Photopheresis Abates the Anti-HLA Antibody Titer and Renal Failure Progression in Chronic Antibody-Mediated Rejection
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Anti-HLA Antibodies
2.3. Mononuclear Cell Isolation
2.4. T-Cell Subset Quantification
2.5. Tregs and Th17 Cells
2.6. Assessment of the Graft Function and Proteinuria
2.7. IL 6 Serum Levels
2.8. ECP Procedures
2.9. Statistical Methods
3. Results
3.1. Baseline Patient Characteristics
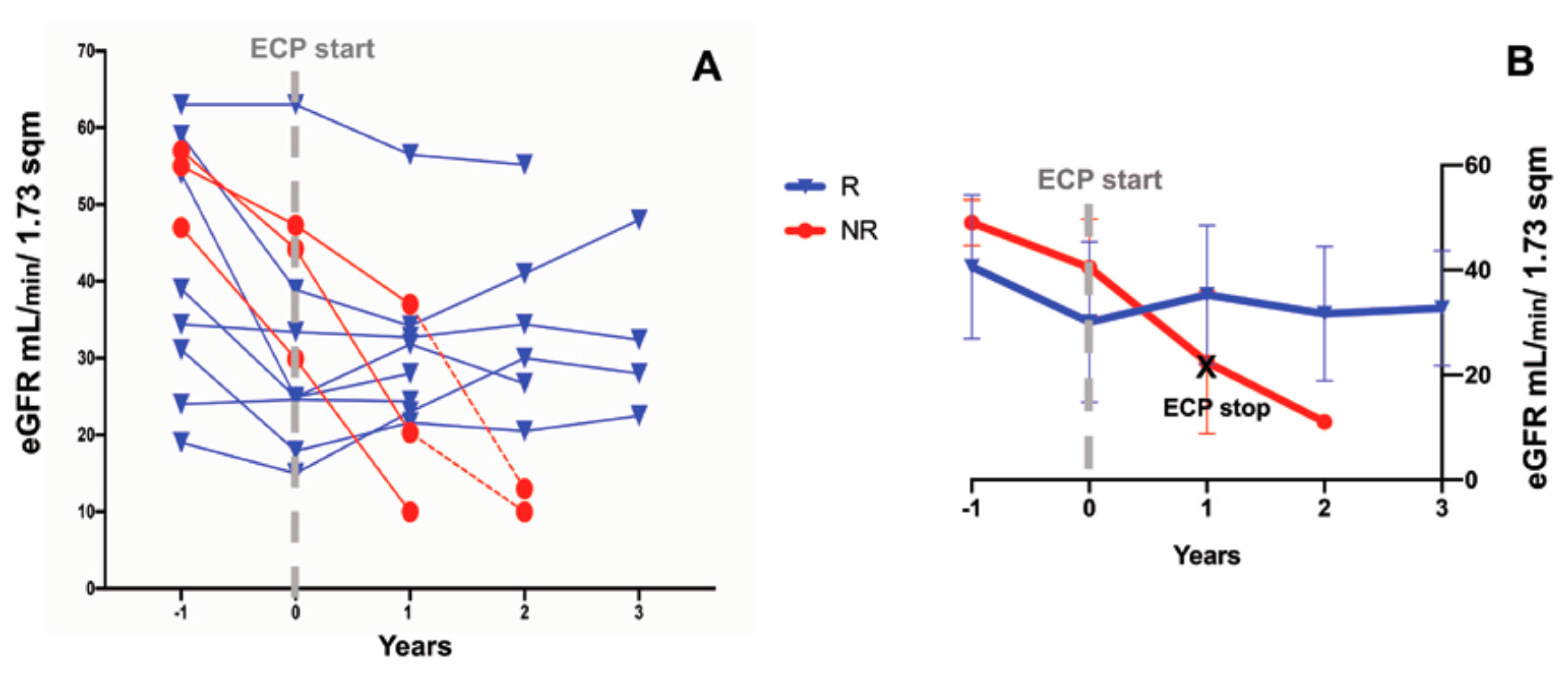
3.2. Effects of ECP Treatment on the Estimated Glomerular Filtration Rate and Proteinuria
3.3. Anti-HLA Antibodies
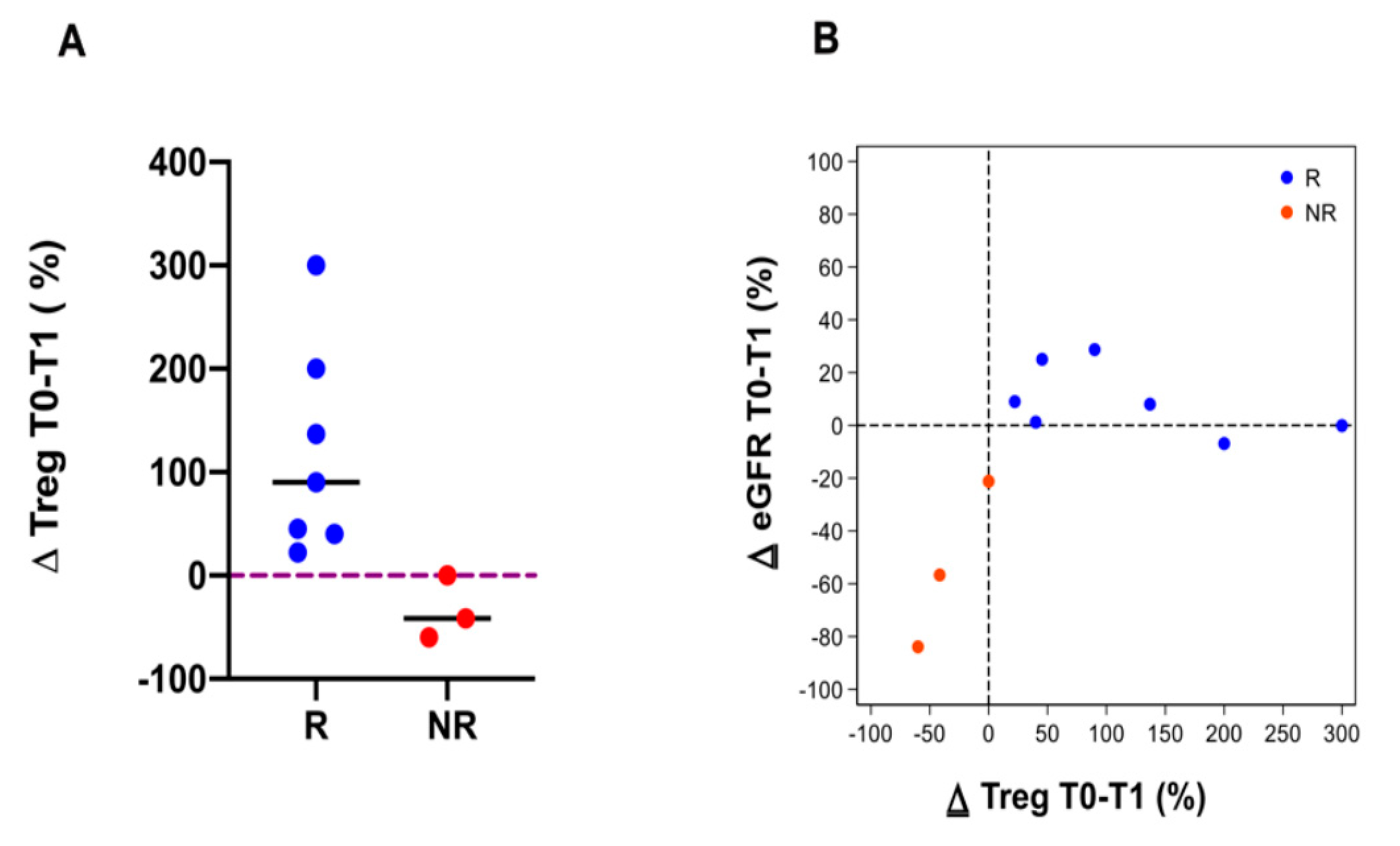
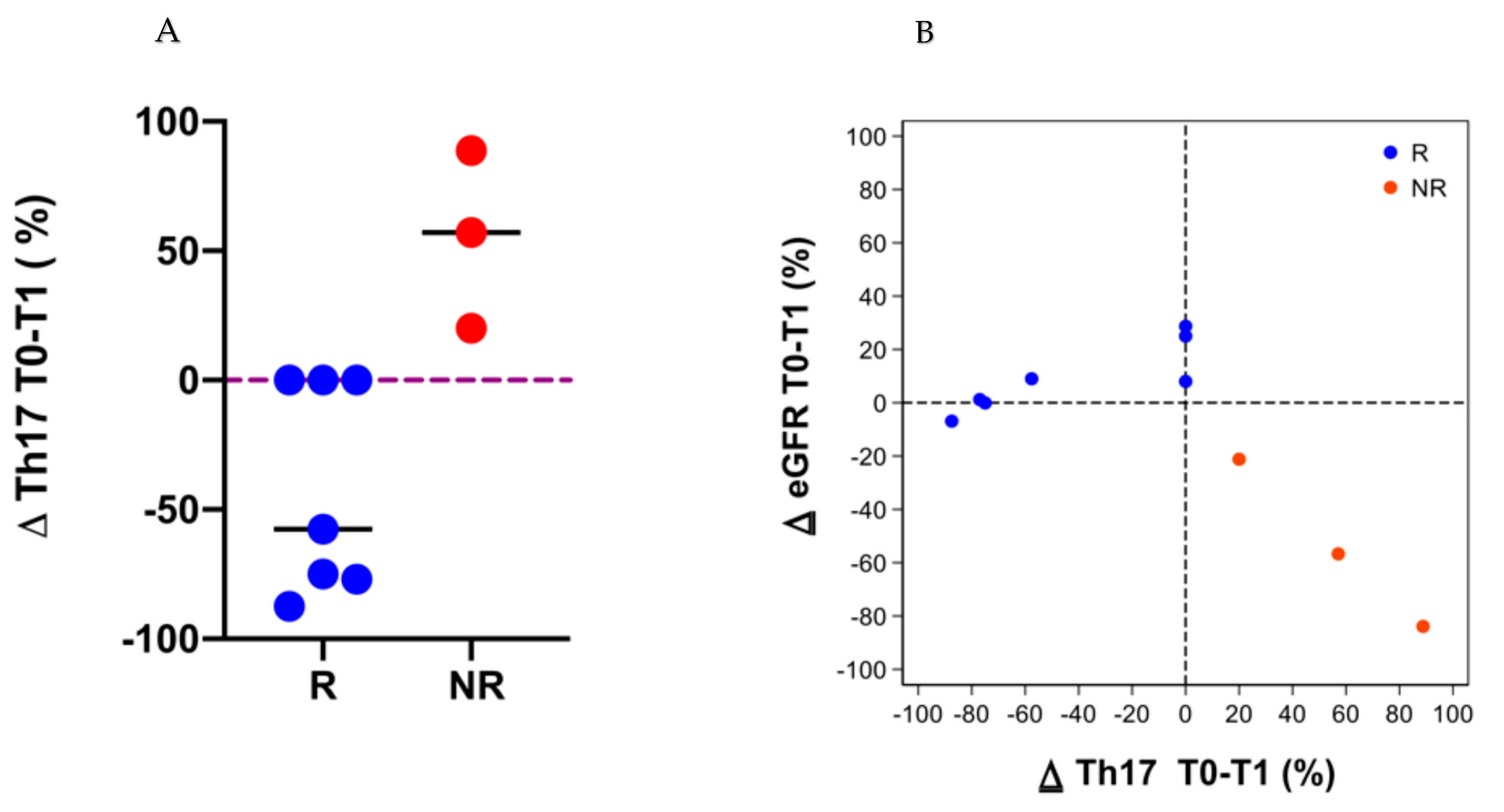
3.4. Immune Cell Subpopulations
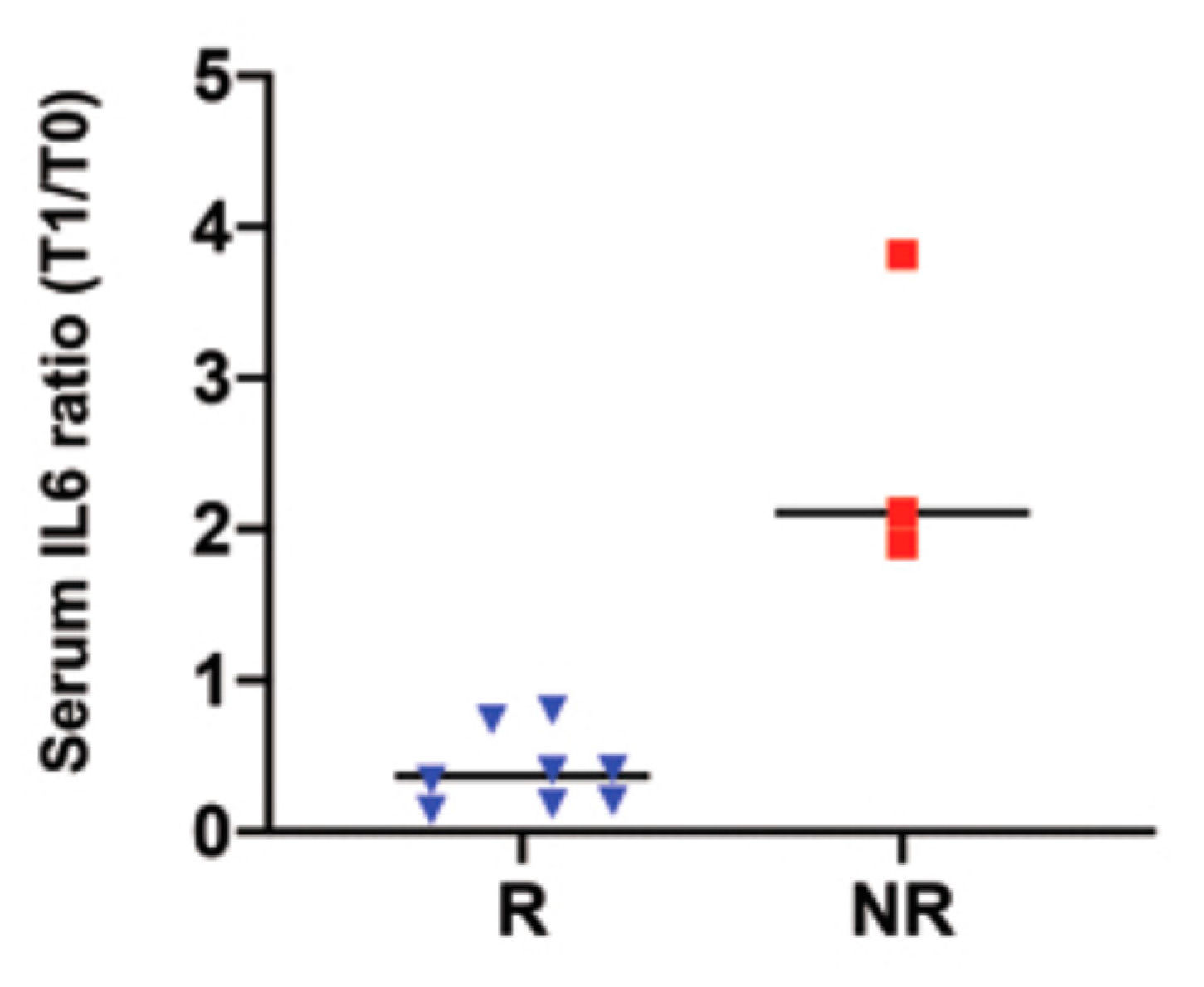
3.5. Serum IL 6 Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Meier-Kriesche, H.-U.; Schold, J.D.; Kaplan, B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am. J. Transplant. 2004, 4, 1289–1295. [Google Scholar] [CrossRef]
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef]
- Halloran, P.F.; Melk, A.; Barth, C. Rethinking Chronic Allograft Nephropathy. J. Am. Soc. Nephrol. 1999, 10, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Mauiyyedi, S.; Della Pelle, P.; Saidman, S.; Collins, A.B.; Pascual, M.; Tolkoff-Rubin, N.E.; Williams, W.W.; Cosimi, A.B.; Schneeberger, E.E.; Colvin, R.B. Chronic Humoral Rejection: Identification of Antibody-Mediated Chronic Renal Allograft Rejection by C4d Deposits in Peritubular Capillaries. J. Am. Soc. Nephrol. 2001, 12, 574–582. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; Duong van Huyen, J.-P.; Mooney, N.; Suberbielle, C.; Frémeaux-Bacchi, V.; Méjean, A.; Desgrandchamps, F.; et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar] [CrossRef]
- Loupy, A.; Hill, G.S.; Jordan, S.C. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat. Rev. Nephrol. 2012, 8, 348–357. [Google Scholar] [CrossRef]
