Acute and Sub-Chronic Exposure to Artificial Sweeteners at the Highest Environmentally Relevant Concentration Induce Less Cardiovascular Physiology Alterations in Zebrafish Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
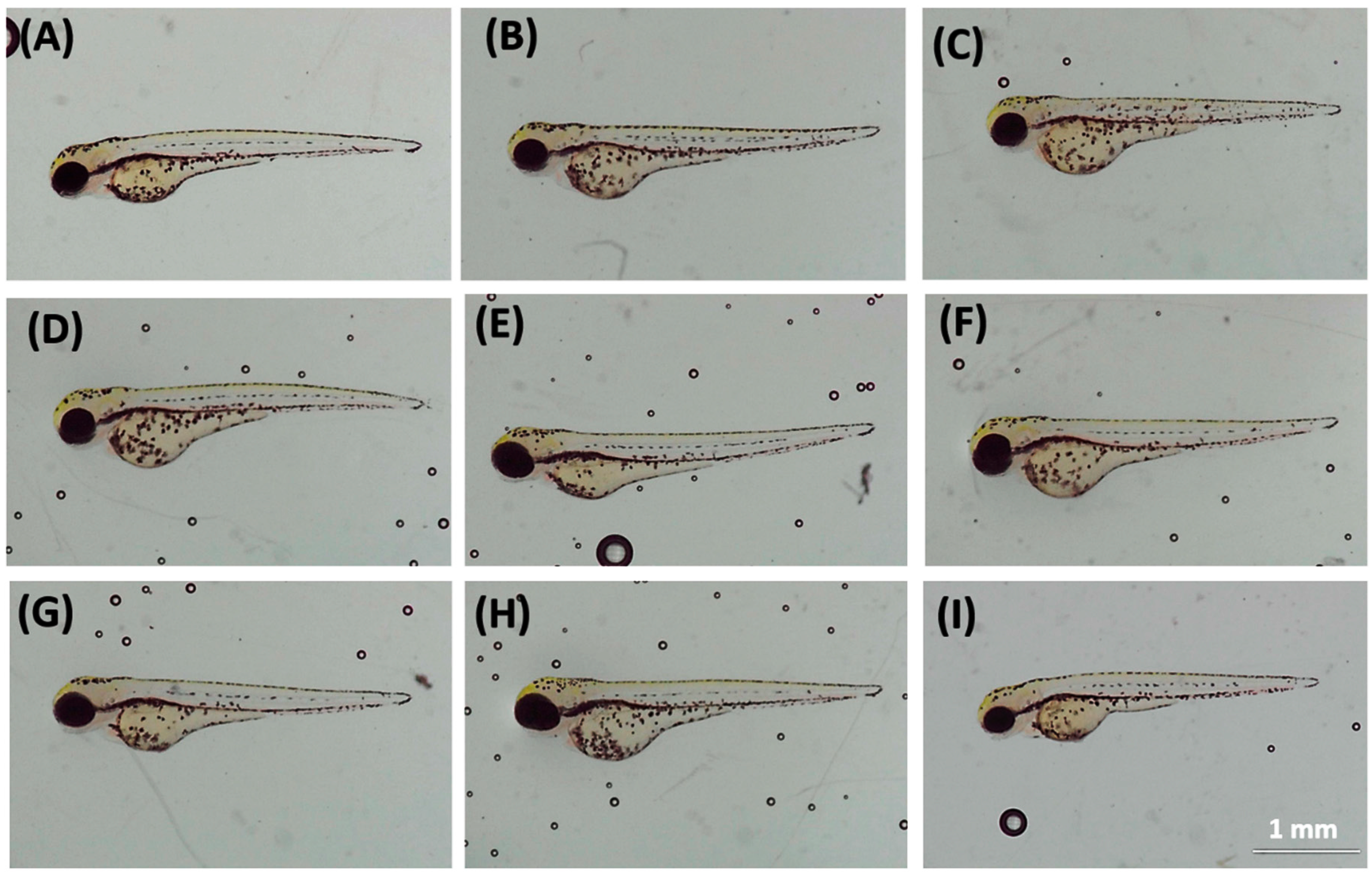
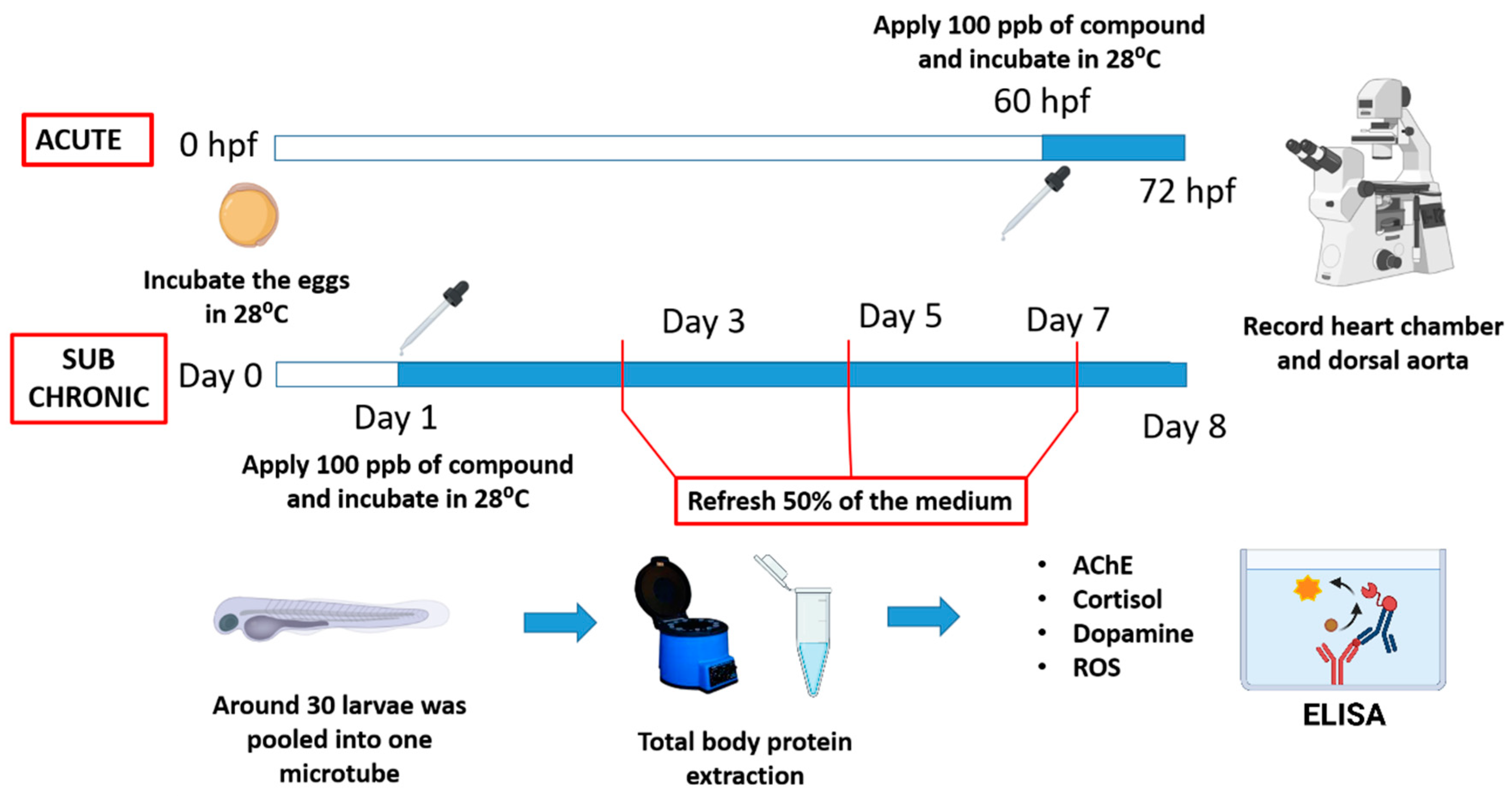
2.1. Animal Ethics and Artificial Sweetener Exposure
2.2. Cardiovascular Performance Measurement
2.3. Determination of Biomarker Content
2.4. Biostatistics
3. Results
3.1. Cardiac Performance in Zebrafish after Acutely Exposed to Artificial Sweeteners
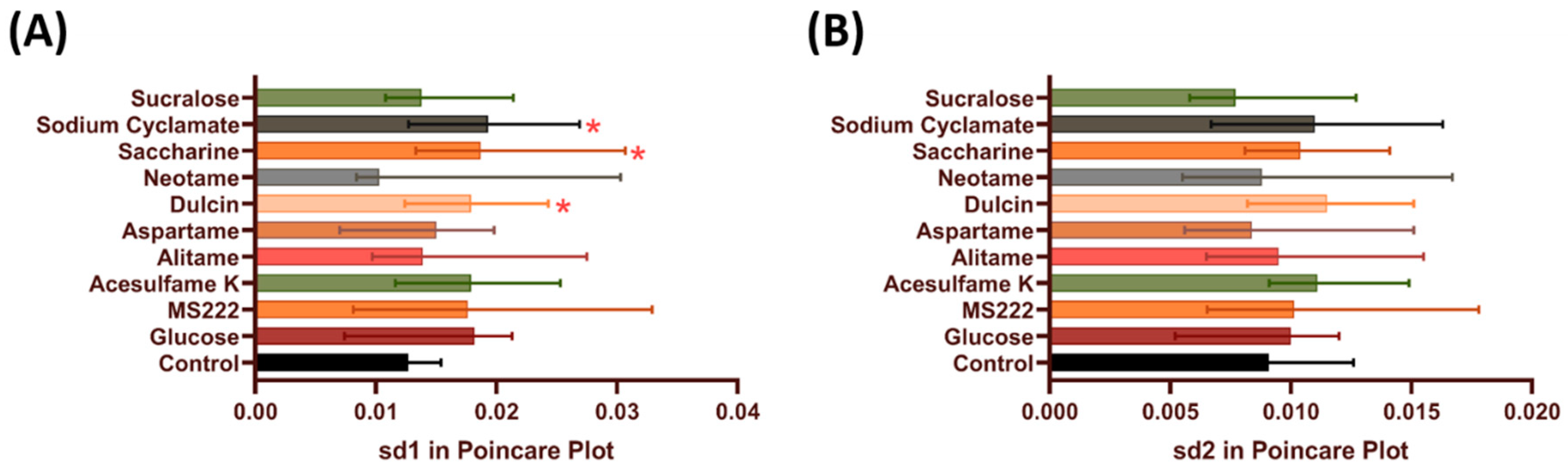
3.2. Heart Rate Regularity in Zebrafish after Acute Exposure to Artificial Sweeteners
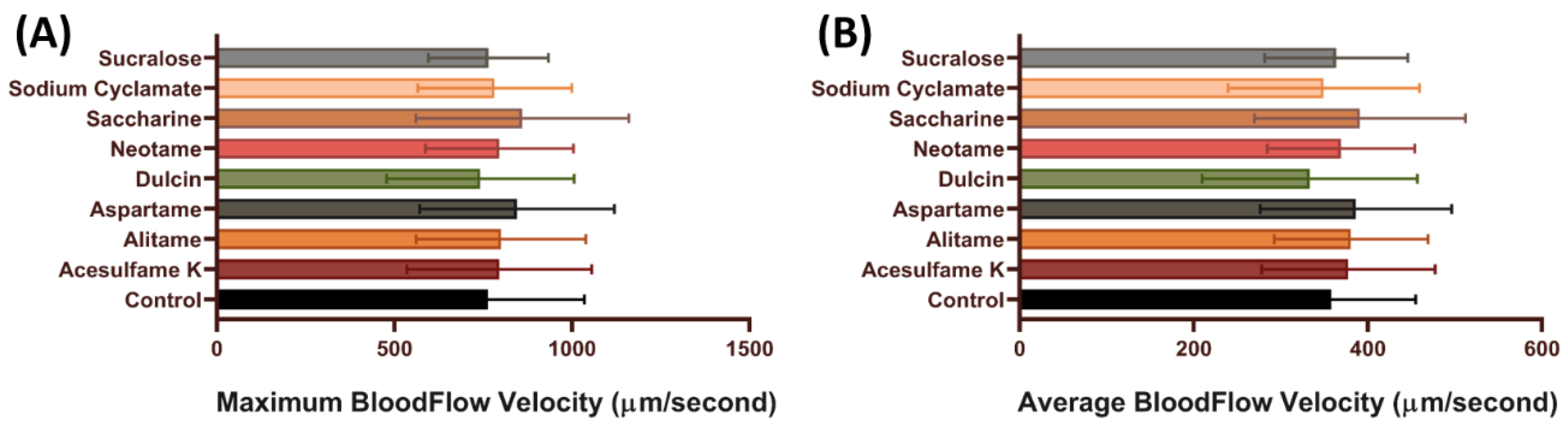
