Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Analysis of ProRoot MTA and Biodentine Disks
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Measurement of Reactive Oxygen Species (ROS)
2.5. Immunofluorescence Microscopy Analysis
2.6. VpSEM and dEDS Analysis
2.7. Evaluation of Antibiofilm Capability
2.8. Statistical Analysis
2.9. In Vivo Evaluation
3. Results
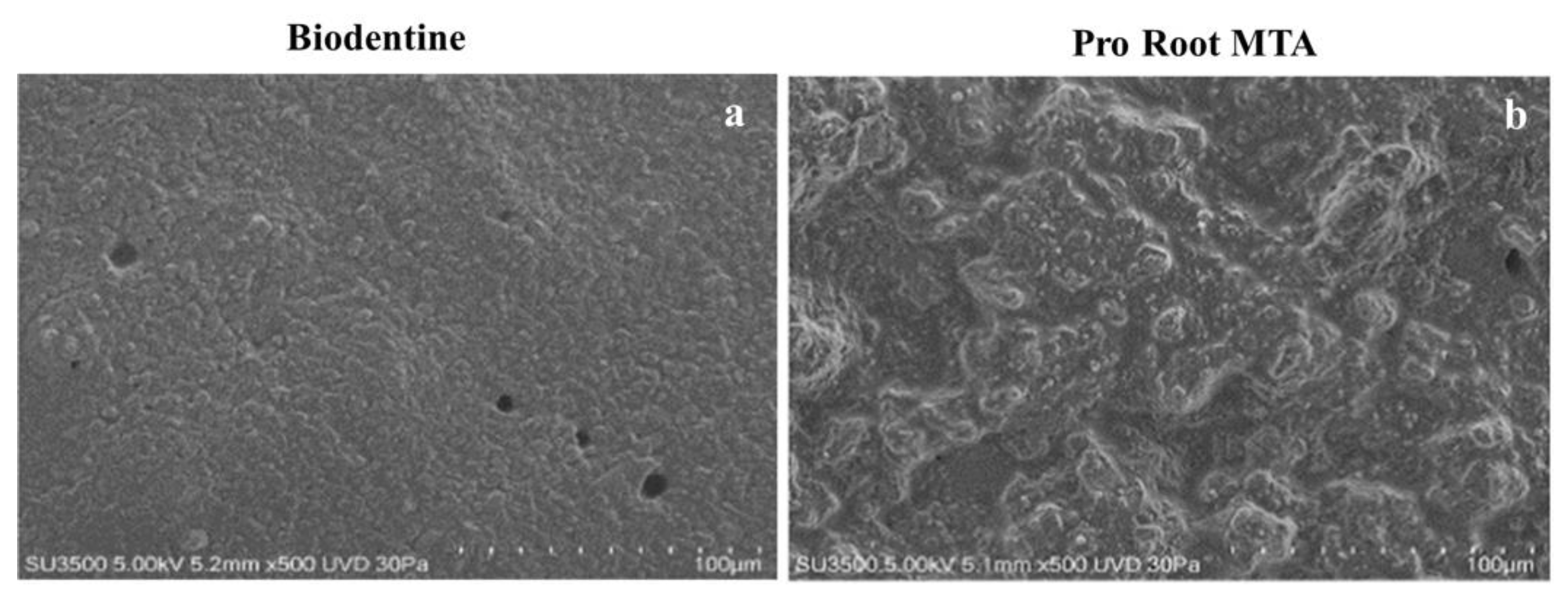
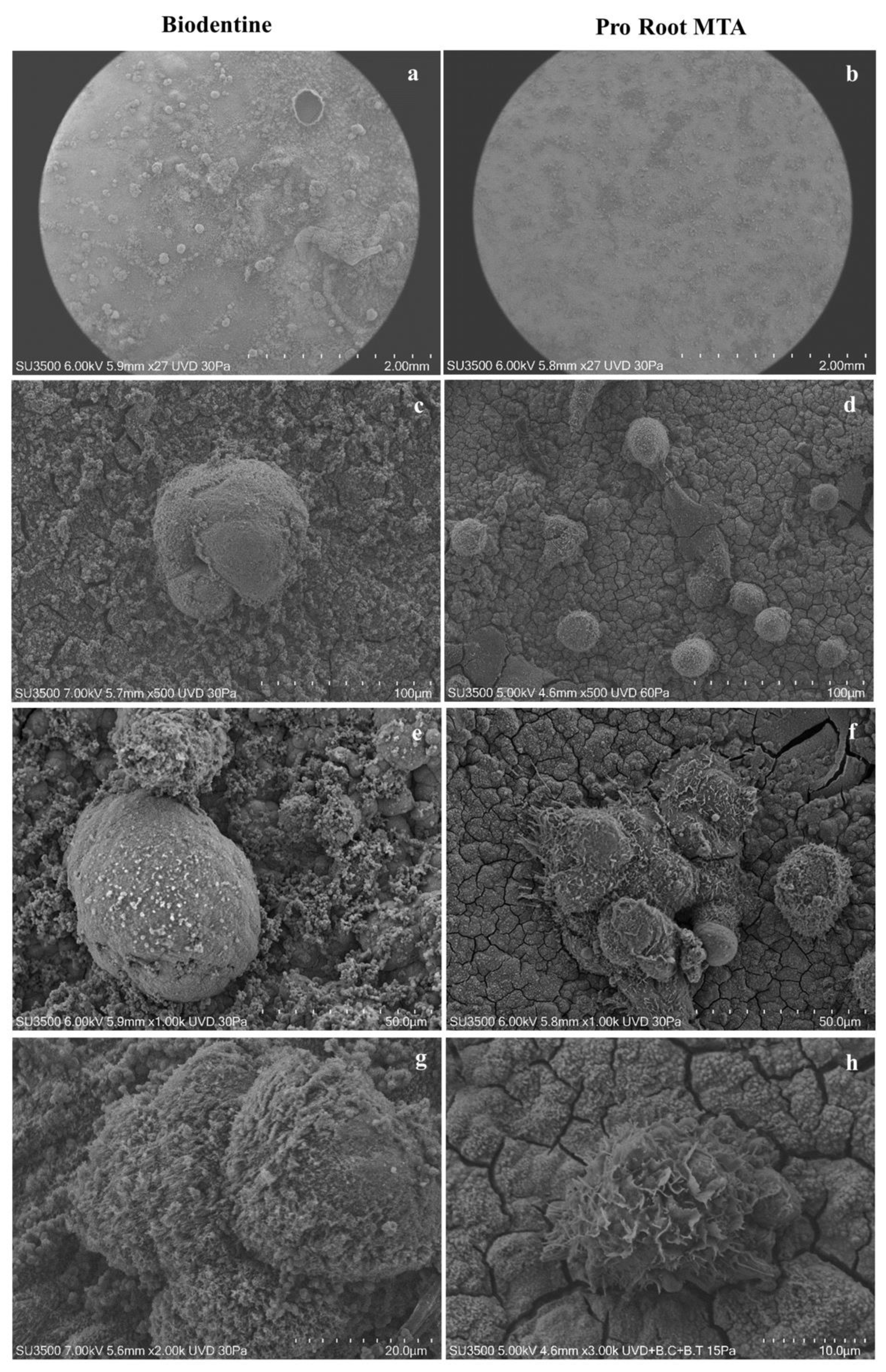
3.1. VpSEM Morphological Analysis of ProRoot MTA and Biodentine Disks
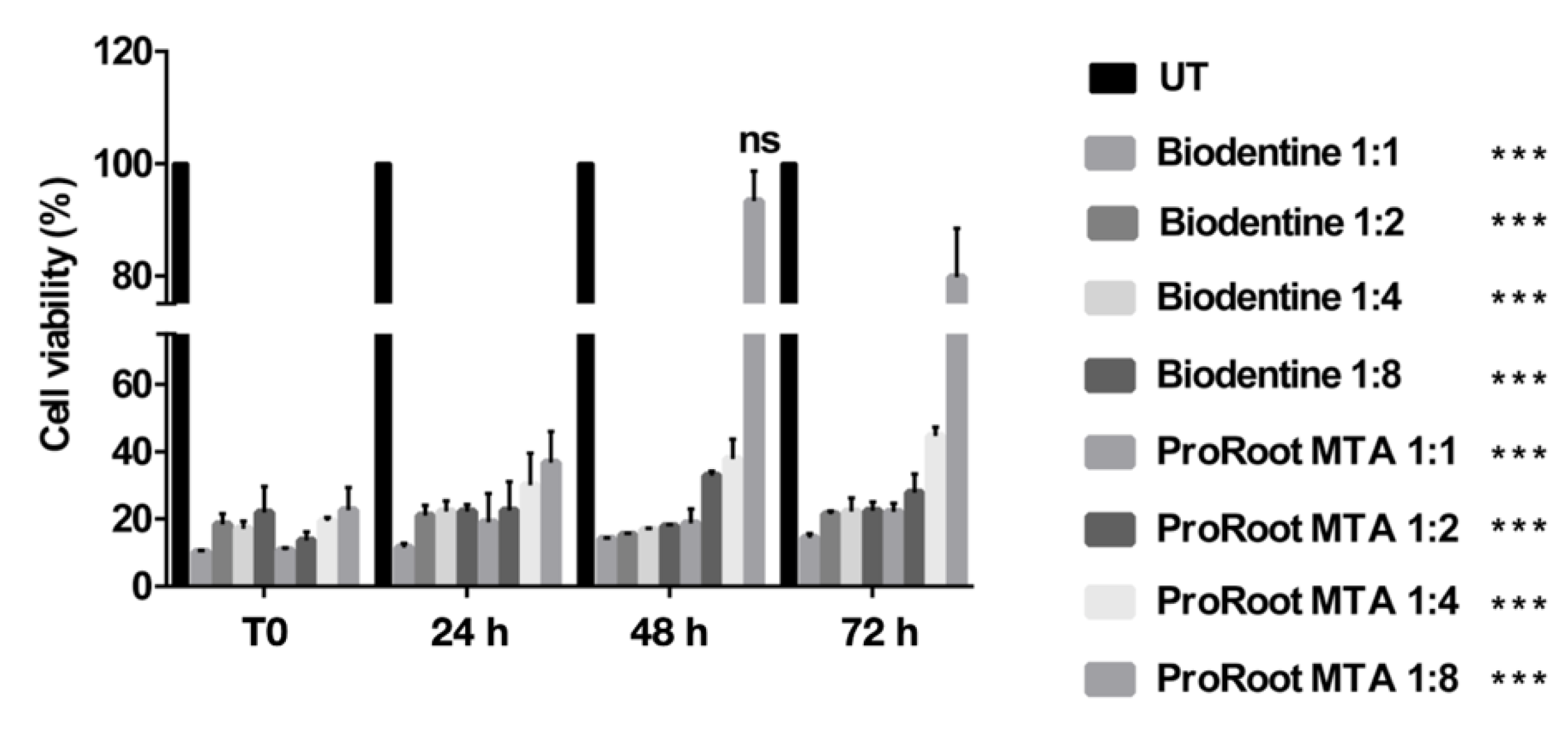
3.2. Effects of Cement Extracts on Cell Viability of Saos-2 Cells
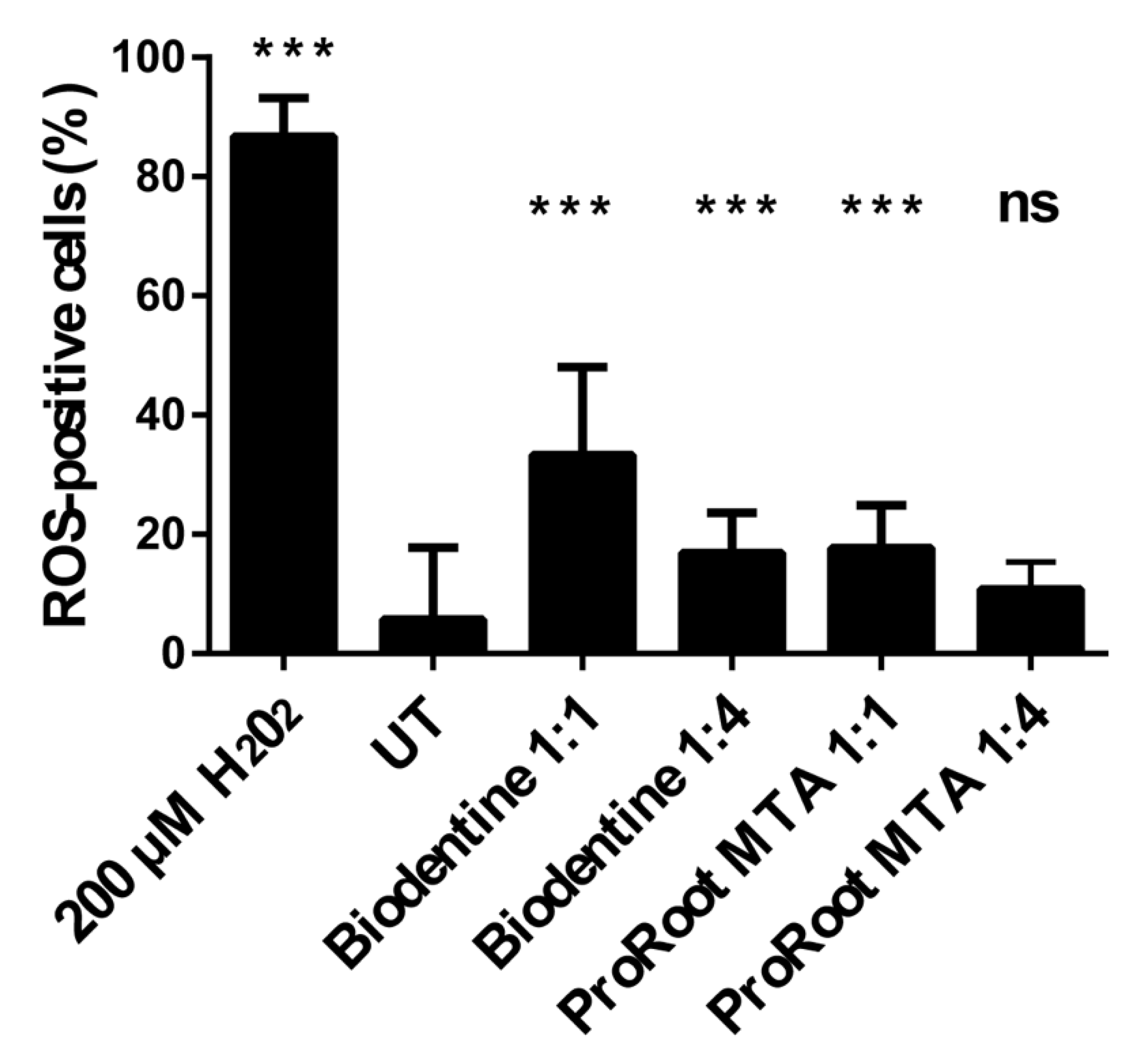
3.3. Effects of Cement Extracts on Oxidative Stress in Saos-2 Cells
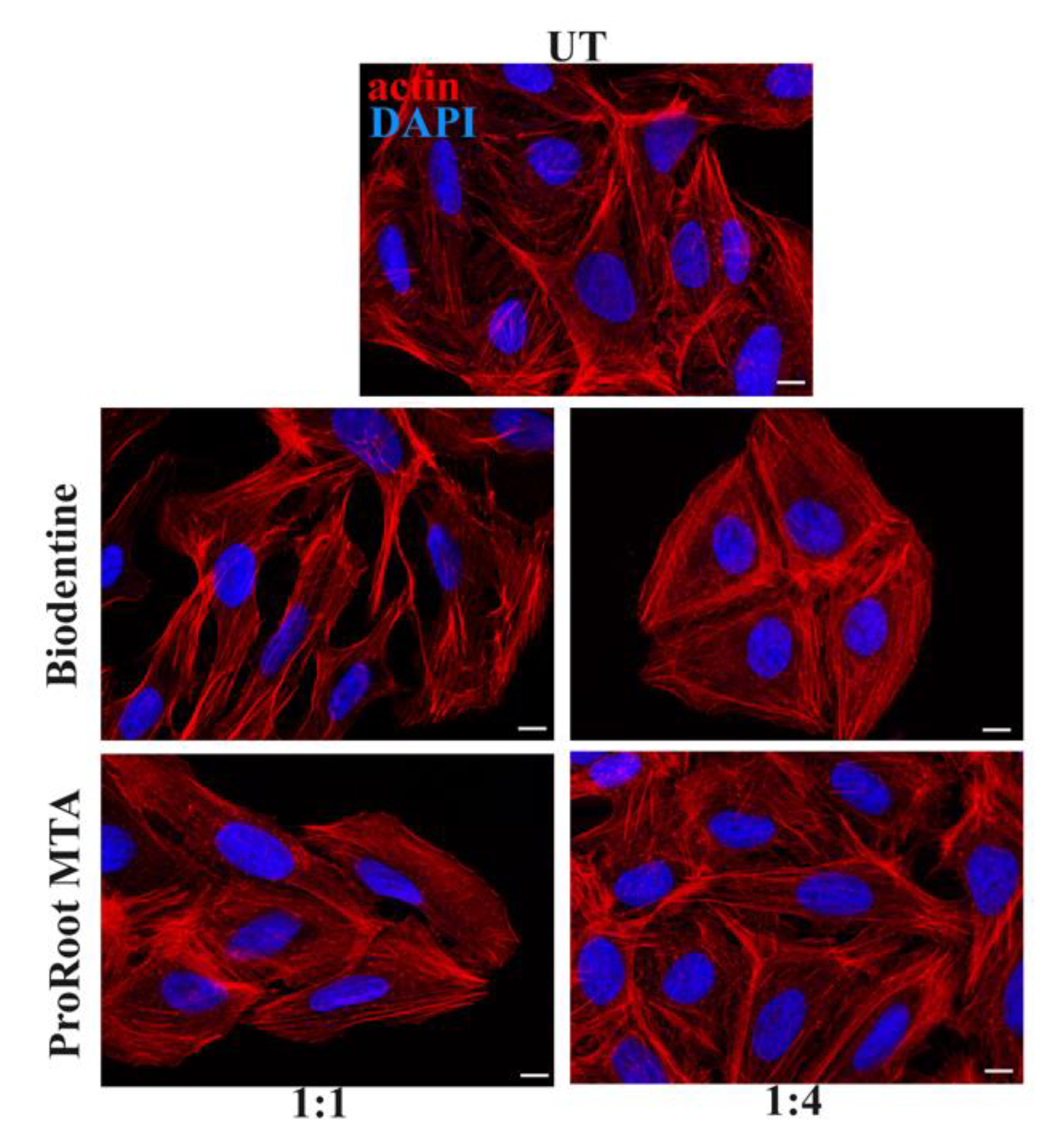
3.4. Effects of Cement Extracts on Actin and Focal Adhesions Organization of Saos-2 Cells
3.5. Biocompatibility of Saos-2 Cells Cultured on ProRoot MTA and Biodentine Disks
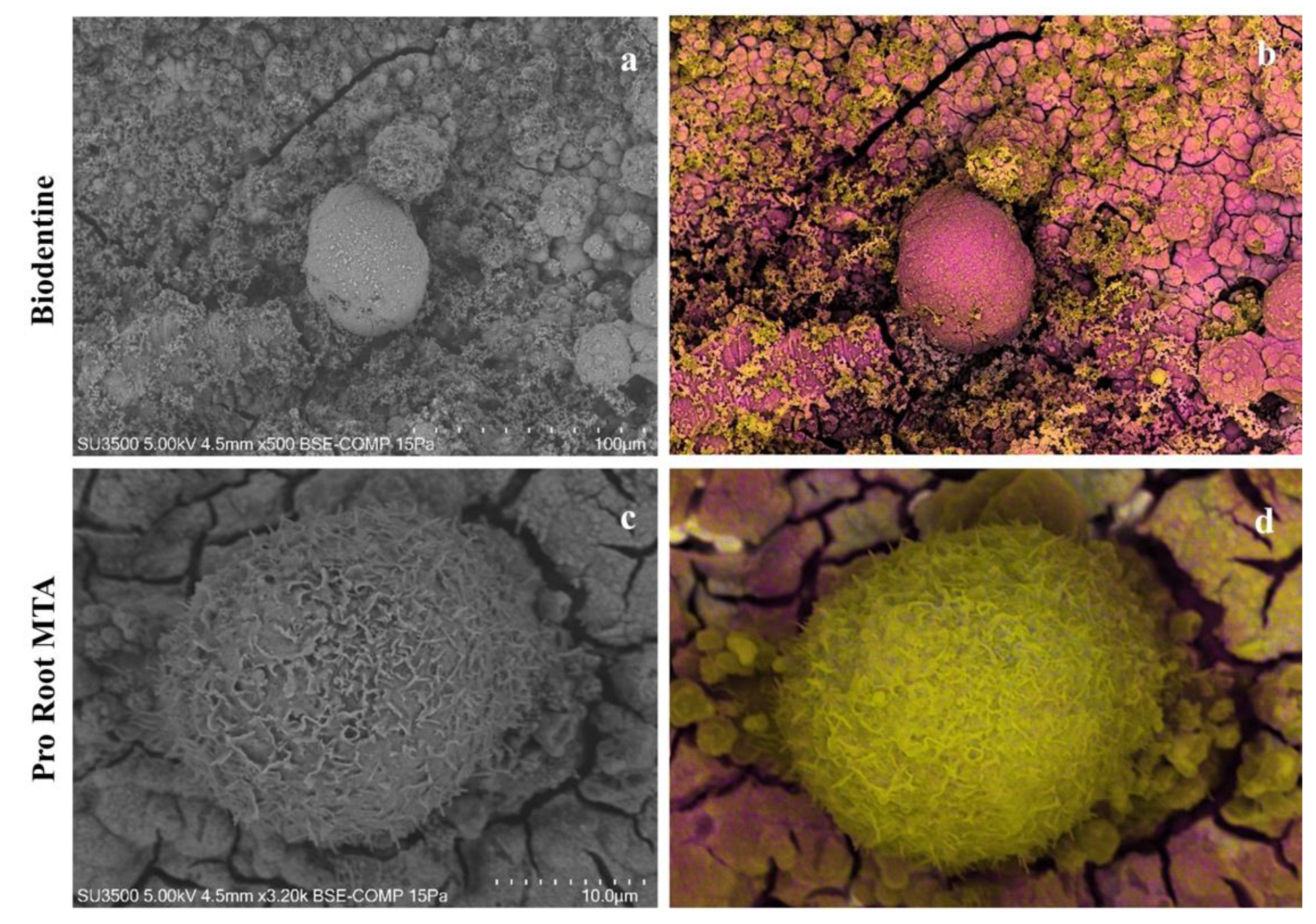
3.6. VpSEM and dEDS Analysis of Saos-2 Cells Cultured on ProRoot MTA and Biodentine Disks
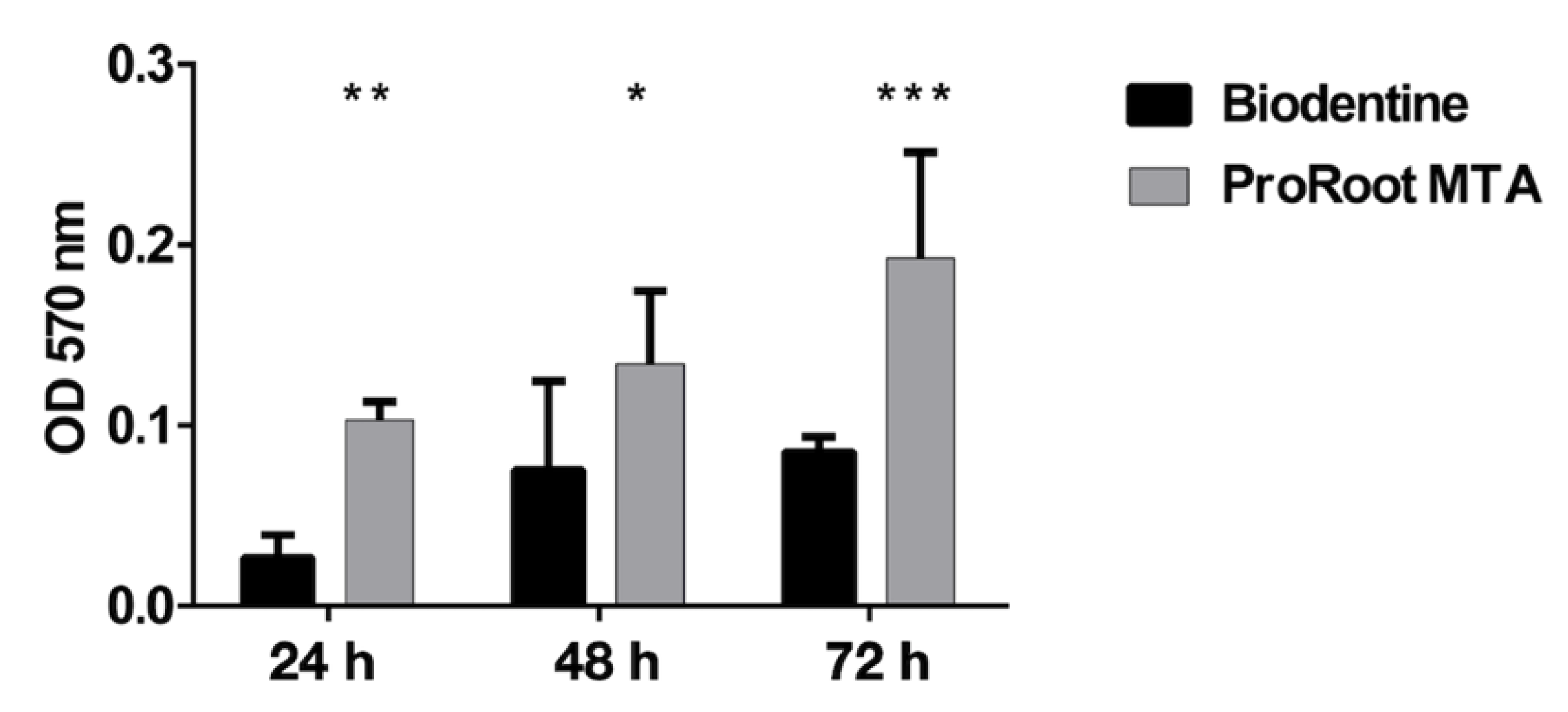
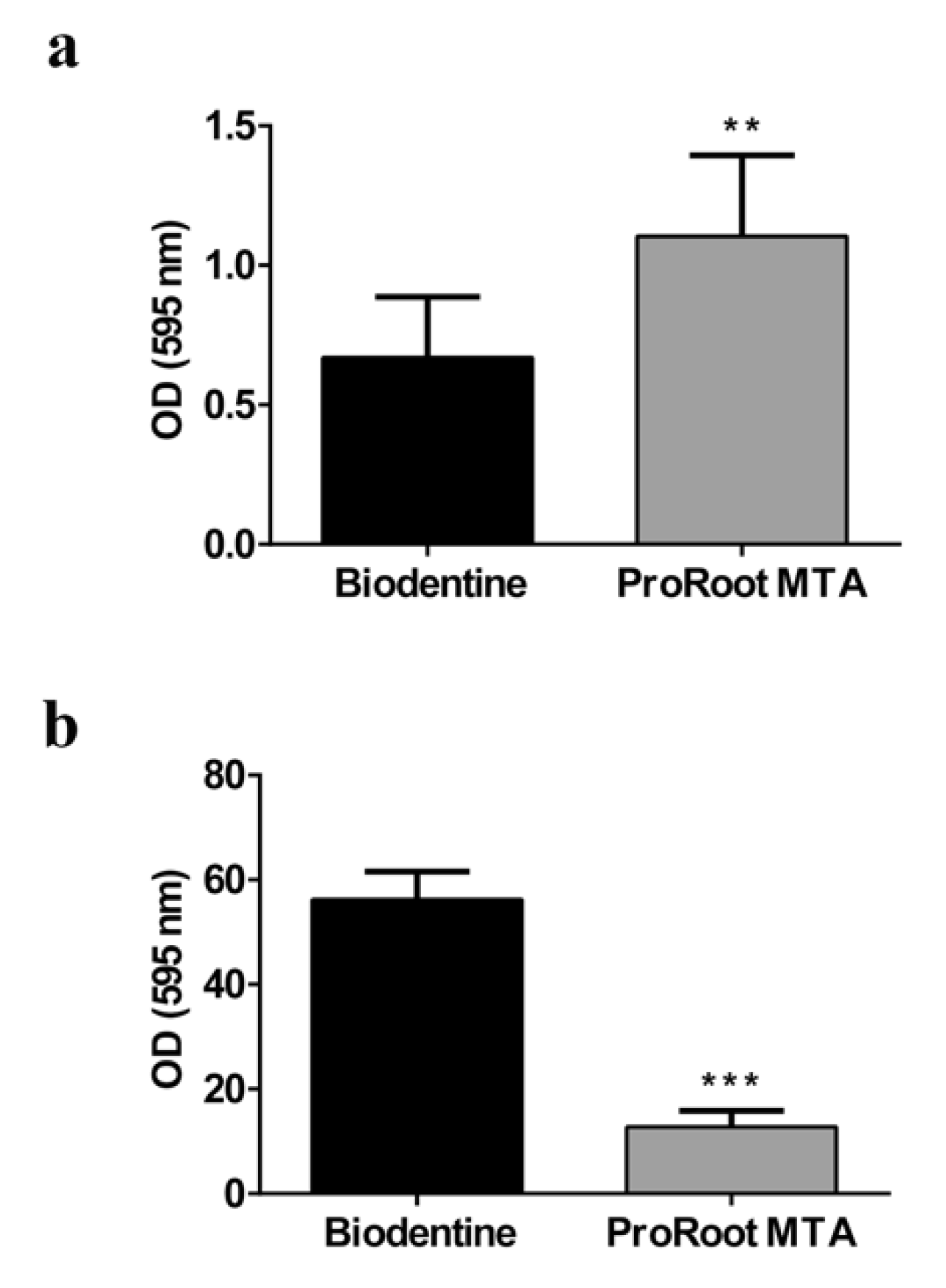
3.7. Antibiofilm Activity
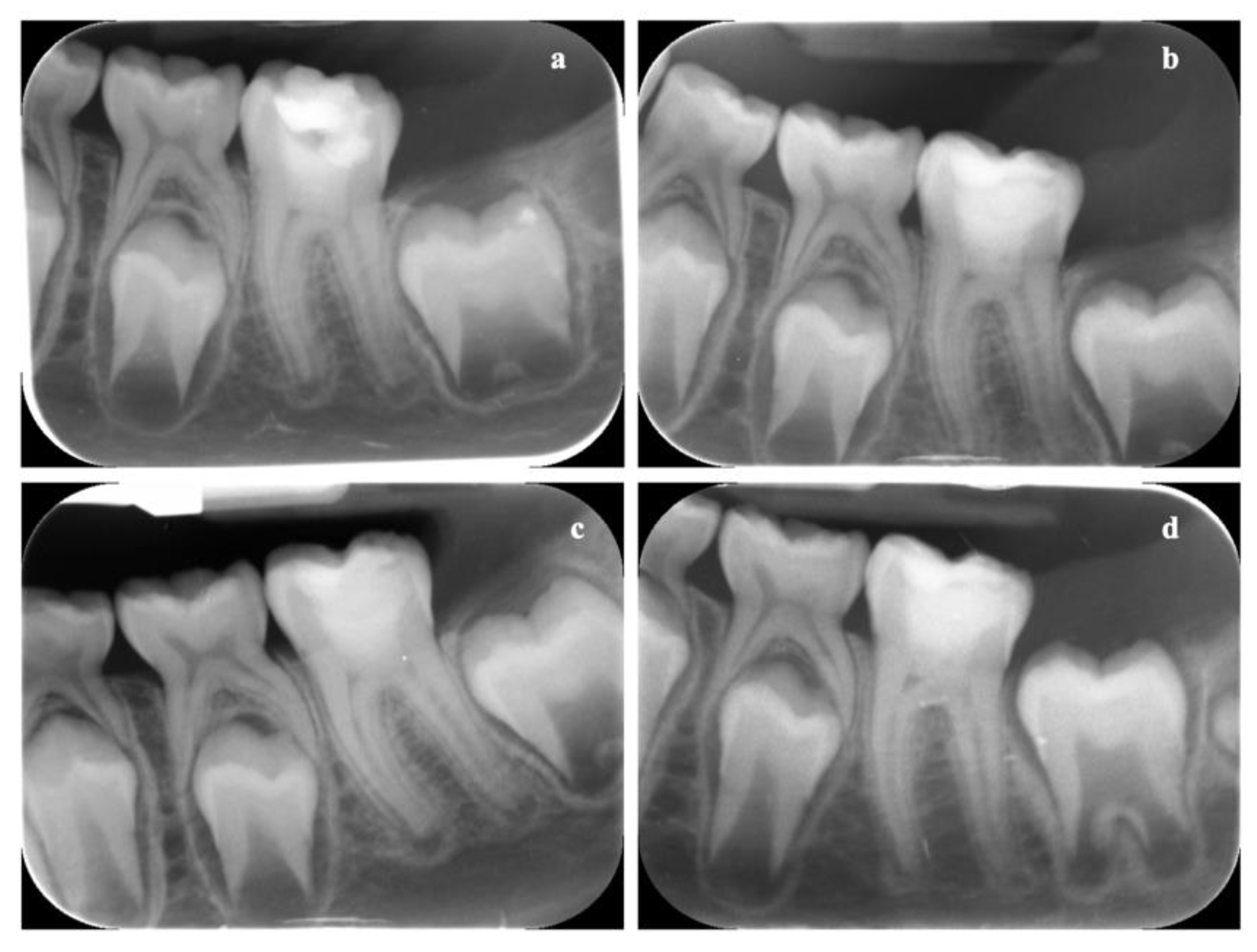
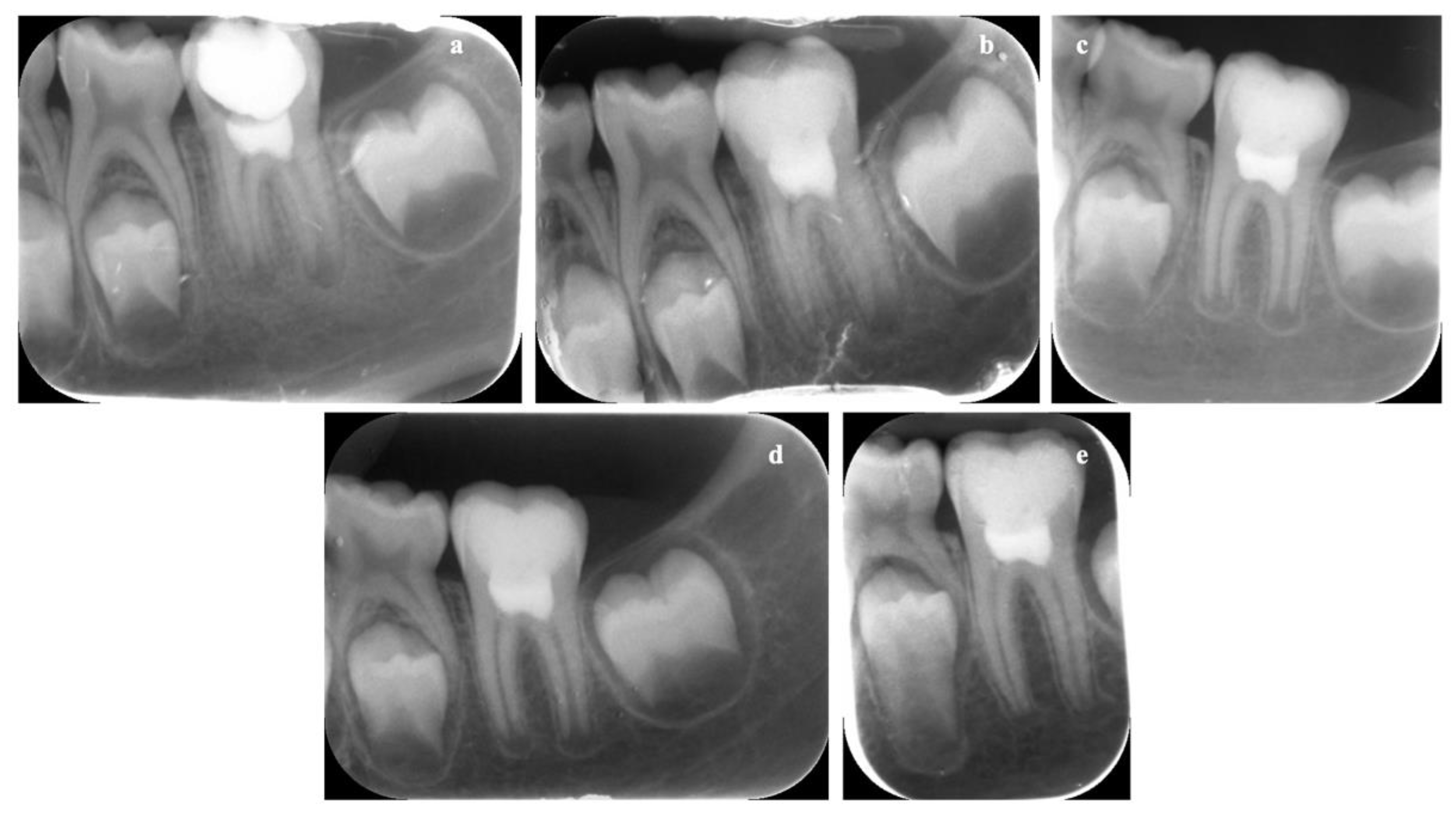
3.8. In Vivo Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.J.; Monsef, M.; Torabinejad, M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef]
- Dhar, V.; Marghalani, A.A.; Crystal, Y.O.; Kumar, A.; Ritwik, P.; Tulunoglu, O.; Graham, L. Use of vital pulp therapies in primary teeth with deep caries lesions. Pediatr. Dent. 2017, 39, 146–159. [Google Scholar] [PubMed]
