1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver worldwide. Its pathogenesis is multifactorial, with a strong aetiological association with chronic viral infection by hepatotropic viruses, alcohol consumption, exposure to hepatic toxins, as well as genetic disorders such as haemochromatosis and α1-antitrypsin deficiency. Fine mechanisms eliciting tumor development are still poorly understood. In the promotion stage, HCC has been associated with defective apoptosis and increased cell proliferation [
1,
2]. Tumor cells often show altered expression of genes involved in cell proliferation and cell death [
3]. Among them, the 66-kDa isoform of ShcA (p66shc) is an adapter protein that was proposed to downregulate mammalian life span by inhibition of receptor tyrosine kinase signaling and induction of cell differentiation [
4,
5]. This protein has been involved in cellular response to oxidative stress and apoptosis [
5] and its pro-apoptotic functions have been widely confirmed, although its role is complex, since it could act as a double-edged sword in the regulation of apoptosis, depending on the environmental context [
6,
7,
8].
Aberrant expression of p66shc could also be involved in various stages of carcinogenesis. Elevated levels of p66shc protein have been found in estrogen-regulated tumors, including metastatic breast and ovarian tumors, thyroid tumors and stage II colon cancer [
9,
10]. Recently, p66shc has been identified as a novel regulator of autophagy and apoptosis in B lymphocytes, allowing their survival and differentiation [
11]. Very little information is available on p66Shc in HCC, even if some data have reported its correlation with a poor clinical outcome [
12].
SerpinB3, formerly known as Squamous Cell Carcinoma Antigen-1 or SCCA-1, is a cysteine peptidase inhibitor, member of the ovalbumin family of serine proteinase inhibitors [
13]. It is a multifunctional molecule, the activities of which have not yet been completely defined. Beside its anti-protease activity [
14,
15], this serpin has been reported to inhibit apoptosis through the interaction with mitochondrial respiratory complex I [
16] and to increase proliferation, improving survival of tumor cells [
17]. However, it has also been found that it can promote accelerated cell death through Caspase-8-mediated apoptosis in response to endoplasmic reticulum stress [
18]. SerpinB3 has been found to be overexpressed in several types of tumor, especially in those with poor prognosis, including breast, liver, esophagus and colorectal cancer [
19,
20,
21,
22], although some findings have suggested its ability to inhibit cancer cell invasion [
23]. These findings suggest that additional players might modulate the biological activity of this molecule, influencing cell fate.
To date, the available evidence is consistent with the existence of different forms of regulated cell death. Necrosis and apoptosis represent two pathways of genetically encoded necrotic cell death [
24] and apoptotic cell death [
25]. Necroptosis is a non-caspase-dependent and precisely regulated mechanism of cell death. Necroptosis serves as an alternative mode of programmed cell death overcoming apoptosis resistance and may trigger and amplify antitumor immunity in cancer therapy [
26].
In this study we have analyzed the expression of p66shc and SerpinB3 in relation to overall survival in patients with primary liver cancer, and carried out in vitro and in vivo experiments to define the effect of these two molecules, involved in cell proliferation and death, on cell fate and tumor growth.
2. Materials and Methods
2.1. Human HCCs
Surgically obtained liver tumor samples of 67 patients with HCC were analyzed. The specimens were obtained under patient written informed consent, following a procedure that was approved by the local ethical committee. Samples were snap frozen and maintained at −80 °C until use. Demographic and clinical data of the patients are reported in
Supplementary Table S1. After surgery, patients were regularly followed up, and clinical, laboratory and imaging assessment were recorded, as previously described [
27].