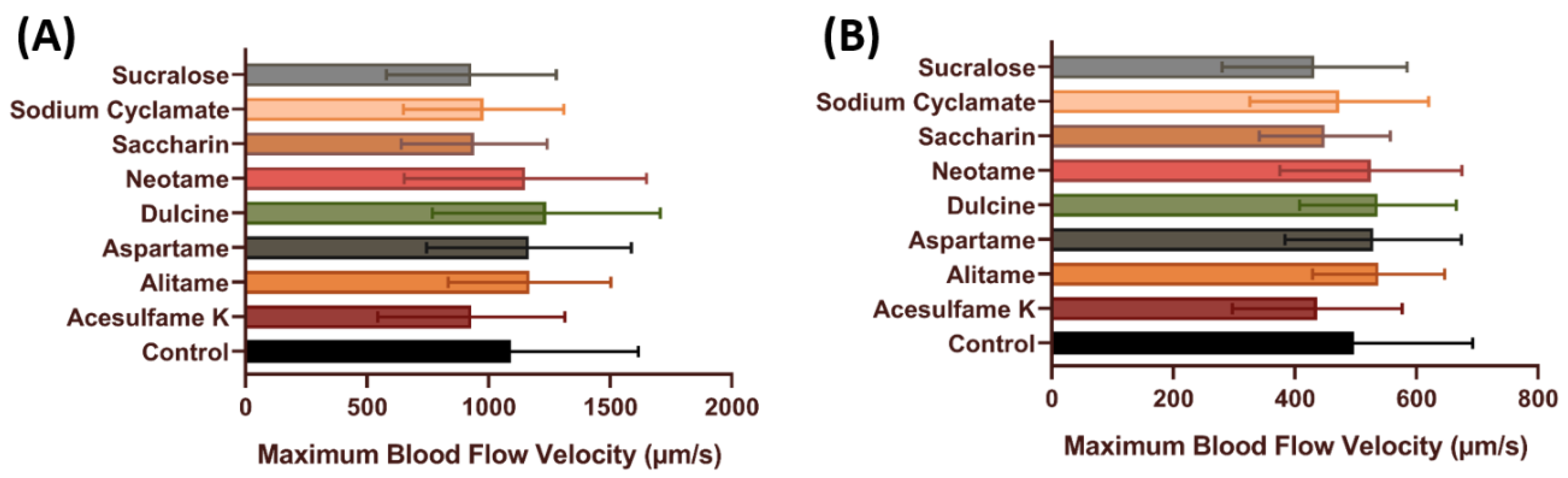
3.3. Blood Flow Velocity of Zebrafish after Exposure to Artificial Sweeteners
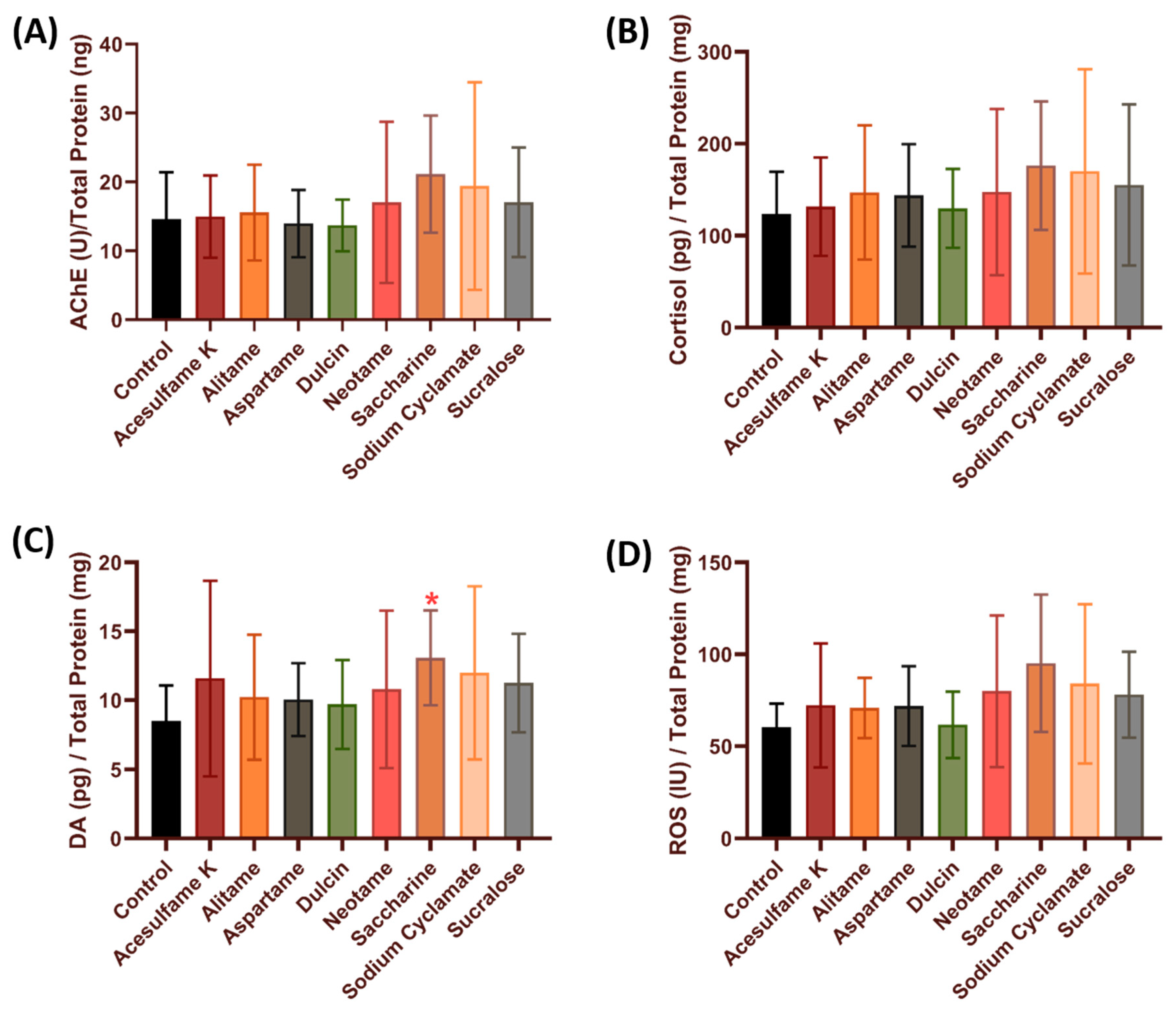
3.4. Comparison of Stress and Oxidative Stress-Related Biomarkers in Zebrafish after Exposure to Artificial Sweeteners
3.5. Cardiovascular Performance of Zebrafish Larvae after Sub-Chronic Exposure of Artificial Sweetener
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Li, D.; O’Brien, J.W.; Tscharke, B.J.; Choi, P.M.; Ahmed, F.; Thompson, J.; Mueller, J.F.; Sun, H.; Thomas, K.V. Trends in artificial sweetener consumption: A 7-year wastewater-based epidemiology study in Queensland, Australia. Sci. Total Environ. 2021, 754, 142438. [Google Scholar] [CrossRef]