- Torabinajad, M.; Chivian, N. Clinical applications of mineral trioxide aggregate. J. Endod. 1999, 25, 197–205. [Google Scholar] [CrossRef]
- Kontakiotis, E.G.; Filippatos, C.G.; Tzanetakis, G.N.; Agrafioti, A. Regenerative endodontic therapy: A data analysis of clinical protocols. J. Endod. 2015, 41, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Staffoli, S.; Plotino, G.; Torrijos, B.G.N.; Grande, N.M.; Bossù, M.; Gambarini, G.; Polimeni, A. Regenerative endodontic procedures using contemporary endodontic materials. Materials 2019, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Felman, D.; Parashos, P. Coronal tooth discoloration and white mineral trioxide aggregate. J. Endod. 2013, 39, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Characterization of set intermediate restorative material, biodentine, bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int. Endod. J. 2013, 46, 632–641. [Google Scholar] [CrossRef]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef]
- Bossù, M.; Iaculli, F.; di Giorgio, G.; Salucci, A.; Polimeni, A.; di Carlo, S. Different pulp dressing materials for the pulpotomy of primary teeth: A systematic review of the literature. J. Clin. Med. 2020, 9, 838. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.G.E.C.; Anthonappa, R.P.; Verbeeck, R.M.H. Biodentine™ material characteristics and clinical applications: A 3 year literature review and update. Eur. Arch. Paediatr. Dent. 2018, 19, 1–22. [Google Scholar] [CrossRef]
