Simplified Iohexol-Based Method for Measurement of Glomerular Filtration Rate in Goats and Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Laboratory Analyses
2.4. Statistical Analysis
2.4.1. Nonlinear Mixed Effects Model to Determine Iohexol Clearance
2.4.2. Simplified Method to Determine Iohexol Clearance
2.5. Software
3. Results
3.1. Statistical Analysis
3.1.1. Data
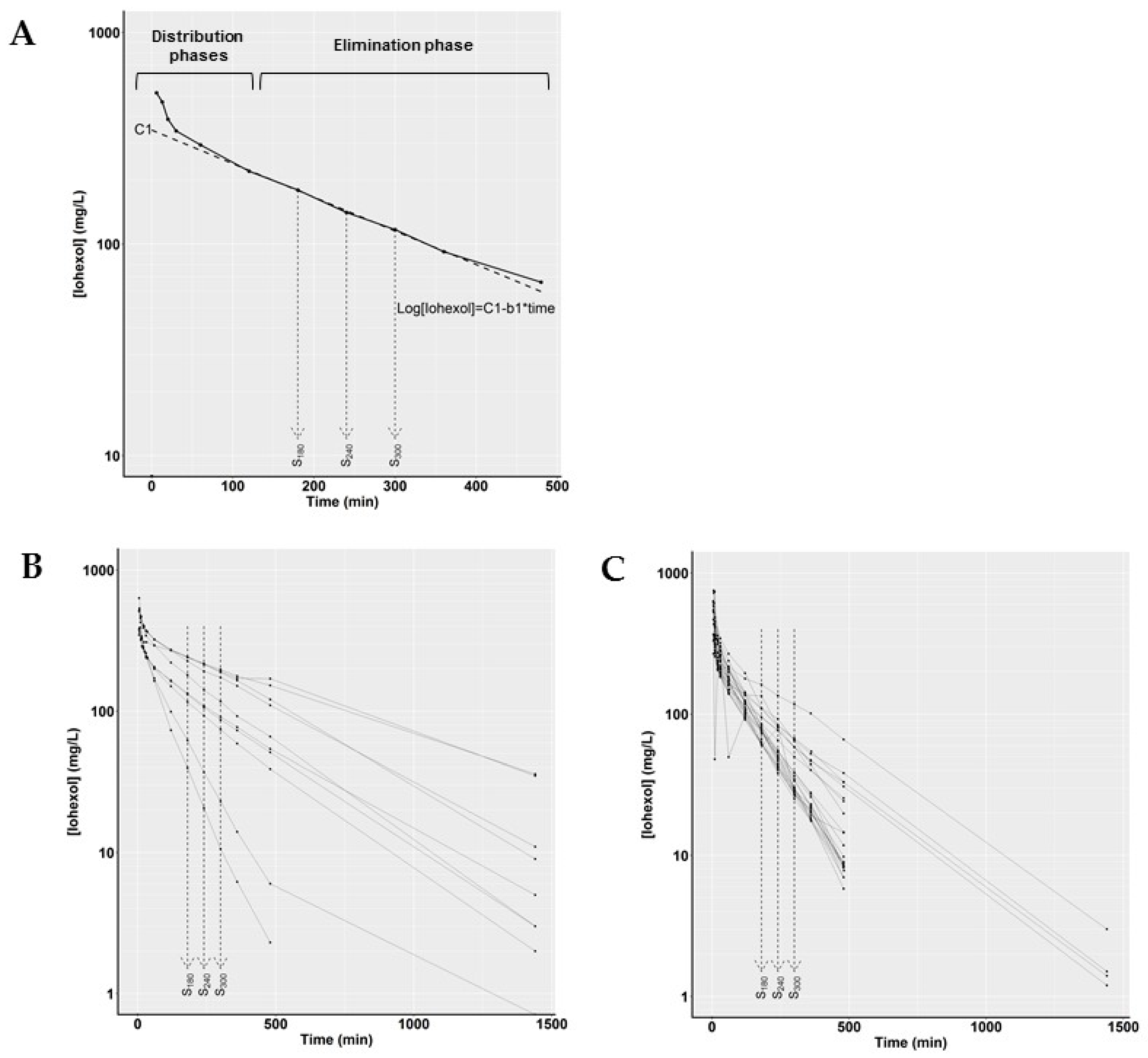
3.1.2. Nonlinear Mixed Effects Model to Determine Iohexol Clearance
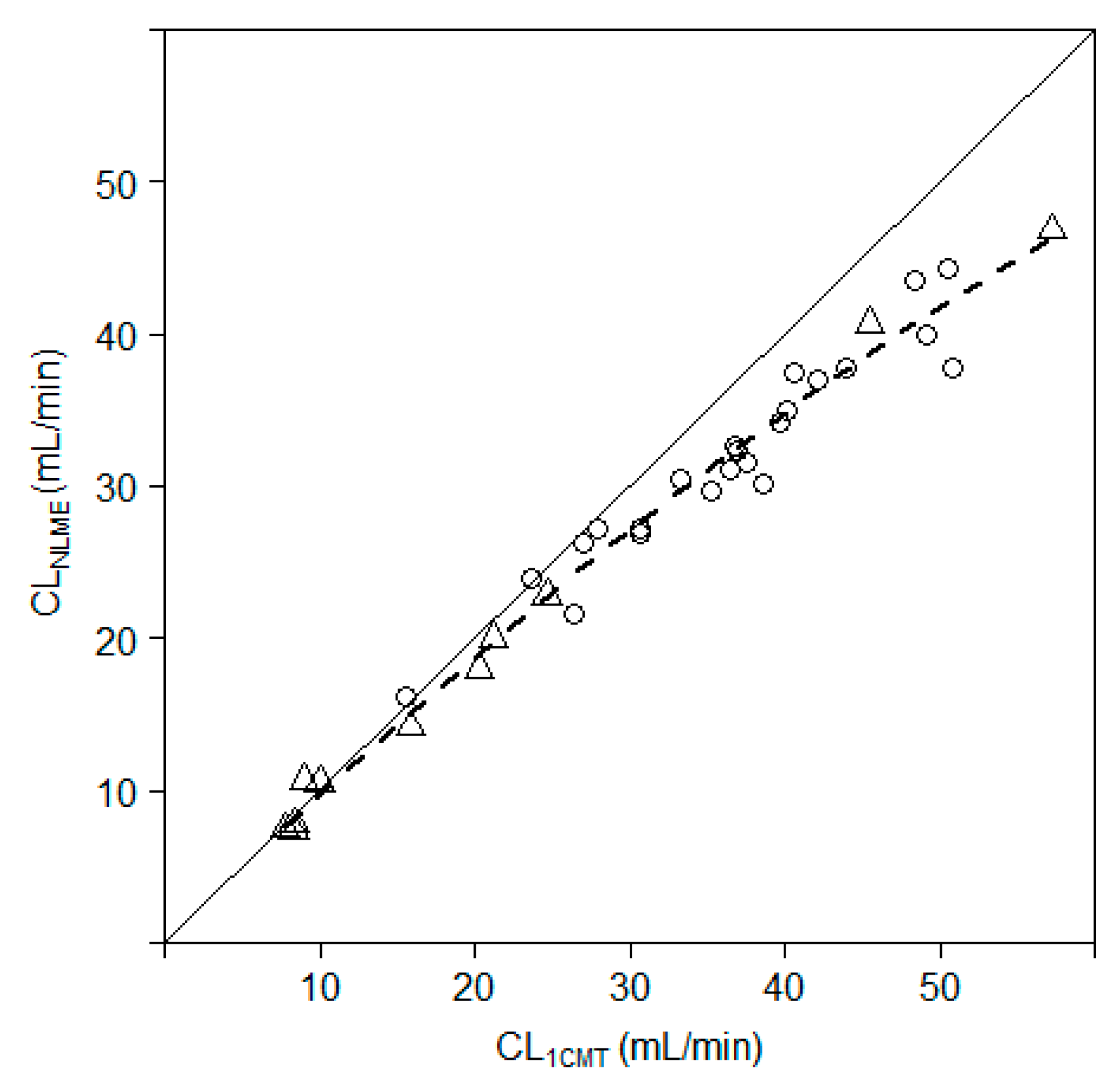
3.1.3. Simplified Method to Determine Iohexol Clearance
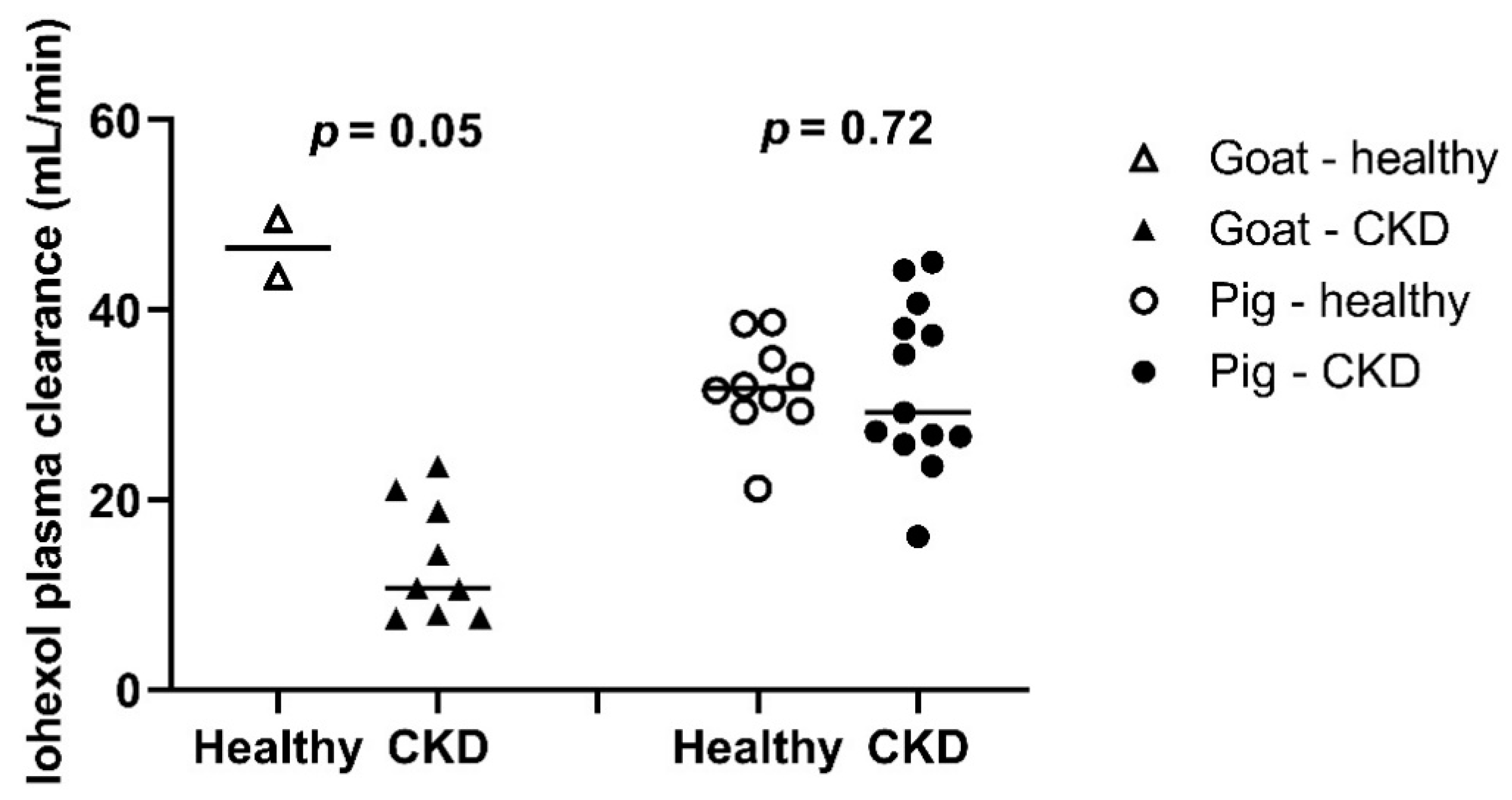
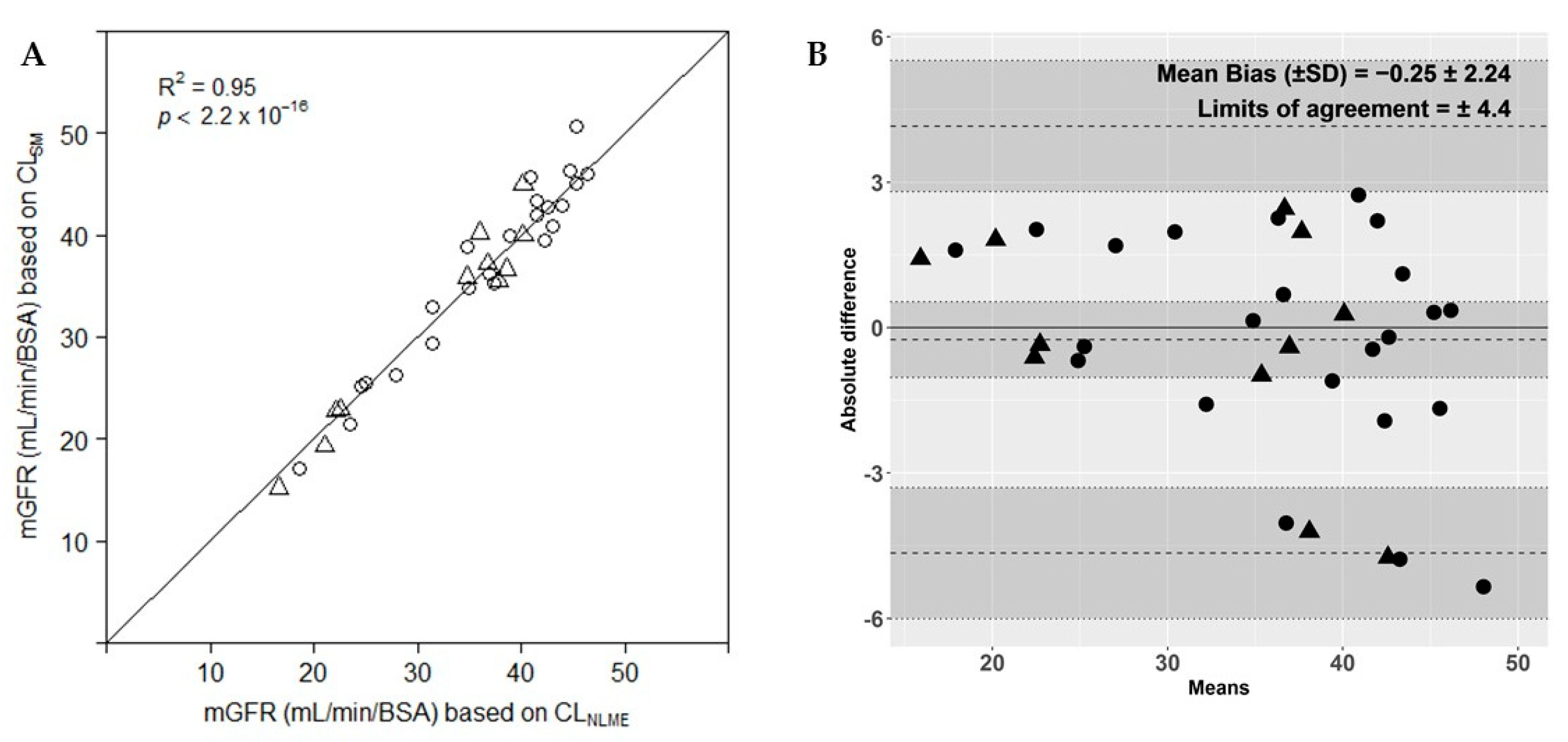
3.1.4. Evaluation of the Simplified Method versus Reference Method
3.1.5. mGFR Results
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75, A6–A7. [Google Scholar] [CrossRef]
- Wester, M.; Gerritsen, K.G.; Simonis, F.; Boer, W.H.; Hazenbrink, D.H.; Vaessen, K.R.; Verhaar, M.C.; Joles, J.A. A regenerable potassium and phosphate sorbent system to enhance dialysis efficacy and device portability: A study in awake goats. Nephrol. Dial. Transplant. 2017, 32, 951–959. [Google Scholar] [CrossRef]
- Giraud, S.; Favreau, F.; Chatauret, N.; Thuillier, R.; Maiga, S.; Hauet, T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: The preclinical model. J. Biomed. Biotechnol. 2011, 2011, 532127. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Williams, M.L.; Engel, J.; Guise, E.; Harvey, T.W. A translational model of chronic kidney disease in swine. Am. J. Physiol. Renal Physiol. 2018, 315, F364–F373. [Google Scholar] [CrossRef]