- Gan, Z.; Sun, H.; Feng, B.; Wang, R.; Zhang, Y. Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Res. 2013, 47, 4928–4937. [Google Scholar] [CrossRef]
- Brorstrom-Lunden, E.; Svenson, A.; Viktor, T.; Woldegiorgis, A.; Remberger, M.; Kai, L.; Dye, C.; Bjerke, A.; Schlabach, M. Measurements of Sucralose in the Swedish Screening Pogram 2007—Part 2: Sucralose in Biota and Regional STP Samples; IVL Report B 1795; IVL Swedish Environmental Research Institute Ltd.: Stockholm, Sweden, 2008. [Google Scholar]
- Ferrer, I.; Thurman, E.M. Analysis of sucralose and other sweeteners in water and beverage samples by liquid chromatography/time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 4127–4134. [Google Scholar] [CrossRef]
- Saucedo-Vence, K.; Elizalde-Velázquez, A.; Dublán-García, O.; Galar-Martínez, M.; Islas-Flores, H.; SanJuan-Reyes, N.; García-Medina, S.; Hernández-Navarro, M.D.; Gómez-Oliván, L.M. Toxicological hazard induced by sucralose to environmentally relevant concentrations in common carp (Cyprinus carpio). Sci. Total Environ. 2017, 575, 347–357. [Google Scholar] [CrossRef]
- Dietrich, A.M.; Pang, Z.; Zheng, H.; Ma, X. Mini Review: Will artificial sweeteners discharged to the aqueous environment unintentionally “sweeten” the taste of tap water? Chem. Eng. J. Adv. 2021, 6, 100100. [Google Scholar] [CrossRef]
- The National Statistical Office. Report on Drinking Water, Sanitation, Hygiene and Housing Condition in India; National Service Scheme: New Delhi, India, 2018.
