The TSPO Ligands MGV-1 and 2-Cl-MGV-1 Differentially Inhibit the Cigarette Smoke-Induced Cytotoxicity to H1299 Lung Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
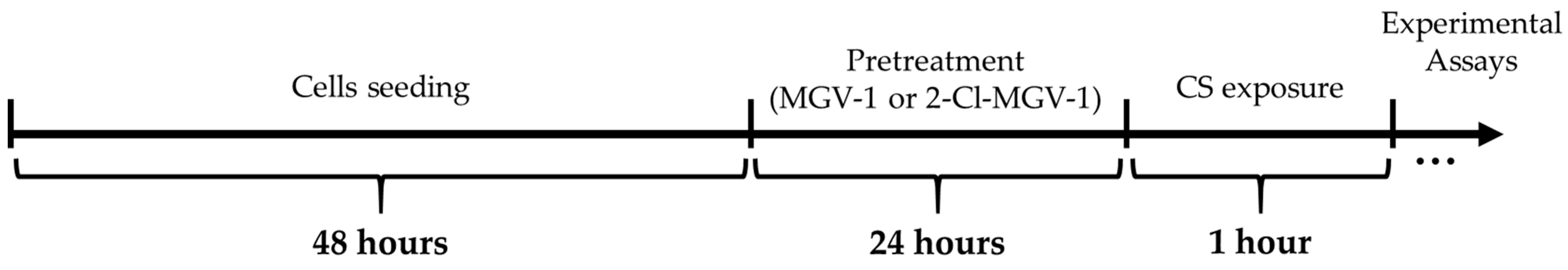
2.1. Cells and Study Design
2.2. Ligand Synthesis
2.3. Exposure of H1299 Cells to Cigarette Smoke
2.4. TSPO and cAMP Levels Measurement Using CyAN ADP
2.5. ADP/ATP Ratio
2.6. Cellular ROS/Superoxide Detection Assay
2.7. Depolarization of the Mitochondrial Membrane Potential (ΔΨm)
2.8. Cell Death Assays
2.8.1. Lactate Dehydrogenase (LDH) Activity
2.8.2. Apoptosis Levels
2.9. Caspase-3, Caspase-8 and Caspase-9 Levels
2.10. Microscopic Imaging
2.10.1. TSPO Levels
2.10.2. Hoechst Staining
2.11. Statistical Analysis
3. Results
3.1. LDH Cytotoxicity Assay
3.1.1. Dose-Response Analysis Using LDH Cytotoxicity Assay
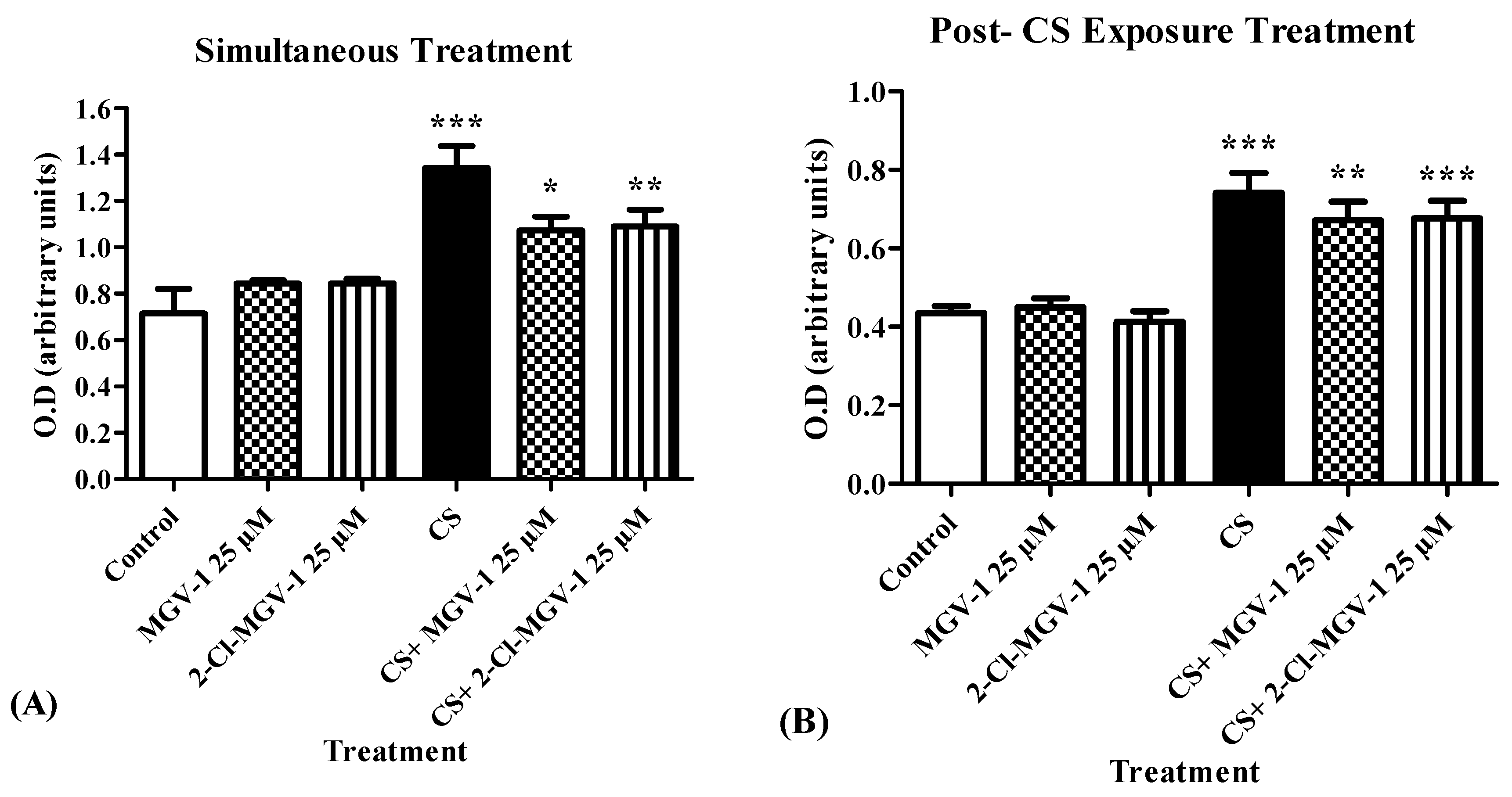
3.1.2. Cytotoxicity Levels Following Simultaneous- and Post-CS Exposure-Treatment
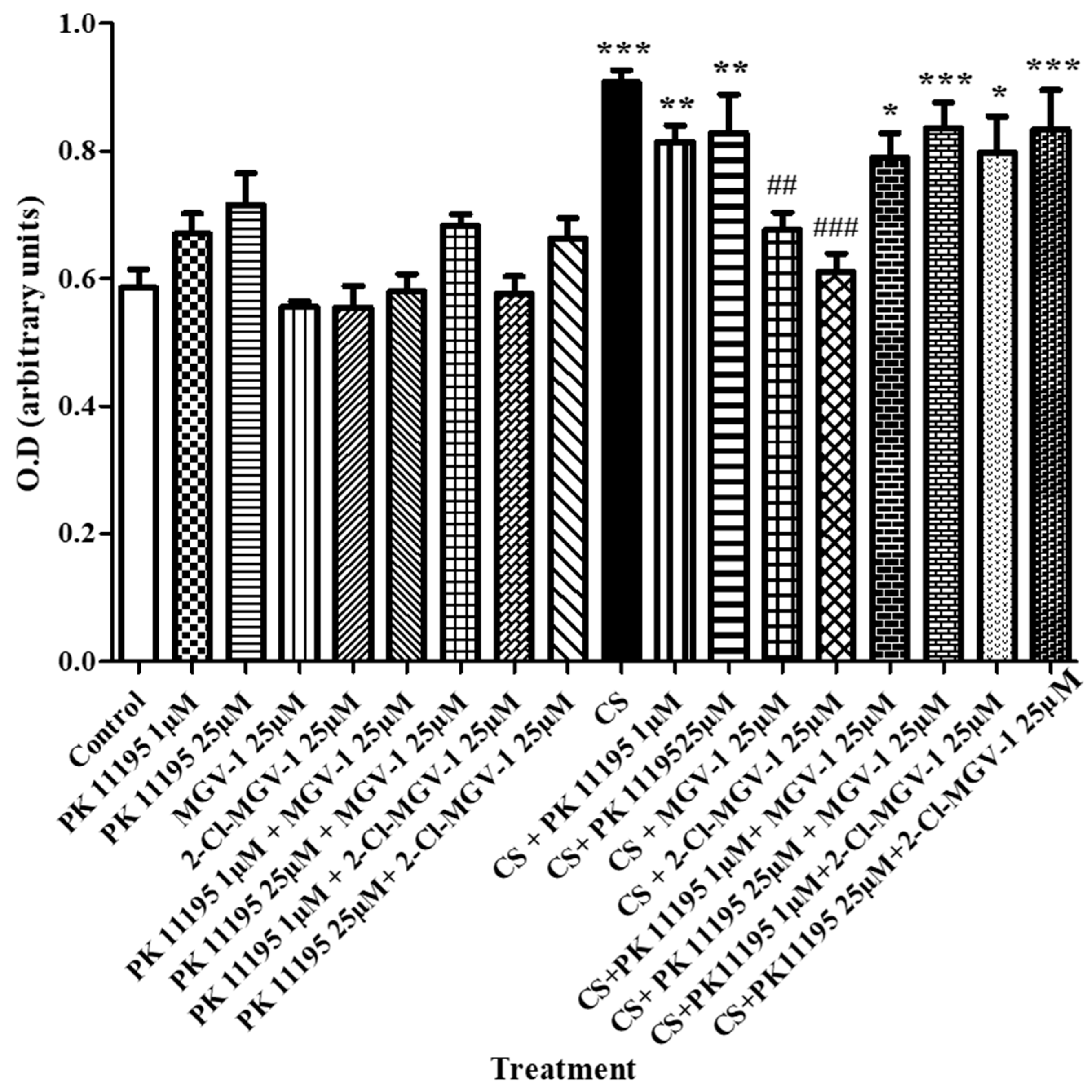
3.1.3. Pharmacological TSPO Knockdown-Like Effect Using PK 11,195 as Antagonist
3.2. TSPO Levels
3.2.1. Flowcytometry
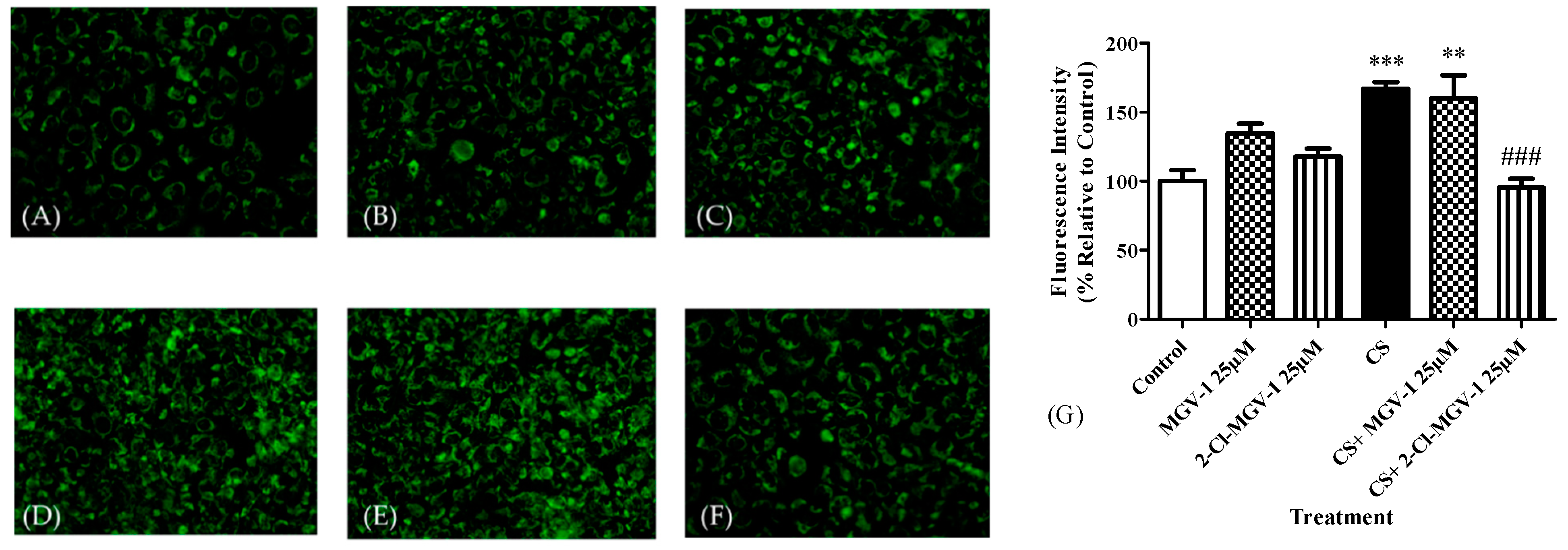
3.2.2. Fluorescence Microscopy
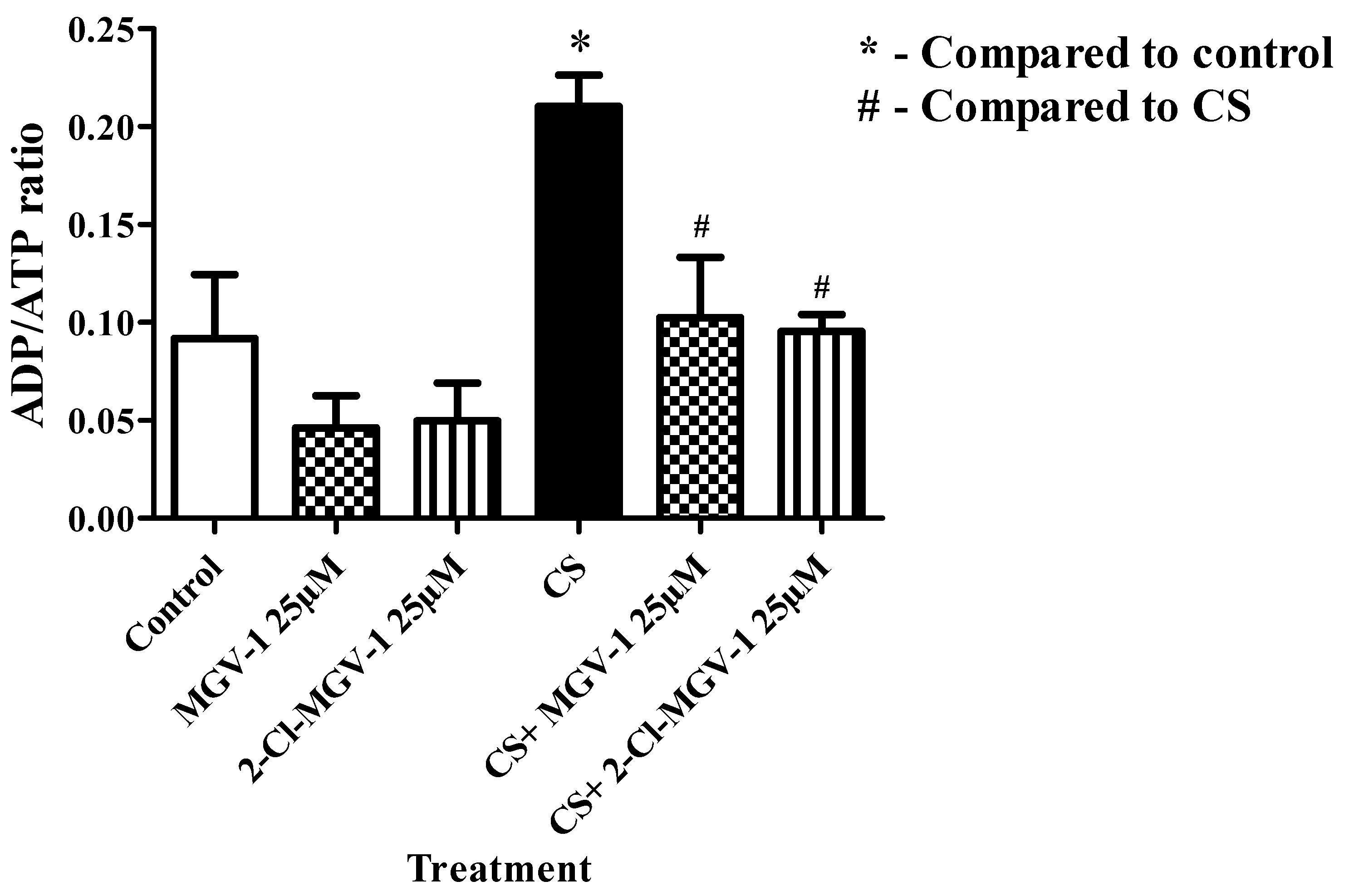
3.3. ADP/ATP Ratio
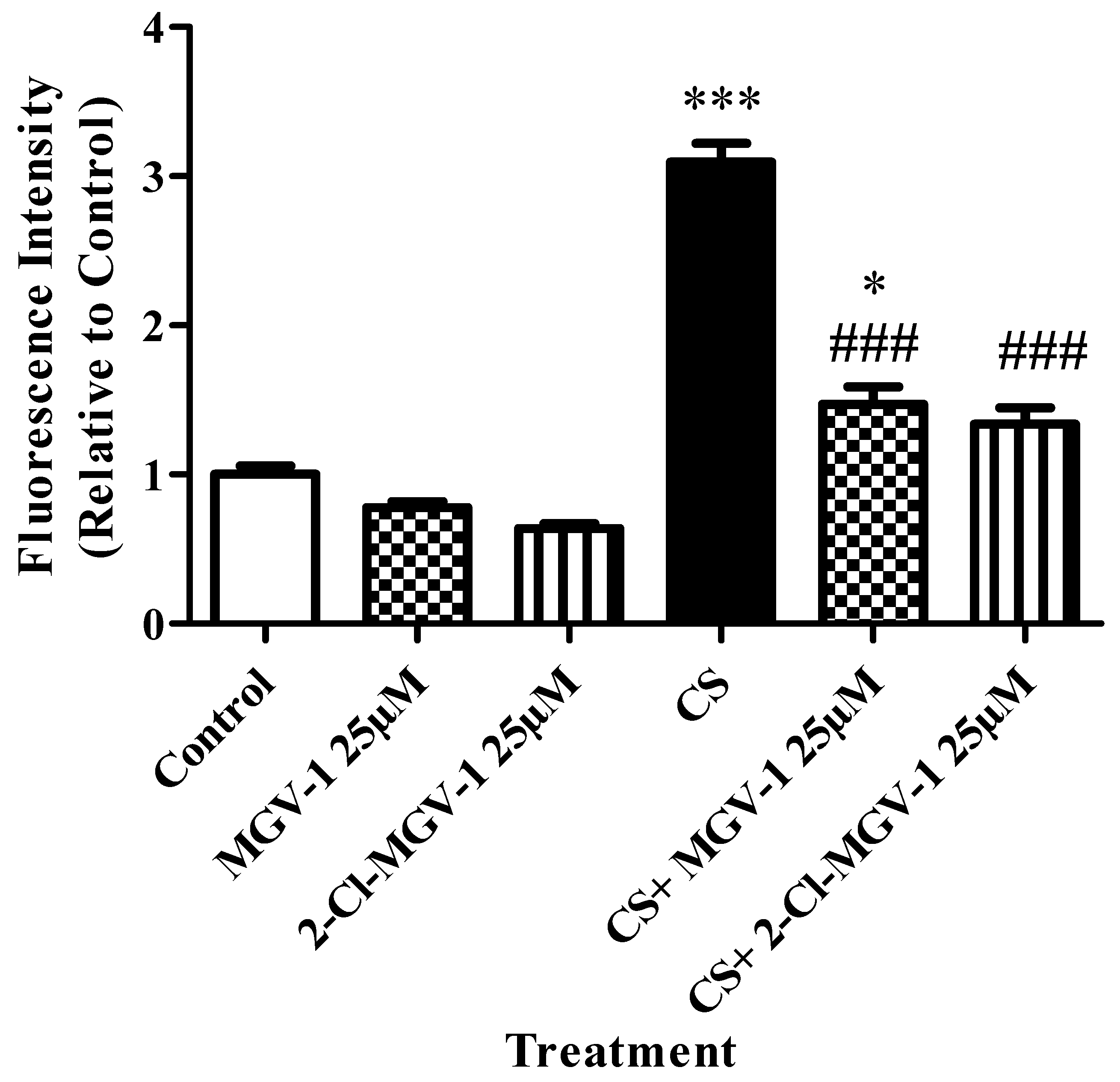
3.4. Oxidative Stress Levels
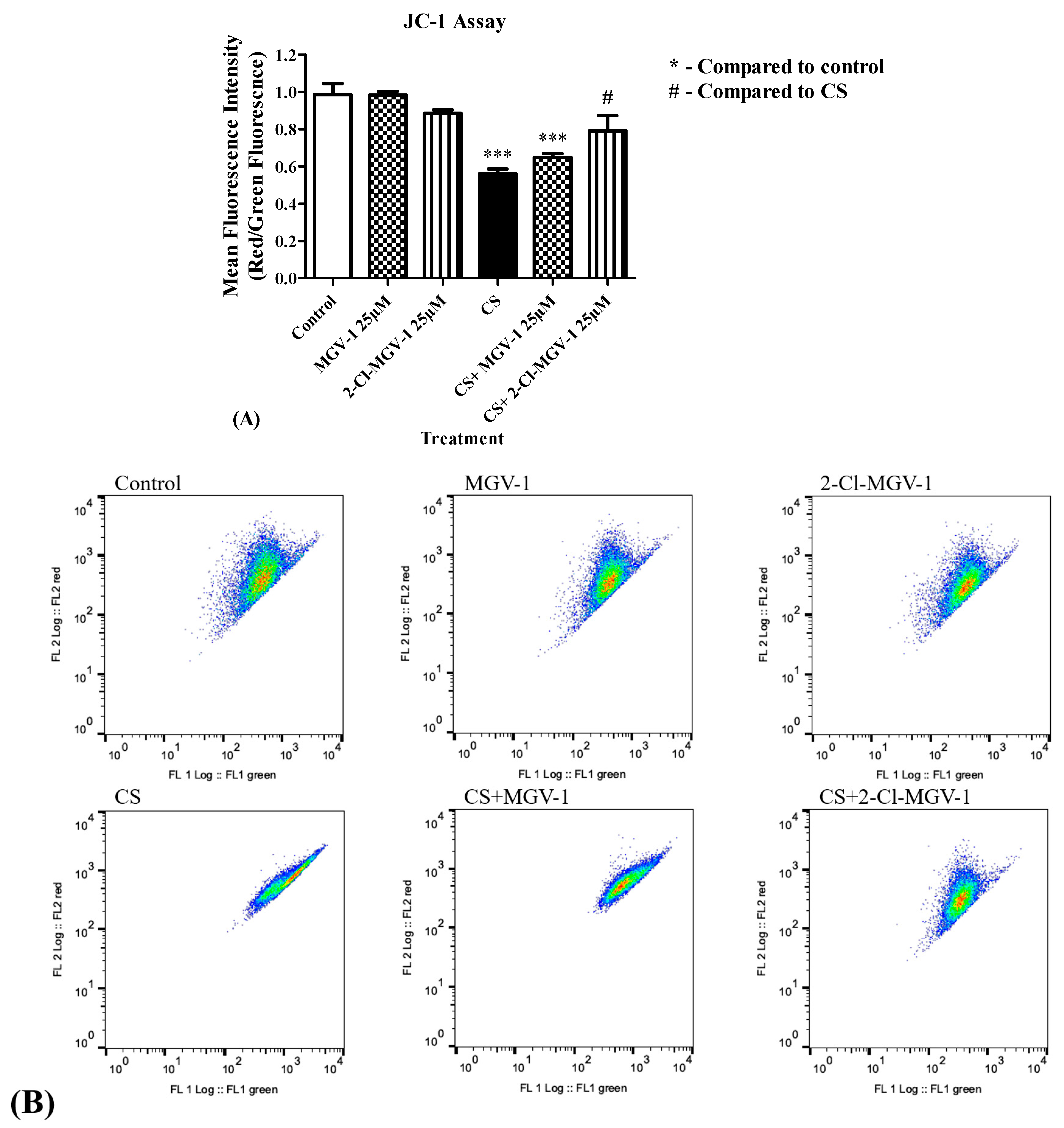
3.5. Depolarization of the Mitochondrial Membrane Potential (ΔΨm)
3.6. Caspase 3, 8 and 9 Levels
3.7. cAMP Levels
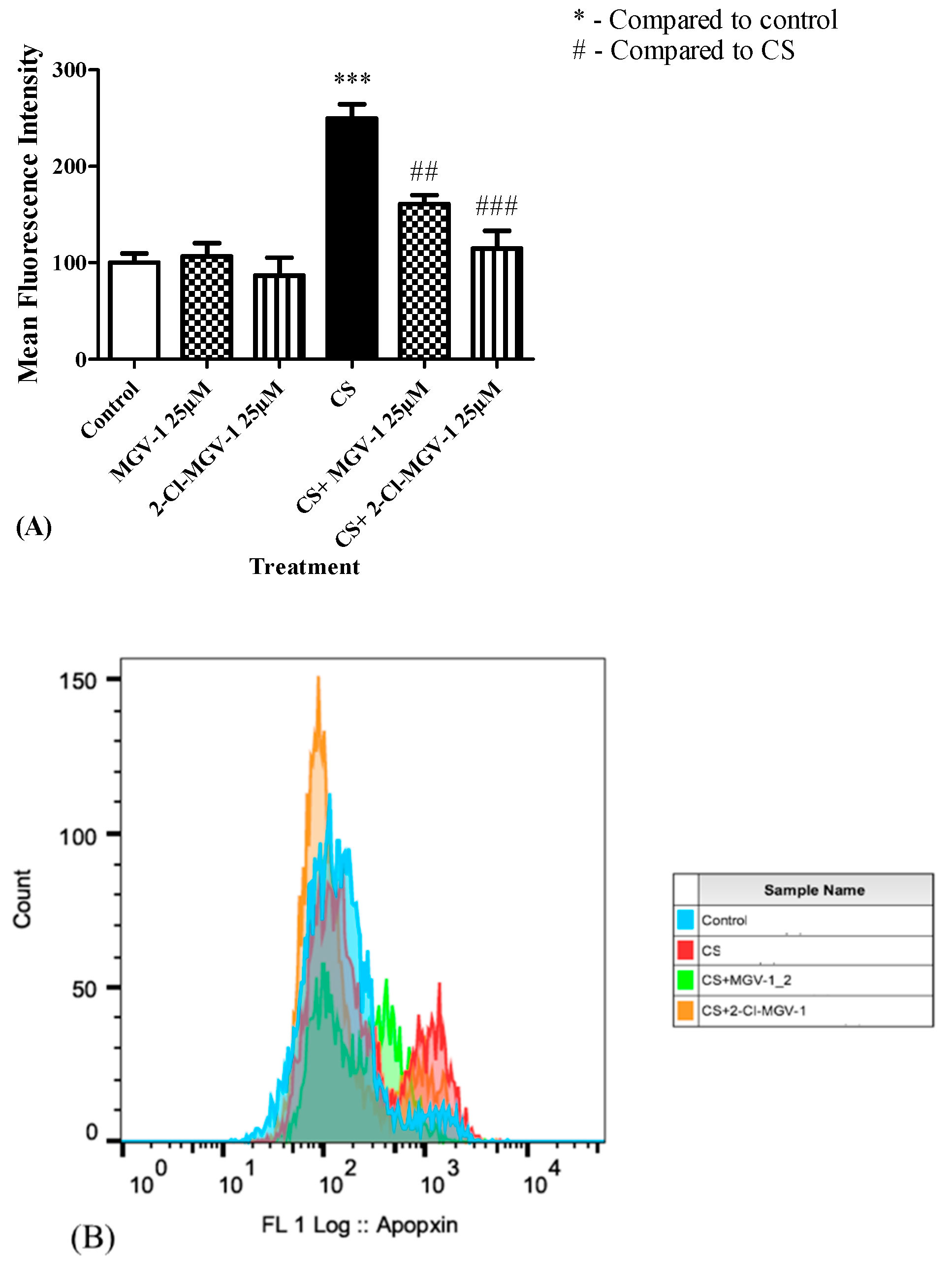
3.8. Apoptosis Levels
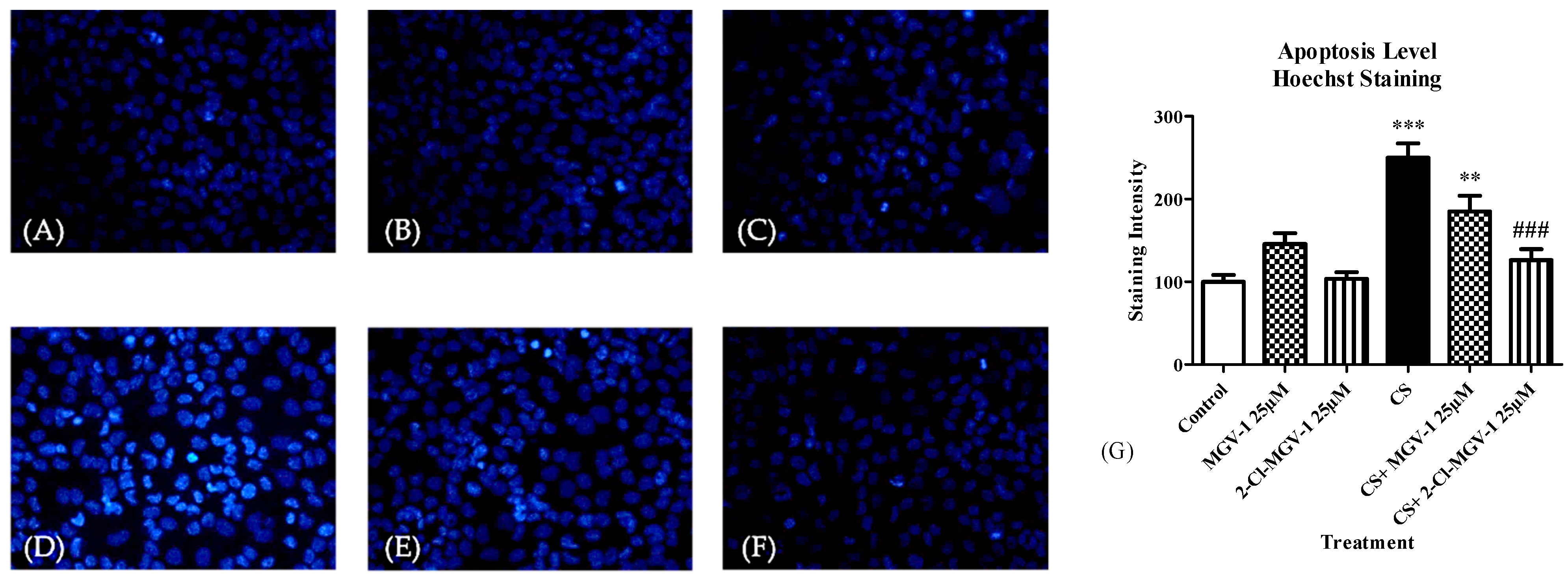
3.8.1. Hoechst Staining
3.8.2. Fluorescence Activated Cell Sorting (FACS) Assay
