Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
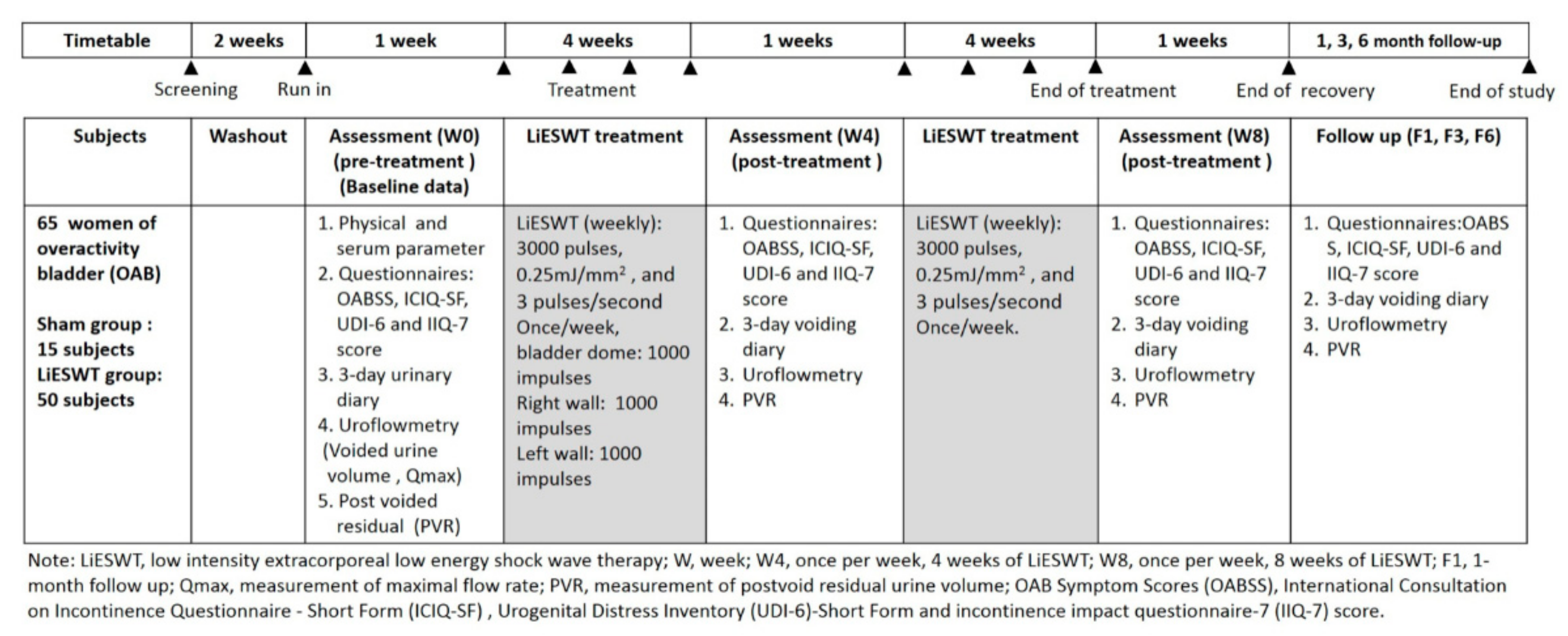
2.1. Design
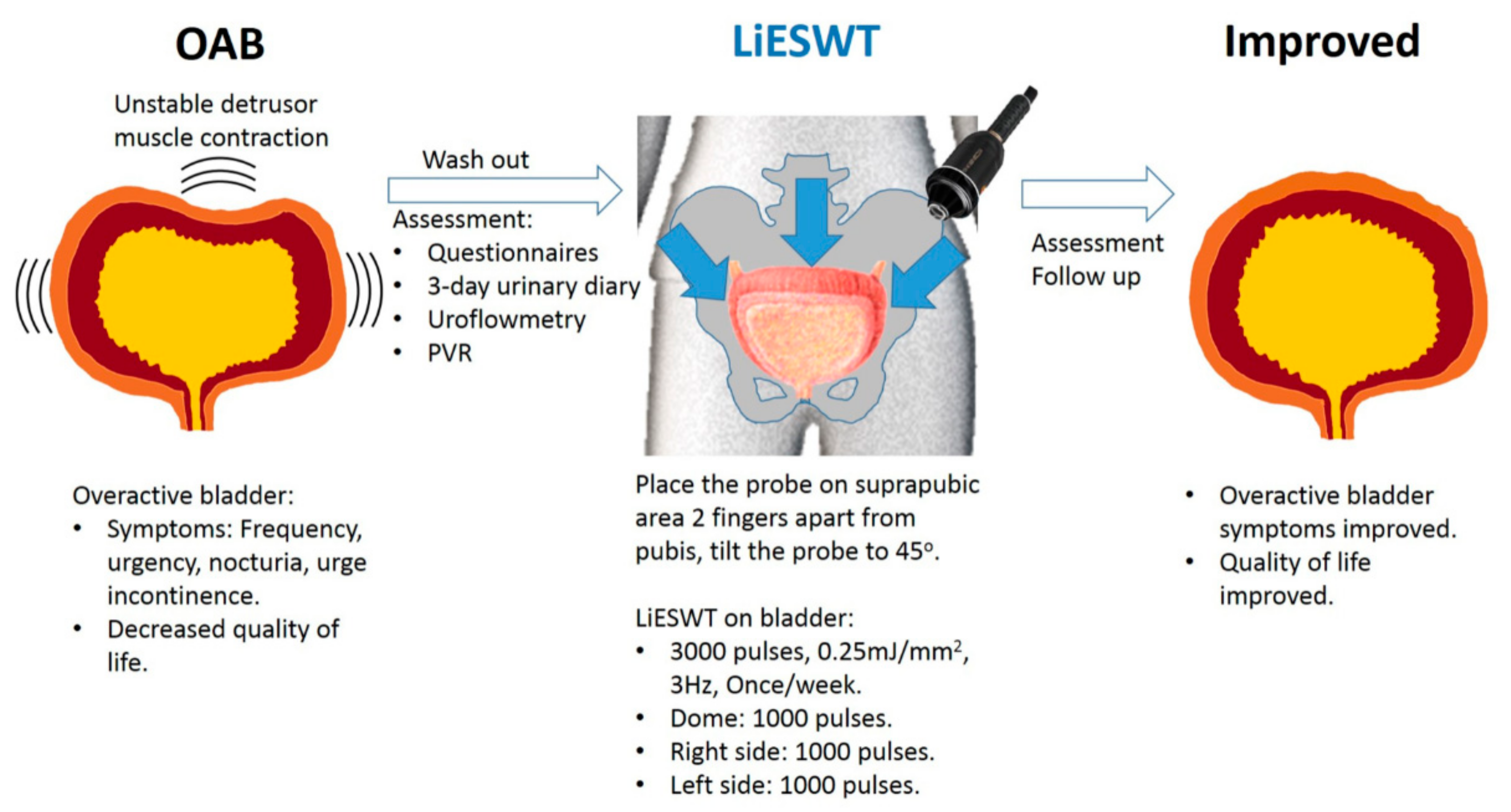
2.2. Procedure and Setting of LiESWT
2.3. Physical and Serum Biochemical Indicators of Studied Participants
2.4. Outcome Measures and Therapeutic Efficacy Assessment for LiESWT
2.5. Statistical Analysis
3. Results
3.1. Diagnoses
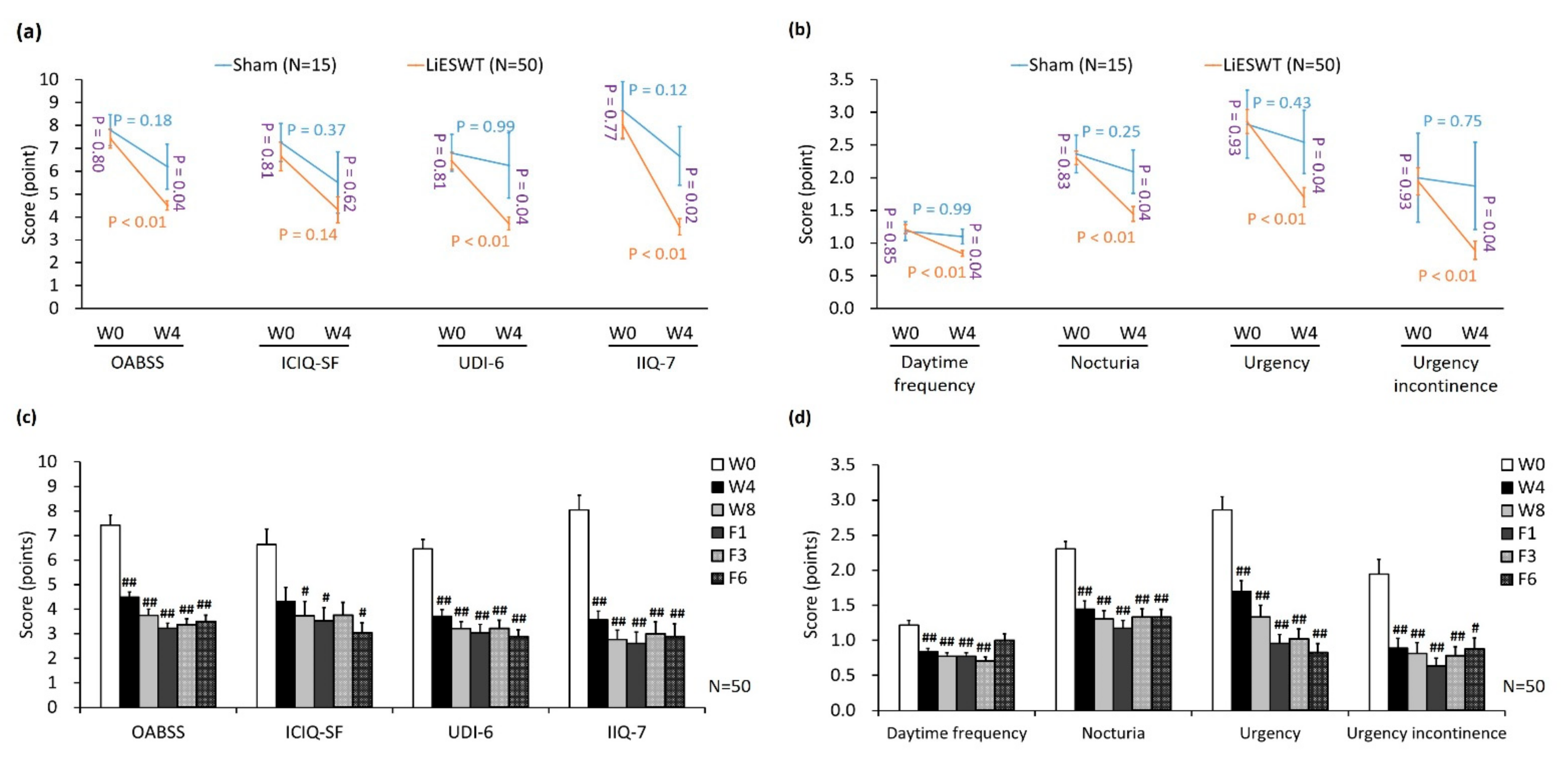
3.2. Primary and Secondary End Points
3.3. Safety of LiESWT Treatments
3.4. A Brief Diagram Proposed for the Potential Effects of LiESWT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Ng, S.; Chen, S.; Huang, Y.; Hu, S.; Chen, G. Overactive bladder in Taiwanese women: Re-analysis of epidemiological database of community from 1999 to 2001. Neurourol. Urodyn. 2012, 31, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, A.A.; Gajewski, J. Urodynamic findings in women with refractory overactive bladder symptoms. Int. J. Urol. 2015, 23, 75–79. [Google Scholar] [CrossRef]
- Jenks, J.C. Overactive bladder in women. Nurs. Stand. 2016, 31, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Kaiho, Y.; Miyazato, M.; Yunoki, T.; Tai, C.; Chancellor, M.B.; Tyagi, P. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Azadzoi, K.M.; Shinde, V.M.; Tarcan, T.; Kozlowski, R.; Siroky, M.B. Increased Leukotriene and Prostaglandin Release, and Overactivity in the Chronically Ischemic Bladder. J. Urol. 2003, 169, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Kihara, K.; Saito, K.; Matsuoka, Y.; Yoshida, S.; Chancellor, M.B.; De Groat, W.C.; Yoshimura, N. Reactive oxygen species mediate detrusor overactivity via sensitization of afferent pathway in the bladder of anaesthetized rats. BJU Int. 2008, 101, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Azadzoi, K.M.; Tarcan, T.; Kozlowski, R.; Krane, R.J.; Siroky, M.B. Overactivity and structural changes in the chronically ischemic bladder. J. Urol. 1999, 162, 1768. [Google Scholar] [CrossRef]