- Mawhinney, D.B.; Young, R.B.; Vanderford, B.J.; Borch, T.; Snyder, S.A. Artificial sweetener sucralose in US drinking water systems. Environ. Sci. Technol. 2011, 45, 8716–8722. [Google Scholar] [CrossRef]
- Ochoa, M.; Lalles, J.-P.; Malbert, C.-H.; Val-Laillet, D. Dietary sugars: Their detection by the gut–brain axis and their peripheral and central effects in health and diseases. Eur. J. Nutr. 2015, 54, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Elkind, M.S. Artificial Sweeteners, Real Risks; American Heart Association, Inc.: Dallas, TX, USA, 2019. [Google Scholar]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef] [PubMed]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler. Thromb. 2017, 25, 27–39. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Al-Eisa, R.A.; Al-Salmi, F.A.; Hamza, R.Z.; El-Shenawy, N.S. Role of L-carnitine in protection against the cardiac oxidative stress induced by aspartame in Wistar albino rats. PLoS ONE 2018, 13, e0204913. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Sundareswaran, L.; Devi, R.S. Effects of aspartame on the evaluation of electrophysiological responses in Wistar albino rats. J. Taibah Univ. Sci. 2016, 10, 505–512. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can. Med. Assoc. J. 2017, 189, E929–E939. [Google Scholar] [CrossRef] [PubMed]
- Negro, F.; Mondardini, A.; Palmas, F. Hepatotoxicity of saccharin. N. Engl. J. Med. 1994, 331, 134–135. [Google Scholar] [CrossRef]
- Blumenthal, H.J.; Vance, D.A. Chewing gum headaches. Headache J. Head Face Pain 1997, 37, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H. Aspartame-induced thrombocytopenia. South. Med. J. 2007, 100, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Cadirci, K.; Tozlu, O.O.; Türkez, H.; Mardinoglu, A. The in vitro cytotoxic, genotoxic, and oxidative damage potentials of the oral artificial sweetener aspartame on cultured human blood cells. Turk. J. Med. Sci. 2020, 50, 448–454. [Google Scholar] [CrossRef]
- Tsakiris, S.; Giannoulia-Karantana, A.; Simintzi, I.; Schulpis, K.H. The effect of aspartame metabolites on human erythrocyte membrane acetylcholinesterase activity. Pharmacol. Res. 2006, 53, 1–5. [Google Scholar] [CrossRef]
- Risdon, S.; Meyer, G.; Marziou, A.; Riva, C.; Roustit, M.; Walther, G. Artificial sweeteners impair endothelial vascular reactivity: Preliminary results in rodents. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 843–846. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Whitehouse, C.R.; Boullata, J.; McCauley, L.A. The potential toxicity of artificial sweeteners. AAOHN J. 2008, 56, 251–261. [Google Scholar] [CrossRef]
- Yin, K.-J.; Xie, D.-Y.; Zhao, L.; Fan, G.; Ren, J.-N.; Zhang, L.-L.; Pan, S.-Y. Effects of different sweeteners on behavior and neurotransmitters release in mice. J. Food Sci. Technol. 2020, 57, 113–121. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Seo, J.; Cho, K.-H. Aspartame-fed zebrafish exhibit acute deaths with swimming defects and saccharin-fed zebrafish have elevation of cholesteryl ester transfer protein activity in hypercholesterolemia. Food Chem. Toxicol. 2011, 49, 2899–2905. [Google Scholar] [CrossRef]
- Weerasooriyagedara, M. Toxicity effects of aspartame on embryonic development of Zebrafish (Danio rerio). Int. J. Eng. Manag. Res. (IJEMR) 2018, 8, 183–188. [Google Scholar]
- Giardoglou, P.; Beis, D. On zebrafish disease models and matters of the heart. Biomedicines 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Reischauer, S.; Stainier, D.Y.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A.; Peterson, R.T. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis. Models Mech. 2014, 7, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.L.; Brand, T. The zebrafish model system in cardiovascular research: A tiny fish with mighty prospects. Glob. Cardiol. Sci. Pract. 2013, 2013, 4. [Google Scholar] [CrossRef]
- Benslimane, F.M.; Alser, M.; Zakaria, Z.Z.; Sharma, A.; Abdelrahman, H.A.; Yalcin, H.C. Adaptation of a mice doppler echocardiography platform to measure cardiac flow velocities for embryonic chicken and adult zebrafish. Front. Bioeng. Biotechnol. 2019, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and function of the developing zebrafish heart. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Feng, H.-Z.; Jin, J.-P. A protocol to study ex vivo mouse working heart at human-like heart rate. J. Mol. Cell. Cardiol. 2018, 114, 175–184. [Google Scholar] [CrossRef]
- Kinsara, A.J.; Najm, H.K.; Al Anazi, M.; Tamim, H. Resting heart rate in patients with ischemic heart disease in Saudi Arabia and Egypt. J. Saudi Heart Assoc. 2011, 23, 225–232. [Google Scholar] [CrossRef][Green Version]
- Gomez, O.; Okumura, K.; Honjo, O.; Sun, M.; Ishii, R.; Bijnens, B.; Friedberg, M.K. Heart rate reduction improves biventricular function and interactions in experimental pulmonary hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H542–H551. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Audira, G.; Siregar, P.; Lin, Y.-C.; Villalobos, O.; Villaflores, O.; Wang, W.-D.; Hsiao, C.-D. Waterborne Exposure of Paclobutrazol at Environmental Relevant Concentration Induce Locomotion Hyperactivity in Larvae and Anxiolytic Exploratory Behavior in Adult Zebrafish. Int. J. Environ. Res. Public Health 2020, 17, 4632. [Google Scholar] [CrossRef] [PubMed]
- Bui Thi, N.H.; Nguyen Thi, N.A.; Audira, G.; Siregar, P.; Liang, S.-T.; Huang, J.-C.; Hsiao, C.-D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. [Google Scholar] [CrossRef]
- Mohrman, D.E.; Heller, L.J. Cardiovascular Physiology, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Crestani, C.C. Emotional stress and cardiovascular complications in animal models: A review of the influence of stress type. Front. Physiol. 2016, 7, 251. [Google Scholar] [CrossRef]