- Salvadori, M.; Tsalouchos, A. Therapeutic apheresis in kidney transplantation: An updated review. World J. Transplant. 2019, 9, 103–122. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Mannon, R.B.; Budde, K.; Chong, A.S.; Haas, M.; Knechtle, S.; Lefaucheur, C.; Montgomery, R.A.; Nickerson, P.; Tullius, S.G.; et al. Recommended Treatment for Antibody-mediated Rejection After Kidney Transplantation: The 2019 Expert Consensus From the Transplantion Society Working Group. Transplantation 2020, 104, 911–922. [Google Scholar] [CrossRef]
- Fehr, T.; Rüsi, B.; Fischer, A.; Hopfer, H.; Wüthrich, R.P.; Gaspert, A. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation 2009, 87, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; Malik, F.; Goes, N.; Farris, A.B.; Zorn, E.; Saidman, S.; Tolkoff-Rubin, N.; Puri, S.; Wong, W. Partial therapeutic response to Rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transpl. Immunol. 2012, 27, 107–113. [Google Scholar] [CrossRef][Green Version]
- Moreso, F.; Crespo, M.; Ruiz, J.C.; Torres, A.; Gutierrez-Dalmau, A.; Osuna, A.; Perelló, M.; Pascual, J.; Torres, I.B.; Redondo-Pachón, D.; et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am. J. Transplant. 2018, 18, 927–935. [Google Scholar] [CrossRef]
- Gupta, G.; Abu Jawdeh, B.G.; Racusen, L.C.; Bhasin, B.; Arend, L.J.; Trollinger, B.; Kraus, E.; Rabb, H.; Zachary, A.A.; Montgomery, R.A.; et al. Late antibody-mediated rejection in renal allografts: Outcome after conventional and novel therapies. Transplantation 2014, 97, 1240–1246. [Google Scholar] [CrossRef]
- Eskandary, F.; Regele, H.; Baumann, L.; Bond, G.; Kozakowski, N.; Wahrmann, M.; Hidalgo, L.G.; Haslacher, H.; Kaltenecker, C.C.; Aretin, M.-B.; et al. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2018, 29, 591–605. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Wen, M.-C.; Wu, M.-J.; Chen, C.-H.; Yu, T.-M.; Chuang, Y.-W.; Huang, S.-T.; Tsai, S.-F.; Lo, Y.-C.; Ho, H.-C.; et al. Treatment of chronic active antibody-mediated rejection in renal transplant recipients - a single center retrospective study. BMC Nephrol. 2020, 21, 6. [Google Scholar] [CrossRef]
- Parajuli, S.; Mandelbrot, D.A.; Muth, B.; Mohamed, M.; Garg, N.; Aziz, F.; Redfield, R.R.; Zhong, W.; Astor, B.C.; Djamali, A. Rituximab and Monitoring Strategies for Late Antibody-Mediated Rejection After Kidney Transplantation. Transplant. Direct 2017, 3, e227. [Google Scholar] [CrossRef]