- Chapple, C.; Khullar, V.; Gabriel, Z.; Dooley, J.A. The Effects of Antimuscarinic Treatments in Overactive Bladder: A Systematic Review and Meta-Analysis. Eur. Urol. 2005, 48, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Carrière, I.; Fourrier-Reglat, A.; Dartigues, J.-F.; Rouaud, O.; Pasquier, F.; Ritchie, K.; Ancelin, M.-L. Drugs With Anticholinergic Properties, Cognitive Decline, and Dementia in an Elderly General Population. Arch. Intern. Med. 2009, 169, 1317–1324. [Google Scholar] [CrossRef]
- Chapple, C.R.; Nazir, J.; Hakimi, Z.; Bowditch, S.; Fatoye, F.; Guelfucci, F.; Khemiri, A.; Siddiqui, E.; Wagg, A. Persistence and Adherence with Mirabegron versus Antimuscarinic Agents in Patients with Overactive Bladder: A Retrospective Observational Study in UK Clinical Practice. Eur. Urol. 2017, 72, 389–399. [Google Scholar] [CrossRef]
- Marcelissen, T.; Cornu, J.-N.; Antunes-Lopes, T.; Geavlete, B.; Delongchamps, N.B.; Rashid, T.; Rieken, M.; Rahnama’I, M.S. Management of Idiopathic Overactive Bladder Syndrome: What Is the Optimal Strategy After Failure of Conservative Treatment? Eur. Urol. Focus 2018, 4, 760–767. [Google Scholar] [CrossRef]
- Orasanu, B.; Mahajan, S.T. The use of botulinum toxin for the treatment of overactive bladder syndrome. Indian J. Urol. 2013, 29, 2–11. [Google Scholar] [CrossRef]
- Chung, E.; Wang, J. A state-of-art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev. Med. Devices 2017, 14, 929–934. [Google Scholar] [CrossRef]
- Chung, E.; Cartmill, R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: An Australian first open-label single-arm prospective clinical trial. BJU Int. 2015, 115, 46–49. [Google Scholar] [CrossRef]
- Clavijo, R.I.; Kohn, T.P.; Kohn, J.R.; Ramasamy, R. Effects of Low-Intensity Extracorporeal Shockwave Therapy on Erectile Dysfunction: A Systematic Review and Meta-Analysis. J. Sex. Med. 2017, 14, 27–35. [Google Scholar] [CrossRef]