- Malkondu, Ö.; Kazandağ, M.K.; Kazazoğlu, E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Camilleri, J. Investigation of biodentine as dentine replacement material. J. Dent. 2013, 41, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus biodentine: Review of literature with a comparative analysis. J. Clin. Diagn. Res. 2017, 11, ZG01–ZG05. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Spagnuolo, G.; Siboni, F.; Procino, A.; Rivieccio, V.; Pelliccioni, G.A.; Prati, C.; Rengo, S. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: Chemical-physical properties and human pulp cells response. Clin. Oral Investig. 2015, 19, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Ficociello, G.; de Caris, M.G.; Trillò, G.; Cavallini, D.; Sarto, M.S.; Uccelletti, D.; Mancini, P. Anti-candidal activity and in vitro cytotoxicity assessment of graphene nanoplates decorated with zinc oxide nanorods. Nanomaterials 2018, 8, 752. [Google Scholar] [CrossRef]
- Zanni, E.; De Palma, S.; Chandraiahgari, C.R.; de Bellis, G.; Cialfi, S.; Talora, C.; Palleschi, C.; Sarto, M.S.; Uccelletti, D.; Mancini, P. In vitro toxicity studies of zinc oxide nano- and microrods on mammalian cells: A comparative analysis. Mater. Lett. 2016, 179, 90–94. [Google Scholar] [CrossRef]
- Vanni, C.; Mancini, P.; Ottaviano, C.; Ognibene, M.; Parodi, A.; Merello, E.; Russo, C.; Varesio, L.; Zheng, Y.; Torrisi, M.R.; et al. Galpha13 regulation of Proto-Dbl signaling. Cell Cycle 2007, 6, 2058–2070. [Google Scholar] [CrossRef]
- Relucenti, M.; Miglietta, S.; Covelli, E.; Familiari, P.; Battaglione, E.; Familiari, G.; Barbara, M. Ciliated cell observation by SEM on the surface of human incudo-malleolar-joint articular cartilage: Are they a new chondrocyte phenotype? Acta Otolaryngol. 2019, 139, 439–443. [Google Scholar] [CrossRef]