3.9. Association between TSPO Expression and Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO urges more countries to require large, graphic health warnings on tobacco packaging: The WHO report on the global tobacco epidemic, 2011 examines anti-tobacco mass-media campaigns. Cent. Eur. J. Public Health 2011, 19, 133–151. [Google Scholar]
- Alberg, A.J.; Samet, J.M. Epidemiology of lung cancer. Chest 2003, 123, 21S–49S. [Google Scholar] [CrossRef]
- Sanner, T.; Grimsrud, T.K. Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment—A Review. Front. Oncol. 2015, 5, 196. [Google Scholar] [CrossRef]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef]
- Caballero, B.; Veenman, L.; Gavish, M. Role of mitochondrial translocator protein (18 kDa) on mitochondrial-related cell death processes. Recent Pat. Endocr Metab. Immune Drug Discov. 2013, 7, 86–101. [Google Scholar] [CrossRef]
- Veenman, L.; Gavish, M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharm. Ther. 2006, 110, 503–524. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Gavish, M. The role of 18 kDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr. Mol. Med. 2012, 12, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Zeno, S.; Zaaroor, M.; Leschiner, S.; Veenman, L.; Gavish, M. CoCl(2) induces apoptosis via the 18 kDa translocator protein in U118MG human glioblastoma cells. Biochemistry 2009, 48, 4652–4661. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J. Effects of Cigarette Smoke on the Lung and Systemic Immunity. J. Physiol. Pharmacol. 2008, 59, 19–34. [Google Scholar]
- Anholt, R.R.; De Souza, E.B.; Oster-Granite, M.L.; Snyder, S.H. Peripheral-type benzodiazepine receptors: Autoradiographic localization in whole-body sections of neonatal rats. J. Pharm. Exp. 1985, 233, 517–526. [Google Scholar]