- Roberts, D.M.; Jiang, S.H.; Chadban, S.J. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation 2012, 94, 775–783. [Google Scholar] [CrossRef]
- Sautenet, B.; Blancho, G.; Büchler, M.; Morelon, E.; Toupance, O.; Barrou, B.; Ducloux, D.; Chatelet, V.; Moulin, B.; Freguin, C.; et al. One-year Results of the Effects of Rituximab on Acute Antibody-Mediated Rejection in Renal Transplantation: RITUX ERAH, a Multicenter Double-blind Randomized Placebo-controlled Trial. Transplantation 2016, 100, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Nigos, J.G.; Arora, S.; Nath, P.; Hussain, S.M.; Marcus, R.J.; Ko, T.Y.; Sureshkumar, K.K. Treatment of antibody-mediated rejection in kidney transplant recipients: A single-center experience with a bortezomib-based regimen. Exp. Clin. Transplant. 2012, 10, 609–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lefaucheur, C.; Nochy, D.; Andrade, J.; Verine, J.; Gautreau, C.; Charron, D.; Hill, G.S.; Glotz, D.; Suberbielle-Boissel, C. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am. J. Transplant. 2009, 9, 1099–1107. [Google Scholar] [CrossRef]
- Orandi, B.J.; Zachary, A.A.; Dagher, N.N.; Bagnasco, S.M.; Garonzik-Wang, J.M.; Van Arendonk, K.J.; Gupta, N.; Lonze, B.E.; Alachkar, N.; Kraus, E.S.; et al. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 2014, 98, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Kirkiles-Smith, N.C.; Deng, Y.H.; Formica, R.N.; Moeckel, G.; Broecker, V.; Bow, L.; Tomlin, R.; Pober, J.S. Eculizumab Therapy for Chronic Antibody-Mediated Injury in Kidney Transplant Recipients: A Pilot Randomized Controlled Trial. Am. J. Transplant. 2017, 17, 682–691. [Google Scholar] [CrossRef]
- Gregorini, M.; Corradetti, V.; Rocca, C.; Pattonieri, E.F.; Valsania, T.; Milanesi, S.; Serpieri, N.; Bedino, G.; Esposito, P.; Libetta, C.; et al. Mesenchymal Stromal Cells Prevent Renal Fibrosis in a Rat Model of Unilateral Ureteral Obstruction by Suppressing the Renin-Angiotensin System via HuR. PLoS ONE 2016, 11, e0148542. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, M.; Bosio, F.; Rocca, C.; Corradetti, V.; Valsania, T.; Pattonieri, E.F.; Esposito, P.; Bedino, G.; Collesi, C.; Libetta, C.; et al. Mesenchymal stromal cells reset the scatter factor system and cytokine network in experimental kidney transplantation. BMC Immunol. 2014, 15, 44. [Google Scholar] [CrossRef]