- Relucenti, M.; Heyn, R.; Petruzziello, L.; Pugliese, G.; Taurino, M.; Familiari, G. Detecting microcalcifications in atherosclerotic plaques by a simple trichromic staining method for epoxy embedded carotid endarterectomies. Eur. J. Histochem. 2010, 54, e33. [Google Scholar] [CrossRef]
- Lo Torto, F.; Relucenti, M.; Familiari, G.; Vaia, N.; Casella, D.; Matassa, R.; Miglietta, S.; Marinozzi, F.; Bini, F.; Fratoddi, I.; et al. The effect of postmastectomy radiation therapy on breast implants: Material analysis on silicone and polyurethane prosthesis. Ann. Plast. Surg. 2018, 81, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Relucenti, M.; Miglietta, S.; Bove, G.; Donfrancesco, O.; Battaglione, E.; Familiari, P.; Barbaranelli, C.; Covelli, E.; Barbara, M.; Familiari, G. SEM BSE 3D image analysis of human incus bone affected by cholesteatoma ascribes to osteoclasts the bone erosion and VpSEM dEDX analysis reveals new bone formation. Scanning 2020, 2020, 9371516. [Google Scholar] [CrossRef] [PubMed]
- Cottignoli, V.; Relucenti, M.; Agrosì, G.; Cavarretta, E.; Familiari, G.; Salvador, L.; Maras, A. Biological niches within human calcified aortic valves: Towards understanding of the pathological biomineralization process. Biomed. Res. Int. 2015, 2015, 542687. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Primus, C.M.; Tay, F.R.; Niu, L.N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef]
- Jain, P.; Ranjan, M. The rise of biocramics in endodontics: A review. Int. J. Pharma Bio Sci. 2015, 6, 416–422. [Google Scholar]
- Sanz, J.L.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Melo, M.; Rengo, S.; Spagnuolo, G.; Rodríguez-Lozano, F.J. Cytocompatibility and bioactive properties of hydraulic calcium silicate-based cements (HCSCs) on stem cells from human exfoliated deciduous teeth (SHEDs): A systematic review of in vitro studies. J. Clin. Med. 2020, 9, 3872. [Google Scholar] [CrossRef]