- Nagler, R.; Cohen, S.; Gavish, M. The Effect of Cigarette Smoke on the Translocator Protein (TSPO) in Cultured Lung Cancer Cells. J. Cell Biochem. 2015, 116, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Gavish, M.; Bachman, I.; Shoukrun, R.; Katz, Y.; Veenman, L.; Weisinger, G.; Weizman, A. Enigma of the peripheral benzodiazepine receptor. Pharm. Rev. 1999, 51, 629–650. [Google Scholar] [PubMed]
- Gavish, M.; Bar-Ami, S.; Weizman, R. The endocrine system and mitochondrial benzodiazepine receptors. Mol. Cell Endocrinol. 1992, 88, 1–13. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharm. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Cai, L.; Kirchleitner, S.V.; Zhao, D.; Li, M.; Tonn, J.C.; Glass, R.; Kalin, R.E. Glioblastoma Exhibits Inter-Individual Heterogeneity of TSPO and LAT1 Expression in Neoplastic and Parenchymal Cells. Int. J. Mol. Sci. 2020, 21, 612. [Google Scholar] [CrossRef]
- Habermeier, A.; Graf, J.; Sandhofer, B.F.; Boissel, J.P.; Roesch, F.; Closs, E.I. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids 2015, 47, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Eitan, A.; Amiri, Z.; Gavish, M. Dramatic increase in peripheral benzodiazepine binding sites in human colonic adenocarcinoma as compared to normal colon. Eur. J. Pharm. 1988, 148, 483–484. [Google Scholar] [CrossRef]
- Kugler, W.; Veenman, L.; Shandalov, Y.; Leschiner, S.; Spanier, I.; Lakomek, M.; Gavish, M. Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine. Cell Oncol. 2008, 30, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Van Dort, M.E.; Ciliax, B.J.; Gildersleeve, D.L.; Sherman, P.S.; Rosenspire, K.C.; Young, A.B.; Junck, L.; Wieland, D.M. Radioiodinated benzodiazepines: Agents for mapping glial tumors. J. Med. Chem. 1988, 31, 2081–2086. [Google Scholar] [CrossRef]