2.2. Cell Lines
HepG2 cell line was authenticated by BMR Genomics S.r.l. (Padova, Italy), according to PowerPlex
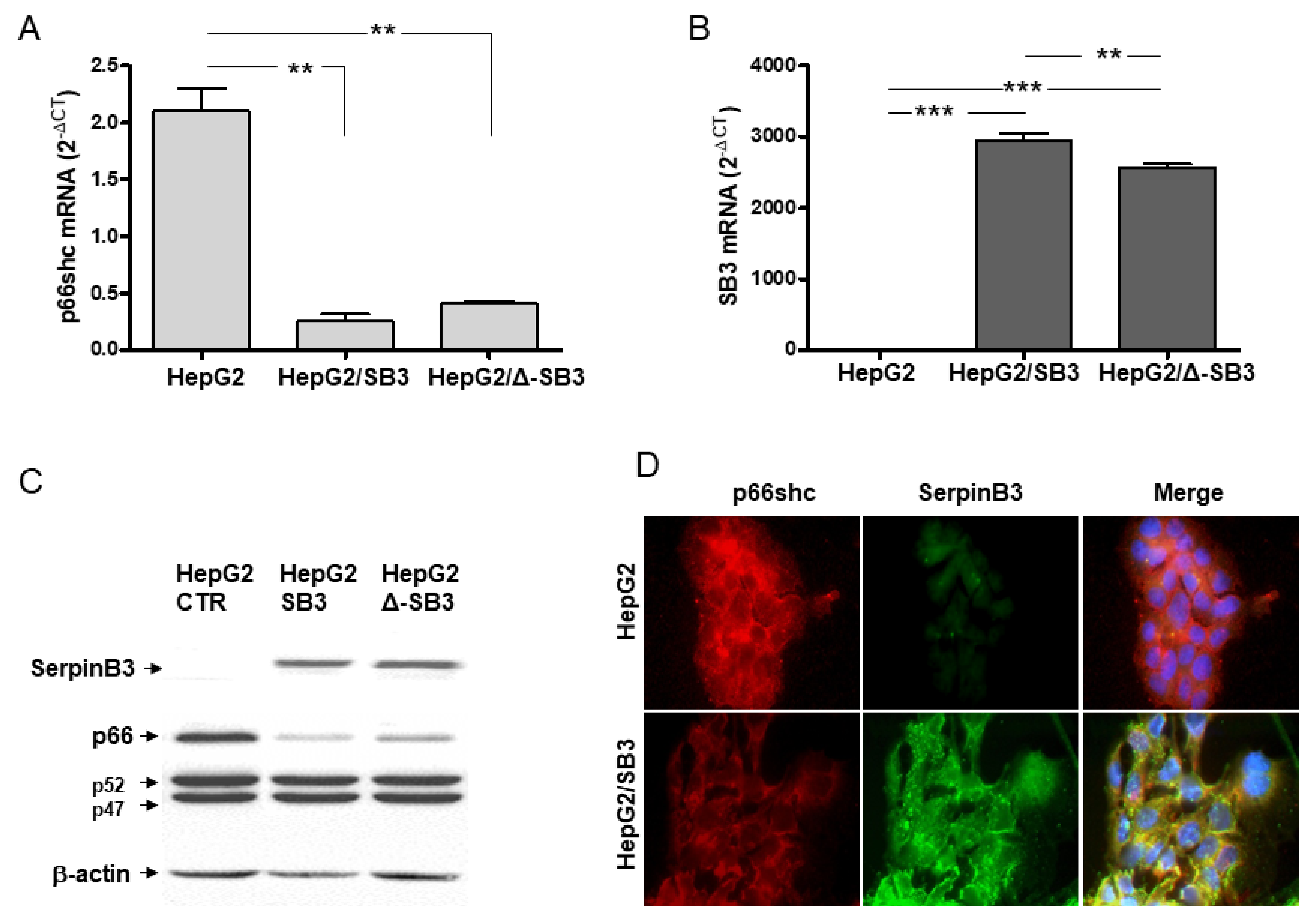
® Fusion System protocol (Promega) and was regularly tested for mycoplasma contamination. This cell line was stably transfected with the full-length human SERPINB3 genomic sequence or with the plasmid vector alone, which were used as previously described [
16]. The experiments were carried out using transfected clone 2 (HepG2/SB3) and were confirmed using transfected HepG2 clone 3 [
16]. An additional HepG2 clone, stably transfected with a Reactive Site Loop (RSL) deleted-SerpinB3 plasmid (Δ-SerpinB3) (provided by Dr. Tim J. Harrison, UCL Medical School, London), was also used to assess the functional role of the antiprotease activity of this serpin in our experimental conditions. Cells were maintained at 37 °C in a humidified chamber with 5% CO
2 and cultured in minimum essential medium with the addition of G418 as selective agent.