- De Martino, M.; Zonta, S.; Rampino, T.; Gregorini, M.; Frassoni, F.; Piotti, G.; Bedino, G.; Cobianchi, L.; Dal Canton, A.; Dionigi, P.; et al. Mesenchymal stem cells infusion prevents acute cellular rejection in rat kidney transplantation. Transplant. Proc. 2010, 42, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, Z.; Liu, Y.; Xiao, Z.; Guo, Y.; Li, M.; Zhao, M. Marrow Mesenchymal Stem Cells Effectively Reduce Histologic Changes in a Rat Model of Chronic Renal Allograft Rejection. Transplant. Proc. 2017, 49, 2194–2203. [Google Scholar] [CrossRef]
- Ramirez-Bajo, M.J.; Rovira, J.; Lazo-Rodriguez, M.; Banon-Maneus, E.; Tubita, V.; Moya-Rull, D.; Hierro-Garcia, N.; Ventura-Aguiar, P.; Oppenheimer, F.; Campistol, J.M.; et al. Impact of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Rat Model of Kidney Rejection. Front. Cell Dev. Biol. 2020, 8, 10. [Google Scholar] [CrossRef]
- Choi, J.; Aubert, O.; Vo, A.; Loupy, A.; Haas, M.; Puliyanda, D.; Kim, I.; Louie, S.; Kang, A.; Peng, A.; et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am. J. Transplant. 2017, 17, 2381–2389. [Google Scholar] [CrossRef]
- Lavacca, A.; Presta, R.; Gai, C.; Mella, A.; Gallo, E.; Camussi, G.; Abbasciano, I.; Barreca, A.; Caorsi, C.; Fop, F.; et al. Early effects of first-line treatment with anti-interleukin-6 receptor antibody tocilizumab for chronic active antibody-mediated rejection in kidney transplantation. Clin. Transplant. 2020, 34, e13908. [Google Scholar] [CrossRef]
- Marques, M.B.; Adamski, J. Extracorporeal photopheresis: Technique, established and novel indications. J. Clin. Apher. 2014, 29, 228–234. [Google Scholar] [CrossRef]
- Marques, M.B.; Tuncer, H.H. Photopheresis in solid organ transplant rejection. J. Clin. Apher. 2006, 21, 72–77. [Google Scholar] [CrossRef]
- Jardine, M.J.; Bhandari, S.; Wyburn, K.R.; Misra, A.K.; McKenzie, P.R.; Eris, J.M. Photopheresis therapy for problematic renal allograft rejection. J. Clin. Apher. 2009, 24, 161–169. [Google Scholar] [CrossRef]