- Zimmermann, R.; Cumpanas, A.; Miclea, F.; Janetschek, G. Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome in Males: A Randomised, Double-Blind, Placebo-Controlled Study. Eur. Urol. 2009, 56, 418–424. [Google Scholar] [CrossRef]
- Guu, S.-J.; Geng, J.-H.; Chao, I.-T.; Lin, H.-T.; Lee, Y.-C.; Juan, Y.-S.; Liu, C.-C.; Wang, C.-J.; Tsai, C.-C. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy on Men with Chronic Pelvic Pain Syndrome Refractory to 3-As Therapy. Am. J. Men’s Health 2017, 12, 441–452. [Google Scholar] [CrossRef]
- Long, C.-Y.; Lin, K.-L.; Lee, Y.-C.; Chuang, S.-M.; Lu, J.-H.; Wu, B.-N.; Chueh, K.-S.; Ker, C.-R.; Shen, M.-C.; Juan, Y.-S. Therapeutic effects of Low intensity extracorporeal low energy shock wave therapy (LiESWT) on stress urinary incontinence. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Wang, C.-J. An overview of shock wave therapy in musculoskeletal disorders. Chang. Gung. Med. J. 2003, 26, 220–232. [Google Scholar]
- Homma, Y.; Yoshida, M.; Seki, N.; Yokoyama, O.; Kakizaki, H.; Gotoh, M.; Yamanishi, T.; Yamaguchi, O.; Takeda, M.; Nishizawa, O. Symptom assessment tool for overactive bladder syndrome—Overactive bladder symptom score. Urology 2006, 68, 318–323. [Google Scholar] [CrossRef]
- Tervonen, T.; Karjalainen, K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J. Clin. Periodontol. 1997, 24, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-Based Survey of Urinary Incontinence, Overactive Bladder, and Other Lower Urinary Tract Symptoms in Five Countries: Results of the EPIC Study. Eur. Urol. 2006, 50, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, S.M.; Dmochowski, R.R. Gender specific pharmacokinetic and pharmacodynamic considerations for antimuscarinic drugs for overactive bladder treatment. Expert Opin. Drug Metab. Toxicol. 