2.3. Quantitative Real-Time RT–PCR
Total RNA was extracted using RNasy Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After determination of the purity and the integrity, total RNA, complementary DNA synthesis and quantitative real-time PCR reactions (RT-PCR) were carried out as previously described [
19] using the CFX96 real-time instrument (Bio-Rad Laboratories Inc, Hercules, CA, USA). In hepatoma cells and HCC samples the relative expression was generated for each sample by calculating 2-Δ Ct [
28]. Primers sequences used in the study are reported in
Supplementary Table S2.
2.4. Western Blot Analysis
Total protein contents (50 μg) from each cellular extract, prepared at 4 °C in lysis buffer (150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 2% Triton X-100) in the presence of phosphatase and protease inhibitors (Roche, IN, USA), were loaded onto 10% polyacrylamide gel. The blots were probed with the following primary antibodies: rabbit polyclonal anti-p66shc (Upstate Cell Signaling Solution, NY, USA), rabbit oligoclonal anti-SerpinB3 (Hepa Ab, Xeptagen, Venice, Italy), mouse anti-p66 shc (Upstate Cell Signaling Solution, NY, USA), rabbit polyclonal anti-β-Catenin (GeneTex, Irvine, CA, USA), rabbit polyclonal anti-LC3B (Abcam, Cambridge, UK), rabbit polyclonal anti-active-Caspase-8 (Novus Biologicals-Bio-Techne, Centennial, CO, USA), mouse monoclonal anti-RIP3K (Santa Cruz biotechnology, Dallas, TX, USA). Mouse monoclonal anti-β actin (Sigma-Aldrich, St. Louis, MO, USA) was used as housekeeping control. Anti-mouse and anti-rabbit horseradish peroxides-conjugated antibodies (Amersham, Arlington Heights, IL, USA) were used as secondary antibodies. Antigenic detection was carried out by enhanced chemiluminescence (Amersham, Arlington Heights, IL, USA) and densitometric analysis was assessed using the VersaDoc Imaging System (Bio-Rad Laboratories, Hercules, CA, USA). Relative density units were obtained by comparing ratios of intensities (volume as sum of the intensities of the pixels inside the volume boundary x area of a single pixel (in mm2)) of a reference band (β-Actin).
2.5. Immunohistochemistry
Quantification of macrophage infiltration was assessed by immunohistochemistry on paraffin-embedded tumor xenografts using anti-F4/80 monoclonal antibody (Abcam). Results were analyzed in 5 slides per group, counting positive cells in 10 fields per slide. The slides were observed blindly by two operators and then by a third operator using a bright field microscope.
2.6. Immunofluorescence
The expression of SerpinB3 and of p66shc in SerpinB3 transfected cells and in controls was assessed by immunofluorescence. Cells were seeded on slides (2 × 105 HepG2 cells/slide) and cultured for 48 h. After fixation with 4% paraformaldehyde and permeabilization with 0.2% Tryton X100, cells were blocked with 5% BSA in PBS. The slides were incubated with anti-SerpinB3 antibody (8 μg/mL), or with anti-p66 Shc antibody (1:50 dilution), washed with 0.1% Tween 20 in phosphate-buffered saline (PBS) and incubated with Alexa Fluor 488 Goat anti-mouse and Alexa Fluor 546 Goat anti-rabbit (Invitrogen, Thermo Fisher, Waltham, MA, USA) as secondary antibodies.
Cellular nuclei were counter-stained with Hoechst 33,342 (Sigma-Aldrich, St. Louis, MO, USA). The slides were mounted with ELVANOL (Sigma-Aldrich, St. Louis, MO, USA) and observed under a fluorescence microscope (Axiovert 200M, Carl Zeiss MicroImaging GmbH, Gottingen, Germany).