- Misra, S. The Porcine Remnant Kidney Model of Chronic Renal Insufficiency. J. Surg. Res. 2006, 135, 370–379. [Google Scholar] [CrossRef]
- Robbins, M.E.C.; Robinson, M.; Rezvani, M.; Golding, S.J.; Hopewell, J.W. The response of the pig kidney to the combined effects of cisplatin and unilateral renal irradiation. Radiother. Oncol. 1988, 11, 271–278. [Google Scholar] [CrossRef]
- Boele-Schutte, E.; Gansevoort, R.T. Measured GFR: Not a gold, but a gold-plated standard. Nephrol. Dial. Transplant. 2017, 32, ii180–ii184. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Decreased Renal Function; European Medicines Agency: London, UK, 2014. [Google Scholar]
- Soveri, I.; Berg, U.B.; Bjork, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Back, S.E.; Group, S.G.R. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Ebert, N.; Melsom, T.; Gaspari, F.; Mariat, C.; Cavalier, E.; Bjork, J.; Christensson, A.; Nyman, U.; Porrini, E.; et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 1: How to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016, 9, 682–699. [Google Scholar] [CrossRef]
- Brochner-Mortensen, J. A simple method for the determination of glomerular filtration rate. Scand. J. Clin. Lab. Investig. 1972, 30, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Belis, J.A.; Horton, J.A. Renal artery embolization with polyvinyl alcohol foam particles. Urology 1982, 19, 224–227. [Google Scholar] [CrossRef]