- Miyazawa, N.; Hamel, E.; Diksic, M. Assessment of the peripheral benzodiazepine receptors in human gliomas by two methods. J. Neurooncol. 1998, 38, 19–26. [Google Scholar] [CrossRef]
- Ferrarese, C.; Appollonio, I.; Frigo, M.; Gaini, S.M.; Piolti, R.; Frattola, L. Benzodiazepine receptors and diazepam-binding inhibitor in human cerebral tumors. Ann. Neurol. 1989, 26, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, N.; Nagler, R.; Gabay, M.; Weizman, A.; Gavish, M. Effects of Cigarette Smoke on TSPO-related Mitochondrial Processes. Cells 2019, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Natarajan, V.; Zhen, L.; Medler, T.R.; Richter, A.T.; Cho, C.; Hubbard, W.C.; Berdyshev, E.V.; Tuder, R.M. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 2005, 11, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Jin, X.K.; Yu, A.Q.; Zhu, Y.T.; Li, D.; Li, W.W.; Wang, Q. Caspase-mediated apoptosis in crustaceans: Cloning and functional characterization of EsCaspase-3-like protein from Eriocheir. Fish. Shellfish Immunol. 2014, 41, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, D.; Zhuang, J.; Zhang, F.; Xu, C. Caspase-8 and Caspase-9 Functioned Differently at Different Stages of the Cyclic Stretch-Induced Apoptosis in Human Periodontal Ligament Cells. PLoS ONE 2016, 11, e0168268. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Girnius, N.; Davis, R.J. JNK Promotes Epithelial Cell Anoikis by Transcriptional and Post-translational Regulation of BH3-Only Proteins. Cell Rep. 2017, 21, 1910–1921. [Google Scholar] [CrossRef]