2.7. Apoptosis/Necrosis Detection Assay
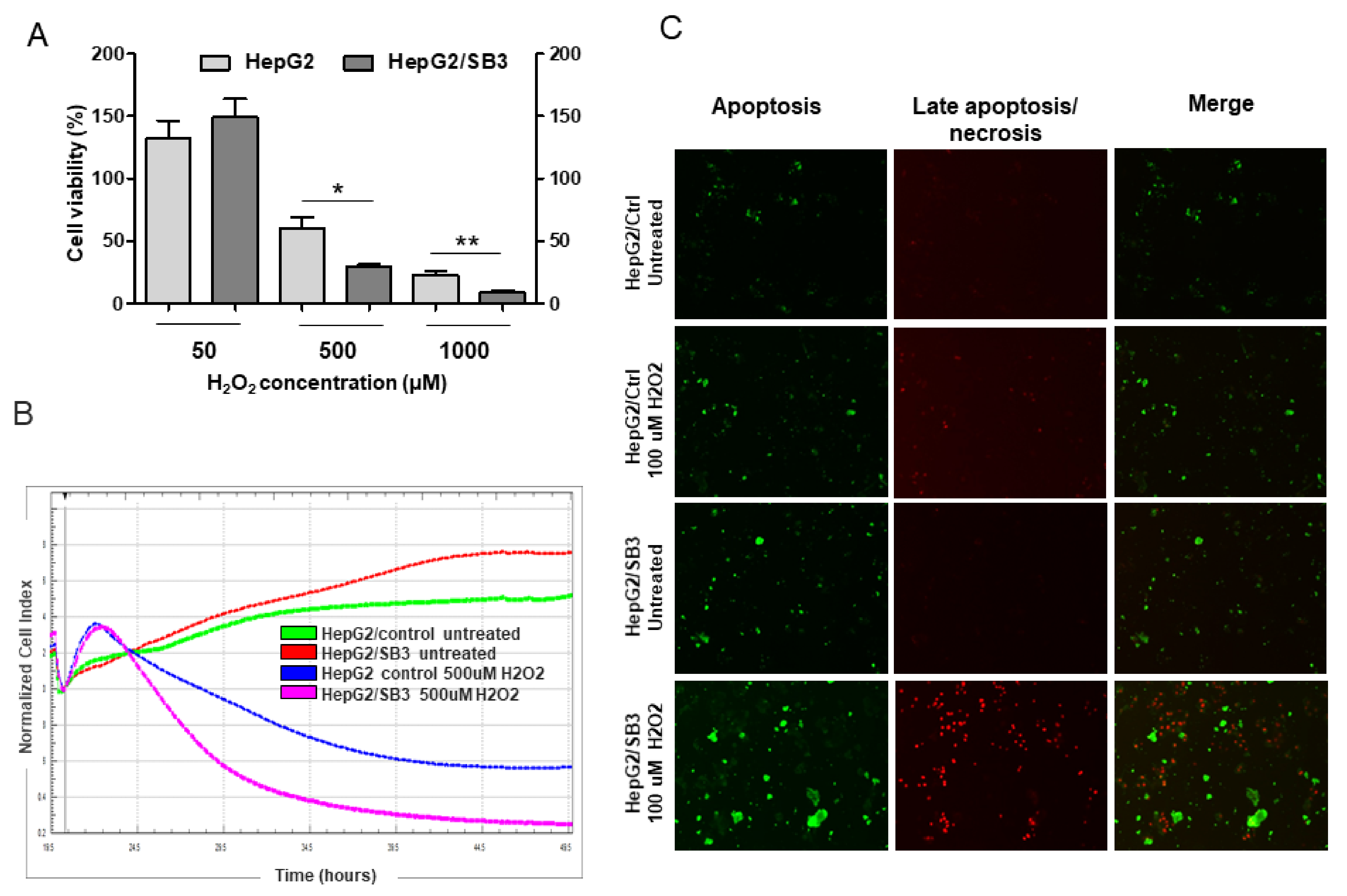
An Apoptosis/Necrosis detection kit (Abcam) was used to simultaneously monitor apoptotic, necrotic and healthy cells through staining with Apopxin Green Solution (green Ex/Em = 490/525 nm) to detect phosphatidylserine (PS) as marker of initial/intermediate stages of apoptosis, with 7-AAD (/-aminoactinomycin D) (red Ex/Em = 546/647 nm) to detect loss of plasma integrity, characteristic of late apoptosis and necrosis. Briefly, 8 × 105 HepG2/SB3 and HepG2/Ctrl cells were seeded onto coverslip and grown until semi-confluence. After overnight treatment with 100 μM H2O2, cells were washed twice and incubated with staining probes and incubated at room temperature for 1 h. After washing, the slides were mounted with ELVANOL (Sigma-Aldrich, St. Louis, MO, USA) and observed under a fluorescence microscope (Axiovert 200M, Carl Zeiss MicroImaging GmbH, Gottingen, Germany).