- Ghilotti, J.; Sanz, J.L.; López-García, S.; Guerrero-Gironés, J.; Pecci-Lloret, M.P.; Lozano, A.; Llena, C.; Rodríguez-Lozano, F.J.; Spagnuolo, G. Comparative surface morphology, chemical composition, and cytocompatibility of bio-C repair, biodentine, and ProRoot MTA on hDPCs. Materials 2020, 13, 2189. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, D.J.; Richardson, M.D.; Bateman, J.F. Matrix deposition by a calcifying human osteogenic sarcoma cell line (SAOS-2). Bone 1995, 16, 415–426. [Google Scholar] [CrossRef]
- Alanezi, A.Z.; Jiang, J.; Safavi, K.E.; Spangberg, L.S.; Zhu, Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e122–e125. [Google Scholar] [CrossRef] [PubMed]
- Keiser, K.; Johnson, C.C.; Tipton, D.A. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J. Endod. 2000, 26, 288–291. [Google Scholar] [CrossRef]
- Zanini, M.; Sautier, J.M.; Berdal, A.; Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 2012, 38, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase ii pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Pelliccioni, G.A.; Ciapetti, G.; Cenni, E.; Granchi, D.; Nanni, M.; Pagani, S.; Giunti, A. Evaluation of osteoblast-like cell response to Proroot™ MTA (mineral trioxide aggregate) cement. J. Mater. Sci. Mater. Med. 2004, 15, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.; Serrano, M.C.; Pagani, R.; Sánchez-Salcedo, S.; Vallet-Regí, M.; Portolés, M.T. Biocompatibility markers for the study of interactions between osteoblasts and composite biomaterials. Biomaterials 2009, 30, 45–51. [Google Scholar] [CrossRef]