- Kumlien, G.; Genberg, H.; Shanwell, A.; Tydén, G. Photopheresis for the treatment of refractory renal graft rejection. Transplantation 2005, 79, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Salvaneschi, L.; Perotti, C.; Zecca, M.; Bernuzzi, S.; Viarengo, G.; Giorgiani, G.; Del Fante, C.; Bergamaschi, P.; Maccario, R.; Pession, A.; et al. Extracorporeal photochemotherapy for treatment of acute and chronic GVHD in childhood. Transfusion 2001, 41, 1299–1305. [Google Scholar] [CrossRef]
- Kusztal, M.; Kościelska-Kasprzak, K.; Gdowska, W.; Zabińska, M.; Myszka, M.; Kłak, R.; Krajewska, M.; Boratyńska, M.; Szyber, P.; Chudoba, P.; et al. Extracorporeal photopheresis as an antirejection prophylaxis in kidney transplant recipients: Preliminary results. Transplant. Proc. 2011, 43, 2938–2940. [Google Scholar] [CrossRef] [PubMed]
- Lamioni, A.; Carsetti, R.; Legato, A.; Landolfo, A.; Isacchi, G.; Emma, F.; Bottazzo, G.F.; Dello Strologo, L. Induction of regulatory T cells after prophylactic treatment with photopheresis in renal transplant recipients. Transplantation 2007, 83, 1393–1396. [Google Scholar] [CrossRef]
- Tamain, M.; Sayegh, J.; Lionet, A.; Grimbert, P.; Philipponnet, C.; Hazzan, M.; Augusto, J.-F.; Büchler, M.; Merlin, E.; Kosmadakis, G.; et al. Extracorporeal photopheresis for the treatment of graft rejection in 33 adult kidney transplant recipients. Transfus. Apher. Sci. 2019, 58, 515–524. [Google Scholar] [CrossRef]
- Del Fante, C.; Scudeller, L.; Oggionni, T.; Viarengo, G.; Cemmi, F.; Morosini, M.; Cascina, A.; Meloni, F.; Perotti, C. Long-Term Off-Line Extracorporeal Photochemotherapy in Patients with Chronic Lung Allograft Rejection Not Responsive to Conventional Treatment: A 10-Year Single-Centre Analysis. Respiration 2015, 90, 118–128. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Haas, M. The Revised (2013) Banff Classification for Antibody-Mediated Rejection of Renal Allografts: Update, Difficulties, and Future Considerations. Am. J. Transplant. 