- Stevens, J.; Wessels, M.A.; Roggeveld, J.; Koster, R.A.; Dekkers, C.C.; van Gelder, M.K.; Gansevoort, R.T.; Heerspink, H.J.; Touw, D.J. UHPLC-MS/MS method for iohexol determination in human EDTA and lithium-heparin plasma, human urine and in goat- and pig EDTA plasma. Bioanalysis 2020, 12, 981–990. [Google Scholar] [CrossRef]
- Karlsson, M.O.; Sheiner, L.B. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J. Pharm. Biopharm. 1993, 21, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development-part 2: Introduction to pharmacokinetic modeling methods. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e38. [Google Scholar] [CrossRef]
- Saito, M.; Tasaki, I.; Annaka, A. Studies on the metabolism of dairy goat. J.-Stage 1954, 25, 25–29. [Google Scholar]
- Swindle, M.M. Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Beal, S.L.; Sheiner, L.B.; Boeckmann, A.J.; Bauer, R.J. (Eds.) NONMEM 7.3.0 Users Guides (1989–2013); ICON Development Solutions: Hanover, MD, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Luis-Lima, S.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Astiz, S.; Carrara, F.; Gaspari, F.; Negrin-Mena, N.; Jimenez-Sosa, A.; Jimenez-Hernandez, H.; Gonzalez-Bulnes, A.; et al. A Simple Method to Measure Renal Function in Swine by the Plasma Clearance of Iohexol. Int. J. Mol. Sci. 2018, 19, 232. [Google Scholar] [CrossRef]