2020, 16, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, I.; Appel, B.; Vardi, Y. Low-Intensity Extracorporeal Shock Wave Therapy—A Novel Effective Treatment for Erectile Dysfunction in Severe ED Patients Who Respond Poorly to PDE5 Inhibitor Therapy. J. Sex. Med. 2012, 9, 259–264. [Google Scholar] [CrossRef]
- Yuan, P.; Ma, D.; Zhang, Y.; Gao, X.; Liu, Z.; Li, R.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Efficacy of low-intensity extracorporeal shock wave therapy for the treatment of chronic prostatitis/chronic pelvic pain syndrome: A systematic review and meta-analysis. Neurourol. Urodyn. 2019, 38, 1457–1466. [Google Scholar] [CrossRef]
- Guu, S.-J.; Liu, C.-C.; Juan, Y.-S.; Li, C.-C.; Tsai, C.-C. The 12-month follow-up of the low-intensity extracorporeal shockwave therapy in the treatment of patients with chronic pelvic pain syndrome refractory to 3-As medications. Aging Male 2020, 23, 793–800. [Google Scholar] [CrossRef]
- I Rudenko, V.; Rapoport, L.M.; A Gazimiev, M.; Demidko, Y.L.; Bayduvaliev, A.M. Initial experience with shock wave therapy in men with chronic pelvic pain syndrome. Urologiia 2015, 26–29. [Google Scholar]
- Zhang, X.; Yan, X.; Wang, C.; Tang, T.; Chai, Y. The dose-effect relationship in extracorporeal shock wave therapy: The optimal parameter for extracorporeal shock wave therapy. J. Surg. Res. 2014, 186, 484–492. [Google Scholar] [CrossRef]
- Chen, Y.T.; Yang, C.C.; Sun, C.K.; Chiang, H.J.; Chen, Y.L.; Sung, P.H.; Zhen, Y.Y.; Huang, T.H.; Chang, C.L.; Chen, H.H.; et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am. J. Transl. Res. 2014, 6, 631–648. [Google Scholar]
- Wang, H.S.; Oh, B.S.; Wang, B.; Ruan, Y.; Zhou, J.; Banie, L.; Lee, Y.C.; Tamaddon, A.; Zhou, T.; Wang, G.; et al. Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int. 2018, 122, 490–500. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, L.; Zhao, Y.; Wang, M.; Jin, X.; Zhang, H. Endogenous Stem Cells Were Recruited by Defocused Low-Energy Shock Wave in Treating Diabetic Bladder Dysfunction. Stem Cell Rev. 2017, 13, 287–298. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Carmack, C.L.; Li, Y.; Brown, J.; Jhingran, A.; Hughes, D.C.; Perkins, H.Y.; Scruggs, S.; Harrison, C.; Baum, G.; et al. Social-cognitive theory predictors of exercise behavior in endometrial cancer survivors. Health Psychol. 2013, 32, 1137–1148. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, J.; Banie, L.; Reed-Maldonado, A.B.; Ning, H.; Lu, Z.; Ruan, Y.; Zhou, T.; Wang, H.S.; Oh, B.S.; et al. Low-intensity extracorporeal shock wave therapy promotes myogenesis through PERK/ATF4 pathway. Neurourol. Urodyn. 2018, 37, 699–707. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Jeon, S.H.; Bae, W.J.; Choi, S.W.; Jeong, H.C.; Kim, K.S.; Kim, S.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Efficient Promotion of Autophagy and Angiogenesis Using Mesenchymal Stem Cell Therapy Enhanced by the Low-Energy Shock Waves in the Treatment of Erectile Dysfunction. Stem Cells Int. 2018, 2018, 1302672. [Google Scholar] [CrossRef]
- Wang, H.J.; Lee, W.C.; Tyagi, P.; Huang, C.C.; Chuang, Y.C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol. Urodyn. 2017, 36, 1440–1447. [Google Scholar] [CrossRef]
- Mariotto, S.; de Prati, A.C.; Cavalieri, E.; Amelio, E.; Marlinghaus, E.; Suzuki, H. Extracorporeal shock wave therapy in inflammatory diseases: Molecular mechanism that triggers anti-inflammatory action. Curr. Med. Chem. 2009, 16, 2366–2372. [Google Scholar] [CrossRef]
- Gotoh, M.; Homma, Y.; Yokoyama, O.; Nishizawa, O. Responsiveness and minimal clinically important change in overactive bladder symptom score. Urology 2011, 78, 768–773. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Female participants aged 20–75 years who were diagnosed with OAB for more than 3 months. 