- Degasne, I.; Baslé, M.F.; Demais, V.; Huré, G.; Lesourd, M.; Grolleau, B.; Mercier, L.; Chappard, D. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif. Tissue Int. 1999, 64, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ayobian-Markazi, N.; Fourootan, T.; Kharazifar, M.J. Comparison of cell viability and morphology of a human osteoblast-like cell line (SaOS-2) seeded on various bone substitute materials: An in vitro study. Dent. Res. J. (Isfahan) 2012, 9, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.; Weinstein, R.; Hynes, R.O. Cell attachment to thrombospondin—The role of Arg-Gly-Asp, calcium, and integrin receptors. J. Cell Biol. 1988, 107, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Neel, E.A.A.; Palmer, G.; Knowles, J.C.; Salih, V.; Young, A.M. Chemical, modulus and cell attachment studies of reactive calcium phosphate filler-containing fast photo-curing, surface-degrading, polymeric bone adhesives. Acta Biomater. 2010, 6, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Giorgi, C.; Pinton, P.; Rizzuto, R. The versatility of mitochondrial calcium signals: From stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta 2008, 1777, 808–816. [Google Scholar] [CrossRef]
- Murgia, M.; Giorgi, C.; Pinton, P.; Rizzuto, R. Controlling metabolism and cell death: At the heart of mitochondrial calcium signaling. J. Mol. Cell Cardiol. 2009, 46, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Chattree, V.; Khanna, N.; Rao, D.N. Alterations in T cell signal transduction by M. leprae antigens is associated with downregulation of second messengers PKC, calcium, calcineurin, MAPK and various transcription factors in leprosy patients. Mol. Immunol. 