- Lodrup, A.B.; Karstoft, K.; Dissing, T.H.; Nyengaard, J.R.; Pedersen, M. The association between renal function and structural parameters: A pig study. BMC Nephrol. 2008, 9, 18. [Google Scholar] [CrossRef]
- Gasthuys, E.; Devreese, M.; Millecam, J.; Sys, S.; Vanderperren, K.; Delanghe, J.; Vande Walle, J.; Heyndrickx, M.; Croubels, S. Postnatal Maturation of the Glomerular Filtration Rate in Conventional Growing Piglets As Potential Juvenile Animal Model for Preclinical Pharmaceutical Research. Front. Pharmacol. 2017, 8, 431. [Google Scholar] [CrossRef]
- Palnaes Hansen, C.; Bie, P.; Stadil, F. Assessment of renal function by 51Cr-EDTA and endogenous creatinine clearances in the pig. Acta Physiol. Scand. 1997, 161, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Frennby, B.; Sterner, G.; Almen, T.; Chai, C.M.; Jonsson, B.A.; Mansson, S. Clearance of iohexol, 51Cr-EDTA and endogenous creatinine for determination of glomerular filtration rate in pigs with reduced renal function: A comparison between different clearance techniques. Scand. J. Clin. Lab. Investig. 1997, 57, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Skotnicka, E.; Muszczynski, Z.; Dudzinska, W.; Suska, M. A review of the renal system and diurnal variations of renal activity in livestock. Ir. Vet. J. 2007, 60, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G. Beitrage zur Kenntius der Nierenphysiologic einiger Haussaugetiere [Contribution to the knowledge on kidney physiology in some domestic mammals]. Zentl Vet. Med. Beiheft 1962, 3. (In German) [Google Scholar] [CrossRef]