2. OAB symptoms included daytime frequency of micturition ≥ 8 times, and nocturia, urgency or urgency incontinence ≥ 1 times. 3. Patients could understand and follow the instructions and were able to complete the questionnaire. 4. Patients with OAB symptom who did not take antimuscarinic or ß3 agonist. 5. OAB patient with antimuscarinic or ß3 agonist treatment could also be included after 3 months of medication withdrawal 6. Signature of informed consent form. | 1. Urinary tract infection detected at screening, and recurrent for urinary tract infections more than 3 episodes in the past 3 months. 2. Neuropathic diseases. 3. Lower urinary tract surgery within last 6 months. 4. Significant bladder outflow obstruction. 5. Drug or nondrug treatments of OAB in the previous 3 months. 6. History of urolithiasis or urologic cancer. 7. Gross hematuria. 8. Severe cardiopulmonary disease, coagulopathy, liver or renal dysfunction. 9. Previous pelvic radiation therapy. 10. Women with pregnancy or within six months of childbearing. |
| Parameter | Sham (Mean ± SE) | LiESWT (Mean ± SE) | Normal Range |
|---|---|---|---|
| Physical parameter | |||
| Female age (years) | 55.63 ± 2.07 | 57.01 ± 1.14 | 20–75 |
| Height (cm) | 160.68 ± 1.15 | 157.07 ± 0.57 | |
| Weight (kg) | 64.81 ± 3.66 | 58.24 ± 0.95 | |
| BMI (kg/m2) | 24.99 ± 1.19 | 24.39 ± 0.80 | 18.5–26 |
| Waistline (cm) | 88.69 ± 3.20 | 85.07 ± 1.07 | |
| Systolic pressure (mmHg) | 115.50 ± 3.85 | 119.32 ± 1.50 | 100–120 |
| Diastolic pressure (mmHg) | 66.88 ± 2.06 | 72.08 ± 1.03 | 60–80 |
| MAP (mmHg) | 83.08 ± 2.41 | 87.83 ± 1.04 | 70–110 |
| Serum parameter | |||
| HbA1C (%) | 5.75 ± 0.11 | 5.61 ± 0.08 | |
| AC sugar (mg/dl) | 99.75 ± 1.32 | 101.11 ± 2.69 | 65–109 |
| BUN (mg/dl) | 10.98 ± 0.89 | 12.50 ± 0.50 | 8–20 |
| Creatinine (mg/dl) | 0.70 ± 0.04 | 0.72 ± 0.02 | 0.44–1.03 |
| GOT(AST) (IU/L) | 22.25 ± 1.07 | 25.56 ± 1.66 | 10–42 |
| GPT(ALT) (IU/L) | 24.63 ± 2.33 | 22.88 ± 1.60 | 10–40 |