2.8. Cell Viability Assay
The effect of oxidative stress on cell viability was estimated using the MTT assay. Cells were cultured in 96-well plates (40,000 cells/well) and treated with H2O2 as specified, followed by incubation at 37 °C for 5 h to determine cell viability. In the MTT assay, after dissolving formazan crystals, light absorbance was measured at 570 nm using a spectrophotometer (Victor, Perkin Elmer, Waltham, MA, USA). The quantity of formazan product in the culture medium was directly proportional to the number of viable cells.
2.9. Flow Cytometric Analysis of Cell Death
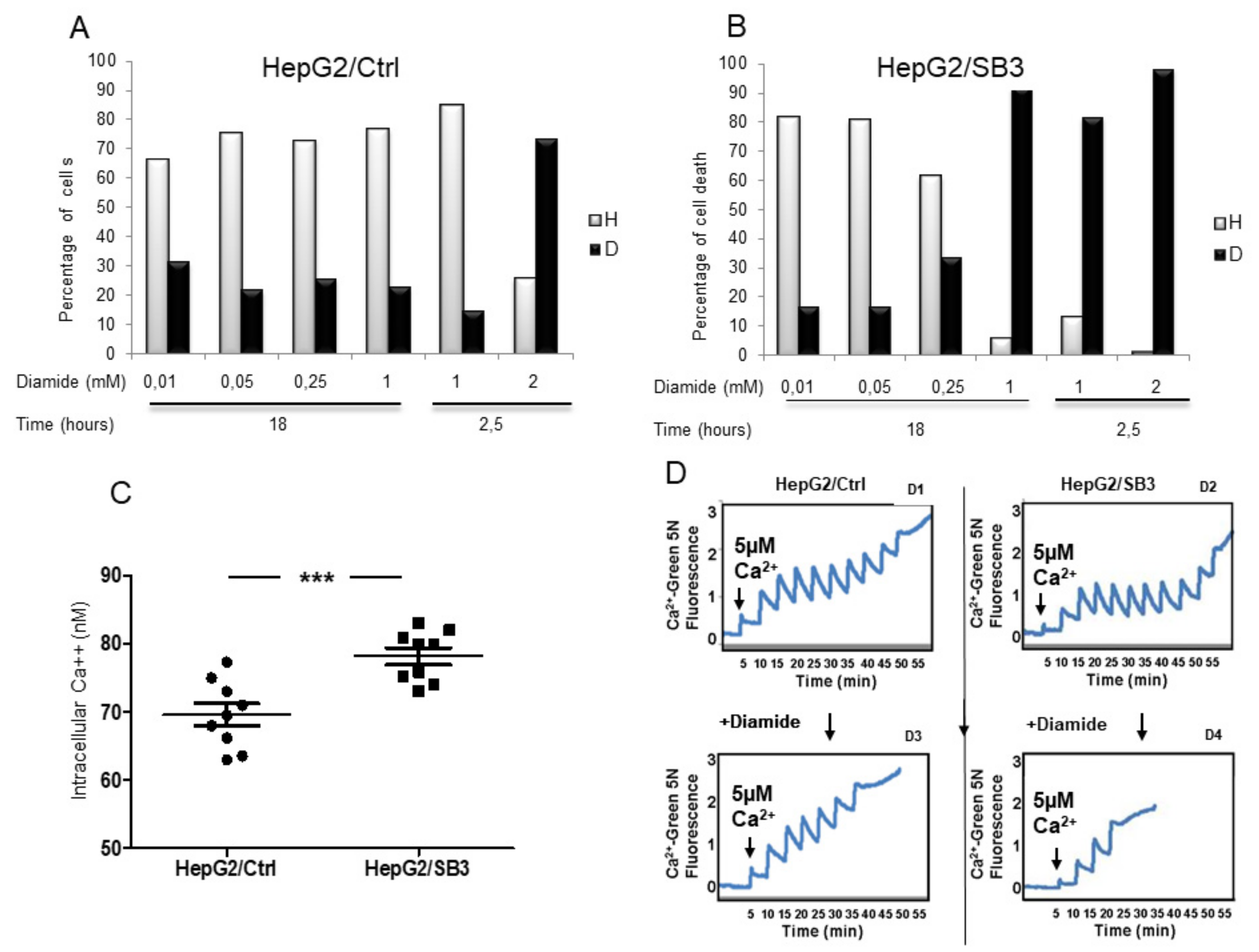
Cells seeded at 2 × 105/well were treated with different concentrations of diamide as indicated and incubated for 2.5 or 18 h. After treatment, cells were washed with phosphate-buffered saline (PBS) and resuspended in 50 µL HEPES buffer (10 mM HEPES, 135 mM NaCl, 5 mM CaCl2). Cells were then incubated for 15 min at 37 °C with Propidium Iodide (PI, 1 µg/mL). Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Data acquisition was performed using CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA) and data analysis was carried out with WinMD free software.