- Ketz, H.A. Vergleichende Betrachtungen zur Nierenfunktion bei den Haustieren [Comparative studies on kidney function in domestic animals]. Arch. Exp. Vet. Med. 1960, 14, 411–419. (In German) [Google Scholar]
- Muszczynski, Z.; Skotnicka, E.; Jankowiak, D. Diurnal variations of renal activity in goats. Turk. J. Vet. Anim. Sci. 2015, 39, 455–464. [Google Scholar] [CrossRef]
- Ladd, M.; Liddle, L.; Gagnon, J.A.; Clarke, R.W. Glomerular and tubular functions in sheep and goats. J. Appl. Physiol. 1957, 10, 249–255. [Google Scholar] [CrossRef]
- Luis-Lima, S.; Mas-Sanmartin, C.; Rodriguez-Rodriguez, A.E.; Porrini, E.; Ortiz, A.; Gaspari, F.; Diaz-Martin, L.; Asberg, A.; Jenssen, T.; Jimenez-Sosa, A.; et al. A Simplified Iohexol-Based Method to Measure Renal Function in Sheep Models of Renal Disease. Biology 2020, 9, 259. [Google Scholar] [CrossRef]
- Nesje, M.; Flaoyen, A.; Moe, L. Estimation of glomerular filtration rate in normal sheep by the disappearance of iohexol from serum. Vet. Res. Commun. 1997, 21, 29–35. [Google Scholar] [CrossRef]
- Frennby, B.; Sterner, G.; Almen, T.; Chai, C.M.; Jonsson, B.A.; Mansson, S. Extrarenal plasma clearance of iohexol, chromium-51-ethylenediaminetetraacetic acid, and inulin in anephric pigs. Acad. Radiol. 1996, 3, 145–153. [Google Scholar] [CrossRef]