2007, 44, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, C.; Han, R.; Pavlos, N.; Phan, T.; Steer, J.H.; Bakker, A.J.; Joyce, D.A.; Zheng, M.H. Evidence of reciprocal regulation between the high extracellular calcium and RANKL signal transduction pathways in RAW cell derived osteoclasts. J. Cell Physiol. 2005, 202, 554–562. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Toka, F.N.; Jurka, P. Role of cadherins in cancer—A review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- Dhavalikar, P.; Robinson, A.; Lan, Z.; Jenkins, D.; Chwatko, M.; Salhadar, K.; Jose, A.; Kar, R.; Shoga, E.; Kannapiran, A. Review of integrin-targeting biomaterials in tissue engineering. Adv. Healthc. Mater. 2020, 16, e2000795. [Google Scholar] [CrossRef] [PubMed]
- Peterková, L.; Rimpelová, S.; Křížová, I.; Slepička, P.; Kasálková, N.S.; Švorčík, V.; Ruml, T. Biocompatibility of Ar plasma-treated fluorinated ethylene propylene: Adhesion and viability of human keratinocytes. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 100, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Peterková, L.; Rimpelová, S.; Slepička, P.; Křížová, I.; Kasálková, N.S.; Švorčík, V.; Ruml, T. Argon plasma-treated fluorinated ethylene propylene: Growth of primary dermal fibroblasts and mesenchymal stem cells. Tissue Cell 2019, 58, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.Y. Salivary biomarkers for dental caries. Periodontol. 2000 2016, 70, 128–141. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Beltrami, R.; Colombo, M.; Ceci, M.; Dagna, A.; Chiesa, M. In vitro antibacterial activity of different pulp capping materials. J. Clin. Exp. Dent. 2015, 7, e584–e588. [Google Scholar] [CrossRef]