- Huang, D.C.; Strasser, A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell 2000, 103, 839–842. [Google Scholar] [CrossRef]
- Pinon, J.D.; Labi, V.; Egle, A.; Villunger, A. Bim and Bmf in tissue homeostasis and malignant disease. Oncogene 2008, 27 (Suppl. 1), S41–S52. [Google Scholar] [CrossRef]
- Jurgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef]
- Puthalakath, H.; Strasser, A. Keeping killers on a tight leash: Transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002, 9, 505–512. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Insel, P.A.; Zhang, L.; Murray, F.; Yokouchi, H.; Zambon, A.C. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol. 2012, 204, 277–287. [Google Scholar] [CrossRef]
- Cross, T.G.; Scheel-Toellner, D.; Henriquez, N.V.; Deacon, E.; Salmon, M.; Lord, J.M. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 2000, 256, 34–41. [Google Scholar] [CrossRef]
- Lerner, A.; Kim, D.H.; Lee, R. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma 2000, 37, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.; Zeineh, N.; Azrad, M.; Yassin, N.; Weizman, A.; Gavish, M. 18-kDa Translocator Protein Ligands Protect H9C2 Cardiomyocytes from Cigarette Smoke-induced Cell Death: In Vitro Study. In Vivo 2020, 34, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Veenman, L.; Shterenberg, A.; Singh, S.; Masarwa, A.; Dutta, B.; Island, B.; Tsoglin, E.; Levin, E.; Leschiner, S.; et al. Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease. Cell Death Discov. 2015, 1. [Google Scholar] [CrossRef]