| Triglycerides (mg/dl) | 88.63 ± 5.79 | 90.30 ± 5.12 | 35–160 |
| Cholesterol (mg/dl) | 215.50 ± 9.84 | 206.54 ± 4.57 | 140–200 |
| HDL (mg/dl) | 63.88 ± 3.32 | 58.38 ± 1.88 | 29–85 |
| LDL (mg/dl) | 126.41 ± 6.84 | 122.54 ± 4.11 | 0–130 |
| Parameter | Sham (N = 15) | LiESWT (N = 50) | ||||||
|---|---|---|---|---|---|---|---|---|
| W0 | W4 | W0 | W4 | W8 | F1 | F3 | F6 | |
| 3-day urinary diary record | ||||||||
| Intake (mL) | 2094.8 ± 208.7 | 2196.5 ± 111.2 | 1987.0 ± 85.0 | 1906.8 ± 82.3 | 2180.9 ± 221.5 | 1921.3 ± 93.0 | 1969.3 ± 97.7 | 1867.0 ± 55.7 |
| Output (mL) | 2321.9 ± 250.4 | 2219.0 ± 166.6 | 2113.4 ± 89.5 | 2017.3 ± 79.4 | 2007.7 ± 83.4 | 2064.2 ± 82.0 | 2034.0 ± 93.4 | 1878.2 ± 64.4 |
| Average voided volume (mL) | 196.4 ± 10.8 | 211.8 ± 10.0 | 186.9 ± 8.4 | 207.1 ± 7.8 | 224.6 ± 8.6 # | 215.2 ± 8.6 | 217.1 ± 9.0 | 198.7 ± 7.6 |
| Functional bladder capacity (mL) | 342.5 ± 17.6 | 345.3 ± 23.5 | 330.5 ± 12.2 | 349.9 ± 12.1 | 375.5 ± 13.2 # | 350.2 ± 12.9 | 358.2 ± 14.6 | 348.7 ± 13.6 |
| Daytime frequency (times) | 12.18 ± 0.99 | 12.00 ± 1.01 | 11.85 ± 0.50 | 9.76 ± 0.37+, ## | 9.48 ± 0.33 ## | 9.65 ± 0.30 ## | 8.89 ± 0.26 ## | 9.46 ± 0.36 ## |
| Nocturia (times) | 1.88 ± 0.43 | 1.57 ± 0.33 | 1.77 ± 0.17 | 1.35 ± 0.12 | 1.20 ± 0.12 ## | 1.22 ± 0.11 # | 1.18 ± 0.11 # | 1.41 ± 0.14 |
| Urgency (times) | 3.07 ± 0.29 | 2.83 ± 0.27 | 3.16 ± 0.39 | 2.13 ± 0.33 | 1.96 ± 0.34 # | 1.90 ± 0.35 # | 1.68 ± 0.31 # | 1.10 ± 0.23 ## |
| Uroflowmetry data | ||||||||
| Voided urine volume (mL) | 293.8 ± 36.3 | 309.1 ± 42.5 | 296.2 ± 16.7 | 357.5 ± 20.1 # | 373.9 ± 21.5 # | 342.4 ± 16.8 | 344.2 ± 15.3 | 340.7 ± 13.4 |
| Maximum flow rate (Qmax) (mL/s) | 27.87 ± 2.59 | 26.46 ± 2.93 | 25.61 ± 1.69 | 27.45 ± 1.60 | 30.51 ± 1.26 # | 30.73 ± 1.37 # | 33.60 ± 3.26 # | 32.74 ± 1.83 # |
| Post voided residual (PVR) (mL) | 43.88 ± 14.27 | 56.43 ± 12.06 | 49.63 ± 6.18 | 40.88 ± 5.39 | 38.19 ± 4.66 | 30.04 ± 3.90 # | 31.24 ± 3.12 # | 23.86 ± 2.66 # |