- Matteo, C.; Beltrami, R.; Chiesa, M.; Colombo, M.; Poggio, C. Biological and chemical-physical properties of root-end filling materials: A comparative study. J. Conserv. Dent. 2015, 18, 94–99. [Google Scholar]
- American Academy of Pediatric Dentistry. Pulp therapy for primary and immature permanent teeth. Pediatr. Dent. 2017, 39, 325–333. [Google Scholar]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossù, M.; Mancini, P.; Bruni, E.; Uccelletti, D.; Preziosi, A.; Rulli, M.; Relucenti, M.; Donfrancesco, O.; Iaculli, F.; Di Giorgio, G.; et al. Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology 2021, 10, 470. https://doi.org/10.3390/biology10060470
Bossù M, Mancini P, Bruni E, Uccelletti D, Preziosi A, Rulli M, Relucenti M, Donfrancesco O, Iaculli F, Di Giorgio G, et al. Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology. 2021; 10(6):470. https://doi.org/10.3390/biology10060470
Chicago/Turabian StyleBossù, Maurizio, Patrizia Mancini, Erika Bruni, Daniela Uccelletti, Adele Preziosi, Marco Rulli, Michela Relucenti, Orlando Donfrancesco, Flavia Iaculli, Gianni Di Giorgio, and et al. 2021. "Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases" Biology 10, no. 6: 470. https://doi.org/10.3390/biology10060470
APA StyleBossù, M., Mancini, P., Bruni, E., Uccelletti, D., Preziosi, A., Rulli, M., Relucenti, M., Donfrancesco, O., Iaculli, F., Di Giorgio, G., Matassa, R., Salucci, A., & Polimeni, A. (2021). Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology, 10(6), 470. https://doi.org/10.3390/biology10060470