- Avezov, K.; Reznick, A.Z.; Aizenbud, D. Oxidative damage in keratinocytes exposed to cigarette smoke and aldehydes. Toxicol. Vitr. 2014, 28, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Cheng, K.S.; Wang, Y.W.; Chen, Y.F.; Wong, K.L.; Su, T.H.; Chan, P.; Leung, Y.M. Perturbation of Akt Signaling, Mitochondrial Potential, and ADP/ATP Ratio in Acidosis-Challenged Rat Cortical Astrocytes. J. Cell Biochem. 2017, 118, 1108–1117. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D.; et al. Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium [corrected]. J. Biotechnol. 1992, 25, 231–243. [Google Scholar] [CrossRef]
- Le Fur, G.; Vaucher, N.; Perrier, M.L.; Flamier, A.; Benavides, J.; Renault, C.; Dubroeucq, M.C.; Gueremy, C.; Uzan, A. Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci. 1983, 33, 449–457. [Google Scholar] [CrossRef]
- Bruey, J.M.; Bruey-Sedano, N.; Luciano, F.; Zhai, D.; Balpai, R.; Xu, C.; Kress, C.L.; Bailly-Maitre, B.; Li, X.; Osterman, A.; et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 2007, 129, 45–56. [Google Scholar] [CrossRef]
- Jin, Y.L.; Xin, L.M.; Zhou, C.C.; Ren, Y. Polydatin exerts anti-tumor effects against renal cell carcinoma cells via induction of caspase-dependent apoptosis and inhibition of the PI3K/Akt pathway. Oncotargets Ther. 2018, 11, 8185–8195. [Google Scholar] [CrossRef] [PubMed]
- Grover, G.; Kini, S.G. Synthesis and evaluation of new quinazolone derivatives of nalidixic acid as potential antibacterial and antifungal agents. Eur. J. Med. Chem. 2006, 41, 256–262. [Google Scholar] [CrossRef]
- Kuyper, L.F.; Baccanari, D.P.; Jones, M.L.; Hunter, R.N.; Tansik, R.L.; Joyner, S.S.; Boytos, C.M.; Rudolph, S.K.; Knick, V.; Wilson, H.R.; et al. High-affinity inhibitors of dihydrofolate reductase: Antimicrobial and anticancer activities of 7,8-dialkyl-1,3-diaminopyrrolo[3,2-f]quinazolines with small molecular size. J. Med. Chem. 1996, 39, 892–903. [Google Scholar] [CrossRef]
