbrodt, J. Birth and life of tissue macrophages and their migration in embryogenesis and inflammation in medaka. J. Leukoc. Biol. 2007, 81, 263–271. [Google Scholar] [CrossRef]
- Hechemy, K.E.; Samsonoff, W.A.; Harris, H.L.; McKee, M. Adherence and entry of Borrelia burgdorferi in Vero cells. J. Med. Microbiol. 1992, 36, 229–238. [Google Scholar] [CrossRef]
- Kazimírová, M.; Štibrániová, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 2013, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Hourcade, D.E.; Akk, A.M.; Mitchell, L.M.; Zhou, H.-F.; Hauhart, R.; Pham, C.T. Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol. Immunol. 2016, 69, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Dulipati, V.; Meri, S.; Panelius, J. Complement evasion strategies of Borrelia burgdorferi sensu lato. FEBS Lett. 2020, 594, 2645–2656. [Google Scholar] [CrossRef]
- Skare, J.T.; Garcia, B.L. Complement Evasion by Lyme Disease Spirochetes. Trends Microbiol. 2020, 28, 889–899. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Frye, A.M.; Nowak, T.A.; Kraiczy, P. New Insights Into CRASP-Mediated Complement Evasion in the Lyme Disease Enzootic Cycle. Front. Cell. Infect. Microbiol. 2020, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Salo, J.; Jaatinen, A.; Söderström, M.; Viljanen, M.K.; Hytönen, J. Decorin Binding Proteins of Borrelia burgdorferi Promote Arthritis Development and Joint Specific Post-Treatment DNA Persistence in Mice. PLoS ONE 2015, 10, e0121512. [Google Scholar] [CrossRef]
- Liang, F.T.; Brown, E.L.; Wang, T.; Iozzo, R.V.; Fikrig, E. Protective Niche for Borrelia burgdorferi to Evade Humoral Immunity. Am. J. Pathol. 2004, 165, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Garin, J.; Diez, R.; Kieffer, S.; Dermine, J.F.; Duclos, S.; Gagnon, E.; Sadoul, R.; Rondeau, C.; Desjardins, M. The phagosome proteome: Insight into phagosome functions. J. Cell Biol. 2001, 152, 165–180. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woitzik, P.; Linder, S. Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages. Biology 2021, 10, 567. https://doi.org/10.3390/biology10070567
Woitzik P, Linder S. Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages. Biology. 2021; 10(7):567. https://doi.org/10.3390/biology10070567
Chicago/Turabian StyleWoitzik, Philipp, and Stefan Linder. 2021. "Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages" Biology 10, no. 7: 567. https://doi.org/10.3390/biology10070567
APA StyleWoitzik, P., & Linder, S. (2021). Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages. Biology, 10(7), 567. https://doi.org/10.3390/biology10070567