- Golbidi, S.; Frisbee, J.C.; Laher, I. Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, H1476–H1498. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. JoVE (J. Vis. Exp.) 2012, 69, e4196. [Google Scholar] [CrossRef]
- Bunescu, A.; Garric, J.; Vollat, B.; Canet-Soulas, E.; Graveron-Demilly, D.; Fauvelle, F. In vivo proton HR-MAS NMR metabolic profile of the freshwater cladoceran Daphnia magna. Mol. Biosyst. 2010, 6, 121–125. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; Terry, M.I.; Felizardo, V.O.; Vera, L.M. Daily rhythms of toxicity and effectiveness of anesthetics (MS222 and eugenol) in zebrafish (Danio rerio). Chronobiol. Int. 2011, 28, 109–117. [Google Scholar] [CrossRef]
- Monnard, C.R.; Grasser, E.K. Perspective: Cardiovascular responses to sugar-sweetened beverages in humans: A narrative review with potential hemodynamic mechanisms. Adv. Nutr. 2018, 9, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Grasser, E.K.; Dulloo, A.; Montani, J.-P. Cardiovascular responses to the ingestion of sugary drinks using a randomised cross-over study design: Does glucose attenuate the blood pressure-elevating effect of fructose? Br. J. Nutr. 2014, 112, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Santoso, F.; Sampurna, B.P.; Lai, Y.-H.; Liang, S.-T.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Development of a Simple ImageJ-Based Method for Dynamic Blood Flow Tracking in Zebrafish Embryos and Its Application in Drug Toxicity Evaluation. Inventions 2019, 4, 65. [Google Scholar] [CrossRef]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A simple imagej-based method to measure cardiac rhythm in zebrafish embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Hoage, T.; Ding, Y.; Xu, X. Quantifying cardiac functions in embryonic and adult zebrafish. In Cardiovascular Development; Springer: Berlin/Heidelberg, Germany, 2012; pp. 11–20. [Google Scholar]
- Costanzo, L.S. Physiology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- McGrath, P. Zebrafish: Methods for Assessing Drug Safety and Toxicity; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ehrman, J.K.; Kerrigan, D.; Keteyian, S. Advanced Exercise Physiology: Essential Concepts and Applications; Human Kinetics: Champaign, IL, USA, 2018. [Google Scholar]
- Mourot, L.; Bouhaddi, M.; Perrey, S.; Rouillon, J.-D.; Regnard, J. Quantitative Poincare plot analysis of heart rate variability: Effect of endurance training. Eur. J. Appl. Physiol. 2004, 91, 79–87. [Google Scholar] [CrossRef]
- Chan, P.K.; Lin, C.C.; Cheng, S.H. Noninvasive technique for measurement of heartbeat regularity in zebrafish (Danio rerio) embryos. BMC Biotechnol. 2009, 9, 11. [Google Scholar] [CrossRef]
- Charlet, A.; Rodeau, J.-L.; Poisbeau, P. Poincare plot descriptors of heart rate variability as markers of persistent pain expression in freely moving rats. Physiol. Behav. 2011, 104, 694–701. [Google Scholar] [CrossRef]
- Liu, J.; Wei, W.; Kuang, H.; Tsien, J.Z.; Zhao, F. Heart rate and heart rate variability assessment identifies individual differences in fear response magnitudes to earthquake, free fall, and air puff in mice. PLoS ONE 2014, 9, e93270. [Google Scholar] [CrossRef] [PubMed]
- Torpy, J.M.; Lynm, C.; Glass, R.M. Chronic stress and the heart. JAMA 2007, 298, 1722. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, K.-H.; Kim, J.; Choi, I.; Cho, K.-H. Modified high-density lipoproteins by artificial sweetener, aspartame, and saccharin, showed loss of anti-atherosclerotic activity and toxicity in zebrafish. Cardiovasc. Toxicol. 2015, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Graffin, R. Effects of Sucralose, Saccharin, Rebaudioside (in Stevia) and Aspartame on Development in Xenopus laevis (Clawed Frog). Bachelor’s Thesis, Saint Peter’s University, Jersey City, NJ, USA, 2016. [Google Scholar]
- Selderslaghs, I.W.; Blust, R.; Witters, H.E. Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reprod. Toxicol. 2012, 33, 142–154. [Google Scholar] [CrossRef]
- Lee, W.; Wang, Y.-C. Assessing developmental toxicity of caffeine and sweeteners in medaka (Oryzias latipes). SpringerPlus 2015, 4, 486. [Google Scholar] [CrossRef]
- Huggett, D.; Stoddard, K. Effects of the artificial sweetener sucralose on Daphnia magna and Americamysis bahia survival, growth and reproduction. Food Chem. Toxicol. 2011, 49, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Marrs, J.A. Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the study of heart development and disease using zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 13. [Google Scholar] [CrossRef]
- Sampurna, B.P.; Santoso, F.; Lee, J.-H.; Yu, W.-H.; Wu, C.-C.; Audira, G.; Juniardi, S.; Chen, J.-R.; Lin, Y.-T.; Hsiao, C.-D.J.C. Cardiac Rhythm and Molecular Docking Studies of Ion Channel Ligands with Cardiotoxicity in Zebrafish. Cells 2019, 8, 566. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.A.; Michos, E.D.; Jacobs, D.R. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Bauman, V.; Blau, J.E.; Garraffo, H.M.; Walter, P.J.; Rother, K.I. Plasma concentrations of sucralose in children and adults. Toxicol. Environ. Chem. 2017, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, L.; Ziarrusta, H.; Zabaleta, I.; Usobiaga, A.; Olivares, M.; Zuloaga, O.; Etxebarria, N.; Prieto, A. Multiresidue analytical method for the determination of 41 multiclass organic pollutants in mussel and fish tissues and biofluids by liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 493–506. [Google Scholar] [CrossRef]
- Lillicrap, A.; Langford, K.; Tollefsen, K.E. Bioconcentration of the intense sweetener sucralose in a multitrophic battery of aquatic organisms. Environ. Toxicol. Chem. 2011, 30, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- NIH. Dulcin. 2013. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8048 (accessed on 31 March 2021).