- Decker, M. Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur. J. Med. Chem. 2005, 40, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.A.; Adami, M.; Istyastono, E.P.; Zuiderveld, O.P.; van Dam, C.M.; de Kanter, F.J.; Jongejan, A.; Coruzzi, G.; Leurs, R.; de Esch, I.J. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J. Med. Chem. 2010, 53, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Marvania, B.; Lee, P.C.; Chaniyara, R.; Dong, H.; Suman, S.; Kakadiya, R.; Chou, T.C.; Lee, T.C.; Shah, A.; Su, T.L. Design, synthesis and antitumor evaluation of phenyl N-mustard-quinazoline conjugates. Bioorg. Med. Chem. 2011, 19, 1987–1998. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, Y.; Ip, M.S.M.; Mak, J.C.W. Cigarette smoke induces apoptosis via 18 kDa translocator protein in human bronchial epithelial cells. Life Sci. 2021, 265, 118862. [Google Scholar] [CrossRef]
- Bhoola, N.H.; Mbita, Z.; Hull, R.; Dlamini, Z. Translocator Protein (TSPO) as a Potential Biomarker in Human Cancers. Int. J. Mol. Sci. 2018, 19, 2176. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Zeineh, N.; Weizman, A.; Veenman, L.; Gavish, M. The TSPO Ligands 2-Cl-MGV-1, MGV-1, and PK11195 Differentially Suppress the Inflammatory Response of BV-2 Microglial Cell to LPS. Int. J. Mol. Sci. 2019, 20, 594. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, N.; Chang, I.; Chung, J.H.; Min, Y.K.; Lee, M.K.; Kim, K.W.; Kim, S.J.; Lee, M.S. Effect of PK11195, a peripheral benzodiazepine receptor agonist, on insulinoma cell death and insulin secretion. Apoptosis Int. J. Program. Cell Death 2005, 10, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, M.S.; Faccenda, D.; De Biase, V.; Campanella, M. PK11195 inhibits mitophagy targeting the F1Fo-ATPsynthase in Bcl-2 knock-down cells. Curr. Mol. Med. 2012, 12, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Monga, S.; Weizman, A.; Gavish, M. The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells. Biology 2020, 9, 291. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeineh, N.; Nagler, R.M.; Gabay, M.; Obeid, F.; Kahana, M.; Weizman, A.; Gavish, M. The TSPO Ligands MGV-1 and 2-Cl-MGV-1 Differentially Inhibit the Cigarette Smoke-Induced Cytotoxicity to H1299 Lung Cancer Cells. Biology 2021, 10, 395. https://doi.org/10.3390/biology10050395
Zeineh N, Nagler RM, Gabay M, Obeid F, Kahana M, Weizman A, Gavish M. The TSPO Ligands MGV-1 and 2-Cl-MGV-1 Differentially Inhibit the Cigarette Smoke-Induced Cytotoxicity to H1299 Lung Cancer Cells. Biology. 2021; 10(5):395. https://doi.org/10.3390/biology10050395
Chicago/Turabian StyleZeineh, Nidal, Rafael M. Nagler, Martin Gabay, Fadi Obeid, Meygal Kahana, Abraham Weizman, and Moshe Gavish. 2021. "The TSPO Ligands MGV-1 and 2-Cl-MGV-1 Differentially Inhibit the Cigarette Smoke-Induced Cytotoxicity to H1299 Lung Cancer Cells" Biology 10, no. 5: 395. https://doi.org/10.3390/biology10050395
APA StyleZeineh, N., Nagler, R. M., Gabay, M., Obeid, F., Kahana, M., Weizman, A., & Gavish, M. (2021). The TSPO Ligands MGV-1 and 2-Cl-MGV-1 Differentially Inhibit the Cigarette Smoke-Induced Cytotoxicity to H1299 Lung Cancer Cells. Biology, 10(5), 395. https://doi.org/10.3390/biology10050395