2016, 16, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Perotti, C.; Del Fante, C.; Tinelli, C.; Viarengo, G.; Scudeller, L.; Zecca, M.; Locatelli, F.; Salvaneschi, L. Extracorporeal photochemotherapy in graft-versus-host disease: A longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion 2010, 50, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Horina, J.H.; Müllegger, R.R.; Horn, S.; Holzer, H.; Halwachs, G.; Kerl, H.; Wolf, P. Photopheresis for renal allograft rejection. Lancet 1995, 346, 61. [Google Scholar] [CrossRef]
- Baron, E.D.; Heeger, P.S.; Hricik, D.E.; Schulak, J.A.; Tary-Lehmann, M.; Stevens, S.R. Immunomodulatory effect of extracorporeal photopheresis after successful treatment of resistant renal allograft rejection. Photodermatol. Photoimmunol. Photomed. 2001, 17, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, G.; Tiriveedhi, V.; Ramachandran, S.; Aloush, A.; Grossman, B.; Hachem, R.; Mohanakumar, T. Efficacy of extracorporeal photopheresis in clearance of antibodies to donor-specific and lung-specific antigens in lung transplant recipients. J. Heart Lung Transplant. 2014, 33, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Loupy, A.; Hill, G.S.; Andrade, J.; Nochy, D.; Antoine, C.; Gautreau, C.; Charron, D.; Glotz, D.; Suberbielle-Boissel, C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J. Am. Soc. Nephrol. 2010, 21, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Aubert, O.; Loupy, A.; Hidalgo, L.; Duong van Huyen, J.-P.; Higgins, S.; Viglietti, D.; Jouven, X.; Glotz, D.; Legendre, C.; Lefaucheur, C.; et al. Antibody-Mediated Rejection Due to Preexisting versus De Novo Donor-Specific Antibodies in Kidney Allograft Recipients. J. Am. Soc. Nephrol. 2017, 28, 1912–1923. [Google Scholar] [CrossRef]
- Haas, M.; Mirocha, J.; Reinsmoen, N.L.; Vo, A.A.; Choi, J.; Kahwaji, J.M.; Peng, A.; Villicana, R.; Jordan, S.C. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int. 2017, 91, 729–737. [Google Scholar] [CrossRef]
- Mankarious, M.; Matthews, N.C.; Snowden, J.A.; Alfred, A. Extracorporeal Photopheresis (ECP) and the Potential of Novel Biomarkers in Optimizing Management of Acute and Chronic Graft vs. Host Disease (GvHD). Front. Immunol. 2020, 11, 81. [Google Scholar] [CrossRef]