| OABSS score (points) | ||||||||
| Total score | 7.80 ± 0.68 | 6.20 ± 0.98 | 7.42 ± 0.41 | 4.50 ± 0.20 +,## | 3.75 ± 0.25 ## | 3.23 ± 0.20 ## | 3.38 ± 0.24## | 3.50 ± 0.26## |
| Daytime frequency | 1.18 ± 0.14 | 1.10 ± 0.11 | 1.22 ± 0.07 | 0.84 ± 0.04 +,## | 0.77 ± 0.05 ## | 0.77 ± 0.05 ## | 0.70 ± 0.06 ## | 1.00 ± 0.09 |
| Nocturia | 2.36 ± 0.29 | 2.09 ± 0.33 | 2.30 ± 0.10 | 1.45 ± 0.11 +,## | 1.31 ± 0.12 ## | 1.17 ± 0.11## | 1.33 ± 0.11 ## | 1.33 ± 0.11 ## |
| Urgency | 2.82 ± 0.52 | 2.55 ± 0.48 | 2.86 ± 0.19 | 1.70 ± 0.15 +,## | 1.33 ± 0.16 ## | 0.96 ± 0.13 ## | 1.02 ± 0.14 ## | 0.83 ± 0.13 ## |
| Urgency incontinence | 2.00 ± 0.68 | 1.88 ± 0.67 | 1.94 ± 0.21 | 0.89 ± 0.14 +,## | 0.81 ± 0.15 ## | 0.64 ± 0.11 ## | 0.78 ± 0.13 ## | 0.88 ± 0.16 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.-H.; Chueh, K.-S.; Chuang, S.-M.; Wu, Y.-H.; Lin, K.-L.; Long, C.-Y.; Lee, Y.-C.; Shen, M.-C.; Sun, T.-W.; Juan, Y.-S. Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome. Biology 2021, 10, 540. https://doi.org/10.3390/biology10060540
Lu J-H, Chueh K-S, Chuang S-M, Wu Y-H, Lin K-L, Long C-Y, Lee Y-C, Shen M-C, Sun T-W, Juan Y-S. Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome. Biology. 2021; 10(6):540. https://doi.org/10.3390/biology10060540
Chicago/Turabian StyleLu, Jian-He, Kuang-Shun Chueh, Shu-Mien Chuang, Yi-Hsuan Wu, Kun-Ling Lin, Cheng-Yu Long, Yung-Chin Lee, Mei-Chen Shen, Ting-Wei Sun, and Yung-Shun Juan. 2021. "Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome" Biology 10, no. 6: 540. https://doi.org/10.3390/biology10060540
APA StyleLu, J.-H., Chueh, K.-S., Chuang, S.-M., Wu, Y.-H., Lin, K.-L., Long, C.-Y., Lee, Y.-C., Shen, M.-C., Sun, T.-W., & Juan, Y.-S. (2021). Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome. Biology, 10(6), 540. https://doi.org/10.3390/biology10060540