- Kojima, S.; Ichibagase, H. Studies on synthetic sweetening agents. VIII. Cyclohexylamine, a metabolite of sodium cyclamate. Chem. Pharm. Bull. 1966, 14, 971–974. [Google Scholar] [CrossRef]
- Erbaş, O.; Erdoğan, M.A.; Khalilnezhad, A.; Solmaz, V.; Gürkan, F.T.; Yiğittürk, G.; Eroglu, H.A.; Taskiran, D. Evaluation of long-term effects of artificial sweeteners on rat brain: A biochemical, behavioral, and histological study. J. Biochem. Mol. Toxicol. 2018, 32, e22053. [Google Scholar] [CrossRef]
- Cruz-Rojas, C.; SanJuan-Reyes, N.; Fuentes-Benites, M.P.A.G.; Dublan-García, O.; Galar-Martínez, M.; Islas-Flores, H.; Gómez-Oliván, L.M. Acesulfame potassium: Its ecotoxicity measured through oxidative stress biomarkers in common carp (Cyprinus carpio). Sci. Total Environ. 2019, 647, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, L.G.; Irone, M.; Barbacini, S.; Merlo, F.; Demo, P.; Pellegrin, C.; Dan, M. Heart rate variability and severe brain damage: Preliminary data. Int. J. Clin. Monit. Comput. 1993, 10, 181–185. [Google Scholar] [CrossRef]
- Ikeda, T.; Murata, Y.; Quilligan, E.J.; Parer, J.T.; Theunissen, I.M.; Cifuentes, P.; Doi, S.; Park, S.-D. Fetal heart rate patterns in postasphyxiated fetal lambs with brain damage. Am. J. Obstet. Gynecol. 1998, 179, 1329–1337. [Google Scholar] [CrossRef]
- Savenije, B.; Lambooij, E.; Gerritzen, M.; Korf, J. Development of brain damage as measured by brain impedance recordings, and changes in heart rate, and blood pressure induced by different stunning and killing methods. Poult. Sci. 2002, 81, 572–578. [Google Scholar] [CrossRef]
- Heredia-García, G.; Gómez-Oliván, L.M.; Orozco-Hernández, J.M.; Luja-Mondragón, M.; Islas-Flores, H.; SanJuan-Reyes, N.; Galar-Martínez, M.; García-Medina, S.; Dublán-García, O. Alterations to DNA, apoptosis and oxidative damage induced by sucralose in blood cells of Cyprinus carpio. Sci. Total Environ. 2019, 692, 411–421. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef]
- Brodal, P. The Central Nervous System; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Coulombe, R.A., Jr.; Sharma, R.P. Neurobiochemical alterations induced by the artificial sweetener aspartame (NutraSweet). Toxicol. Appl. Pharmacol. 1986, 83, 79–85. [Google Scholar] [CrossRef]
- Priya, K.A.; Prasath, G.S. Comparison of effect of aspartame (artificial sweetener) and aspartame-sweetened diet drink on autonomic reactivity of volunteers. Nat. J. Physiol. Pharm. Pharmacol. 2018, 8, 1057–1060. [Google Scholar]
- De Araujo, I.E.; Oliveira-Maia, A.J.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A.; Simon, S.A. Food reward in the absence of taste receptor signaling. Neuron 2008, 57, 930–941. [Google Scholar] [CrossRef]
- Mark, G.; Blander, D.; Hoebel, B. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991, 551, 308–310. [Google Scholar] [CrossRef]
- De Araujo, I.E. Sweet taste signaling and the formation of memories of energy sources. Front. Syst. Neurosci. 2011, 5, 99. [Google Scholar] [CrossRef]
- Holmes, J.C.; Fowler, N.O. Direct cardiac effects of dopamine. Circ. Res. 1962, 10, 68–72. [Google Scholar] [CrossRef]
- Ferrari, P.; Van Erp, A.; Tornatzky, W.; Miczek, K. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur. J. Neurosci. 2003, 17, 371–378. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Beauchamp, G.K. Taste receptor genes. Annu. Rev. Nutr. 2007, 27, 389–414. [Google Scholar] [CrossRef]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Okada, S.; Naito, H.; Nagai, T.; Yasuoka, A.; Matsumoto, I.; Abe, K. Two families of candidate taste receptors in fishes. Mech. Dev. 2005, 122, 1310–1321. [Google Scholar] [CrossRef]
- Oike, H.; Nagai, T.; Furuyama, A.; Okada, S.; Aihara, Y.; Ishimaru, Y.; Marui, T.; Matsumoto, I.; Misaka, T.; Abe, K. Characterization of ligands for fish taste receptors. J. Neurosci. 2007, 27, 5584–5592. [Google Scholar] [CrossRef]
- Horne, J.; Lawless, H.T.; Speirs, W.; Sposato, D. Bitter taste of saccharin and acesulfame-K. Chem. Senses 2002, 27, 31–38. [Google Scholar] [CrossRef]
- Kuhn, C.; Bufe, B.; Winnig, M.; Hofmann, T.; Frank, O.; Behrens, M.; Lewtschenko, T.; Slack, J.P.; Ward, C.D.; Meyerhof, W. Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 2004, 24, 10260–10265. [Google Scholar] [CrossRef]
- Bobowski, N.; Reed, D.R.; Mennella, J.A. Variation in the TAS2R31 bitter taste receptor gene relates to liking for the nonnutritive sweetener Acesulfame-K among children and adults. Sci. Rep. 2016, 6, 39135. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; Uchida, N. Multiple dopamine systems: Weal and woe of dopamine. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Cardello, H.; Da Silva, M.; Damasio, M. Measurement of the relative sweetness of stevia extract, aspartame and cyclamate/saccharin blend as compared to sucrose at different concentrations. Plant Foods Hum. Nutr. 1999, 54, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-W.; Kim, S.-B.; Chung, S.-J. Effect of concentration range on the accuracy of measuring sweetness potencies of sweeteners. Food Qual. Prefer. 2020, 79, 103753. [Google Scholar] [CrossRef]
- Wee, M.; Tan, V.; Forde, C. A comparison of psychophysical dose-response behaviour across 16 sweeteners. Nutrients 2018, 10, 1632. [Google Scholar] [CrossRef]