- Yazdani, S.; Callemeyn, J.; Gazut, S.; Lerut, E.; de Loor, H.; Wevers, M.; Heylen, L.; Saison, C.; Koenig, A.; Thaunat, O.; et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 2019, 95, 188–198. [Google Scholar] [CrossRef]
- Afzali, B.; Lombardi, G.; Lechler, R.I.; Lord, G.M. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin. Exp. Immunol. 2007, 148, 32–46. [Google Scholar] [CrossRef]




| Variables | n = 14 Patients |
|---|---|
| Sex | |
| Male (%) | 42.8 |
| Female (%) | 57.2 |
| Age (years) | |
| Mean ± SD | 49.55 ± 8.87 |
| Ethnicity | |
| Caucasian (%) | 13 (92.86) |
| African (%) | 1 (7.14) |
| Cause of ERSD | |
| Unknown (%) | 3 (21.43) |
| GN (%) | 4 (28.57) |
| ADPKD (%) | 1 (7.14) |
| HT (%) | 1 (7.14) |
| DN (%) | 0 (0) |
| Other genetic (%) | 2 (14.29) |
| Other (%) | 3 (21.43) |
| Pre-transplant dialysis duration (years) | |
| Mean ± SD | 3.9 ± 3.0 |
| Donor Type | |
| Deceased (%) | 13 (92.86) |
| Living (%) | 1 (7.14) |
| Retransplantation | |
| Yes (%) | 1 (7.14) |
| No (%) | 13 (92.86) |
| PRA (%) | |
| Median; min-max | 0 (0–30) |
| HLA match | |
| Median; min-max | 2 (2–5) |
| DGF | |
| Yes (%) | 1 (7.14) |
| No (%) | 12 (85.71) |
| Unknown (%) | 1 (7.14) |
| Acute renal allograft rejection | |
| Yes (%) | 3 (21.4) |
| No (%) | 9 (78.6) |
| Transplant age (years) | |
| Median and IQR | 9.25 (6.66–11.9) |
| Immunosuppressive therapy (%) | |
| Induction | 9 (64.29) |
| Basiliximab | 5 (35.71) |
| Anti Thymocyte globulin | |
| Maintenance | 6 (42.86) |
| Cyclosporin | 8 (57.14) |
| Tacrolimus | 1 (7.14) |
| mTOR inhibitor | 12 (85.71) |
| Corticosteroids | 14 (100) |
| Mycophenolate mofetil/Mycophenolic acid/Azathioprine |
| ID Patient | DSA | Anti-HLA-Ab | DSA (MFI) Baseline | DSA (MFI) 1Y | DSA (MFI) 2Y | DSA (MFI) 3Y |
|---|---|---|---|---|---|---|
| # 1 | DQ7 | 36,780 | 31,500 | 18,330 | 26,000 | |
| DQAI | 21,811 | 18,800 | 13,750 | 18,000 | ||
| # 2 | DQ4 | 12,000 | 4890 | Neg | Neg | |
| DQ4 | 6800 | 3800 | Neg | Neg | ||
| DQ6 | 3900 | 1700 | Neg | Neg | ||
| # 3 | CW7 | 3000 | Neg | Neg | Neg | |
| # 4 | B47 | 2197 | Neg | Neg | ||
| # 5 | DQ5 | 5474 | 5000 | Neg | ||
| DQA | 3045 | Neg | Neg | |||
| # 6 | DQ61 | 15,800 | Neg | Neg | ||
| DQ62 | 4000 | Neg | Neg | |||
| DQ53 | 19,120 | Neg | Neg | |||