| Parameter | Method | Precision (%) | Accuracy (%) | |
|---|---|---|---|---|
| P30 * | P10 * | |||
| CL | NLME SM | 80.9 80.7 | 53 | 12 |
| mGFR | NLME SM | 49.2 53.6 | 71 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gelder, M.K.; Stevens, J.; Pieters, T.T.; Vaessen, K.R.D.; Joles, J.A.; Verhaar, M.C.; Gerritsen, K.G.F. Simplified Iohexol-Based Method for Measurement of Glomerular Filtration Rate in Goats and Pigs. Biology 2021, 10, 461. https://doi.org/10.3390/biology10060461
van Gelder MK, Stevens J, Pieters TT, Vaessen KRD, Joles JA, Verhaar MC, Gerritsen KGF. Simplified Iohexol-Based Method for Measurement of Glomerular Filtration Rate in Goats and Pigs. Biology. 2021; 10(6):461. https://doi.org/10.3390/biology10060461
Chicago/Turabian Stylevan Gelder, Maaike K., Jasper Stevens, Tobias T. Pieters, Koen R. D. Vaessen, Jaap A. Joles, Marianne C. Verhaar, and Karin G. F. Gerritsen. 2021. "Simplified Iohexol-Based Method for Measurement of Glomerular Filtration Rate in Goats and Pigs" Biology 10, no. 6: 461. https://doi.org/10.3390/biology10060461
APA Stylevan Gelder, M. K., Stevens, J., Pieters, T. T., Vaessen, K. R. D., Joles, J. A., Verhaar, M. C., & Gerritsen, K. G. F. (2021). Simplified Iohexol-Based Method for Measurement of Glomerular Filtration Rate in Goats and Pigs. Biology, 10(6), 461. https://doi.org/10.3390/biology10060461