- Belloir, C.; Neiers, F.; Briand, L. Sweeteners and sweetness enhancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 279–285. [Google Scholar] [CrossRef]
- Sigala, D.M.; Widaman, A.M.; Hieronimus, B.; Nunez, M.V.; Lee, V.; Benyam, Y.; Bremer, A.A.; Medici, V.; Havel, P.J.; Stanhope, K.L. Effects of Consuming Sugar-Sweetened Beverages for 2 Weeks on 24-h Circulating Leptin Profiles, Ad Libitum Food Intake and Body Weight in Young Adults. Nutrients 2020, 12, 3893. [Google Scholar] [CrossRef]
- Antonaccio, M.J.; Taylor, D.G. Involvement of central GABA receptors in the regulation of blood pressure and heart rate of anesthetized cats. Eur. J. Pharmacol. 1977, 46, 283–287. [Google Scholar] [CrossRef]
- Williford, D.J.; Hamilton, B.L.; Souza, J.D.; Williams, T.P.; Dimicco, J.A.; Gillis, R.A. Central nervous system mechanisms involving GABA influence arterial pressure and heart rate in the cat. Circ. Res. 1980, 47, 80–88. [Google Scholar] [CrossRef]
- Souza, B.R.; Romano-Silva, M.A.; Tropepe, V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J. Neurosci. 2011, 31, 5512–5525. [Google Scholar] [CrossRef]
- Vargas, R.A. Effects of GABA, neural regulation, and intrinsic cardiac factors on heart rate variability in zebrafish larvae. Zebrafish 2017, 14, 106–117. [Google Scholar] [CrossRef]
- Morris, M.C.; Hellman, N.; Abelson, J.L.; Rao, U. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clin. Psychol. Rev. 2016, 49, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N.; Hoehn, K. Anatomy and Physiology; Benjamin-Cummings Publishing Company: San Francisco, CA, USA, 2010. [Google Scholar]
- Burke, H.M.; Davis, M.C.; Otte, C.; Mohr, D.C. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology 2005, 30, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 2005, 1, 291. [Google Scholar] [CrossRef] [PubMed]


| Related Disease | Effect on Biomarker | |
|---|---|---|
| Human | Obesity, diabetes, cardiovascular event (artificial sweetener beverage) [17], hepatotoxicity (saccharine) [18], nausea, vomiting, thrombocytopenia (aspartame) [19,20]. | Lactate dehydrogenase ↑, acetylcholinesterase ↓ (sspartame) [21,22]. |
| Rodent | Obesity, gut biota community shift (acesulfame K) [11], vascular endothelial dysfunction (acesulfame K & sucralose) [23], glucose intolerance (saccharine) [24], thyroid tumor (acesulfame K) [25] (Rat). | Dopamine ↑, hydroxytryptamine ↑, norepinephrine ↑, epinephrine ↑ (acesulfame K) [26], xanthine oxidase ↑, superoxide dismutase ↑, catalase ↑ (aspartame) [15] (rat). |
| Fish | Swimming defect, inflammatory in brain and liver, growth malformation (aspartame) [27,28] (zebrafish). | Reactive oxygen species ↑ (aspartame) [27] (zebrafish), superoxide dismutase ↑, catalase ↑, lipid peroxidase (sucralose) [5] (common carp). |
| Number | Artificial Sweetener | Molecular Formula | Aquatic Acute Toxicity |
|---|---|---|---|
| 1 | acesulfame K | C4H4KNO4S | LC50: 96 hr for fish: (mg/L): >1000 |
| 2 | alitame | C14H25N3O4S | N.A. |
| 3 | aspartame | C14H18N2O5 | N.A. |
| 4 | dulcin | C9H12N2O2 | N.A. |
| 5 | neotame | C20H30N2O5 | N.A. |
| 6 | saccharine | C7H5NO3S | N.A. |
| 7 | sodium cyclamate | C6H12NO3SNa | N.A. |
| 8 | sucralose | C12H19Cl3O8 | N.A. |
| Number | Artificial Sweetener | Absorption in Humans | Metabolic Fate in Humans |
|---|---|---|---|
| 1 | acesulfame K | <1% [76] | Not metabolized [76] |
| 2 | alitame | 100% [76] | Rapidly metabolized [76] |
| 3 | aspartame | 100% [69] | Metabolized into methanol, aspartic acid, and phenylalanine [69] |
| 4 | dulcin | N.A. | Metabolized into 4-aminophenol [77] |
| 5 | neotame | 100% [76] | Rapidly metabolized [76] |
| 6 | saccharine | 0% [69] | Bind to plasma protein and distributed via blood without metabolized [69] |
| 7 | sodium cyclamate | Poorly absorbed [78] | Metabolized into cyclohexamine [76] |
| 8 | sucralose | <10% [76] | Not metabolized [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputra, F.; Lai, Y.-H.; Fernandez, R.A.T.; Macabeo, A.P.G.; Lai, H.-T.; Huang, J.-C.; Hsiao, C.-D. Acute and Sub-Chronic Exposure to Artificial Sweeteners at the Highest Environmentally Relevant Concentration Induce Less Cardiovascular Physiology Alterations in Zebrafish Larvae. Biology 2021, 10, 548. https://doi.org/10.3390/biology10060548
Saputra F, Lai Y-H, Fernandez RAT, Macabeo APG, Lai H-T, Huang J-C, Hsiao C-D. Acute and Sub-Chronic Exposure to Artificial Sweeteners at the Highest Environmentally Relevant Concentration Induce Less Cardiovascular Physiology Alterations in Zebrafish Larvae. Biology. 2021; 10(6):548. https://doi.org/10.3390/biology10060548
Chicago/Turabian StyleSaputra, Ferry, Yu-Heng Lai, Rey Arturo T. Fernandez, Allan Patrick G. Macabeo, Hong-Thih Lai, Jong-Chin Huang, and Chung-Der Hsiao. 2021. "Acute and Sub-Chronic Exposure to Artificial Sweeteners at the Highest Environmentally Relevant Concentration Induce Less Cardiovascular Physiology Alterations in Zebrafish Larvae" Biology 10, no. 6: 548. https://doi.org/10.3390/biology10060548
APA StyleSaputra, F., Lai, Y.-H., Fernandez, R. A. T., Macabeo, A. P. G., Lai, H.-T., Huang, J.-C., & Hsiao, C.-D. (2021). Acute and Sub-Chronic Exposure to Artificial Sweeteners at the Highest Environmentally Relevant Concentration Induce Less Cardiovascular Physiology Alterations in Zebrafish Larvae. Biology, 10(6), 548. https://doi.org/10.3390/biology10060548