| DQ64 | 3785 | Neg | Neg | |||
| DQ69 | 14,000 | Neg | Neg | |||
| # 7 | DQ7 | 47,000 | 27,000 | |||
| # 8 | DR11 | 4000 | Neg | Neg | Neg | |
| DR15 | 1500 | Neg | Neg | Neg | ||
| ID Patient | DSA | Anti-HLA-Ab | DSA (MFI) Baseline | DSA (MFI) 1Y | DSA (MFI) 2Y | DSA (MFI) 3Y |
| # 9 | DQ21 | 17,700 | 17,000 | 15,562 | 19,623 | |
| DQ22 | 5000 | 1900 | 4902 | 6170 | ||
| DR531 | 5500 | 2000 | 2168 | 1962 | ||
| DR533 | 5300 | 4300 | ||||
| # 10 | see Supplementary Figure S2 | |||||
| # 11 | DQ21 | 4215 | 17,660 | |||
| DQ22 | 18,324 | 37,689 | ||||
| DR531 | 6715 | 11,217 | ||||
| DR533 | 0 | 2062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorini, M.; Del Fante, C.; Pattonieri, E.F.; Avanzini, M.A.; Grignano, M.A.; Cassaniti, I.; Baldanti, F.; Comolli, G.; Nocco, A.; Ramondetta, M.; et al. Photopheresis Abates the Anti-HLA Antibody Titer and Renal Failure Progression in Chronic Antibody-Mediated Rejection. Biology 2021, 10, 547. https://doi.org/10.3390/biology10060547
Gregorini M, Del Fante C, Pattonieri EF, Avanzini MA, Grignano MA, Cassaniti I, Baldanti F, Comolli G, Nocco A, Ramondetta M, et al. Photopheresis Abates the Anti-HLA Antibody Titer and Renal Failure Progression in Chronic Antibody-Mediated Rejection. Biology. 2021; 10(6):547. https://doi.org/10.3390/biology10060547
Chicago/Turabian StyleGregorini, Marilena, Claudia Del Fante, Eleonora Francesca Pattonieri, Maria Antonietta Avanzini, Maria Antonietta Grignano, Irene Cassaniti, Fausto Baldanti, Giuditta Comolli, Angela Nocco, Miriam Ramondetta, and et al. 2021. "Photopheresis Abates the Anti-HLA Antibody Titer and Renal Failure Progression in Chronic Antibody-Mediated Rejection" Biology 10, no. 6: 547. https://doi.org/10.3390/biology10060547
APA StyleGregorini, M., Del Fante, C., Pattonieri, E. F., Avanzini, M. A., Grignano, M. A., Cassaniti, I., Baldanti, F., Comolli, G., Nocco, A., Ramondetta, M., Viarengo, G., Sepe, V., Libetta, C., Klersy, C., Perotti, C., & Rampino, T. (2021). Photopheresis Abates the Anti-HLA Antibody Titer and Renal Failure Progression in Chronic Antibody-Mediated Rejection. Biology, 10(6), 547. https://doi.org/10.3390/biology10060547