2.10. Cell Proliferation
The xCELLigence system (Roche Diagnostics GmbH, Mannheim, Germany and ACEA Biosciences, Inc., San Diego, CA, USA) was used to evaluate cell proliferation, according to the instructions of the supplier. A cell suspension of 5 × 104 cells was seeded in each well of E-plate 16 in quadruple, cultured in complete MEM and maintained in a CO2 incubator at 37 °C with 95% humidity and 5.0% carbon dioxide saturation. After 18 h, cells were treated with H2O2 diluted in MEM or with MEM alone as control, and automatically monitored every 5 min for 48 h. Dynamic cell proliferation of cells plated was monitored at 5 min intervals from the time of plating until the end of the experiment, analyzed with RTCA software and expressed as cell index value.
2.11. Measurement of Intracellular Ca2+
HepG2 cells overexpressing SerpinB3 and control HepG2 were seeded onto coverslips (3 × 1 cm) and allowed to grow to confluence. The medium was then changed to medium without serum and the cells were used after 24 h. Before starting the experiments, the cells were loaded with 3 µmol/L Fura-2 AM for 1 h at room temperature. Then, Fura-2 was removed, a physiological medium (containing in mmol/L: NaCl 129, KCl 2.8, KH2PO4 0.8, CaCl2 1, NaHCO3 8.9, MgCl2 0.8, glucose 5.6, HEPES 5.6; pH 7.4) was added, and cells were incubated at room temperature for 30 min. The coverslip was placed into a quartz cuvette (3 mL) inside a fluorescent spectrophotometer (Shimadzu-1501) equipped with a thermostatted cuvette holder and superfused with physiological medium at 37 °C. The baseline fluorescence was obtained by rapidly alternating the excitation wavelength between 340 and 380 nm and recording the 510 nm emission intensity. Ca2+ levels were calculated according to the standard formula: (Ca2+) = Kd (R-Rmin)/(Rmax-R)/(Sf2/Sb2). Kd was taken as 224 nmol/L, and Rmax, Rmin and Sf2/Sb2 were calculated by plotting a calibration curve with buffers containing various Ca2+ concentrations.
2.12. Calcium Retention Capacity (CRC)
The CRC assay has been used to assess the propensity to open of the mitochondrial permeability transition pore (mtPTP), as previously described [
29]. Briefly, HepG2/SB3 and HepG2/Ctrl cells, after diamide treatment, were permeabilized with 100 μM digitonin (15 min, 4 °C) in a 1 mM EGTA buffer. Digitonin was then eliminated and permeabilized cells were placed in 10 μM EGTA in the presence of the Ca
2+ probe Calcium Green-5N (1 µM; λ exc: 505 nm; λ em: 535 nm; Molecular Probes), which does not permeate mitochondria. Cells were then exposed to Ca
2+ spikes (5 μM), and fluorescence drops were used to assess mitochondrial Ca
2+ uptake using a Fluoroskan Ascent FL (Thermo Electron Corp. Thermo Fisher, Waltham, MA USA) plate reader. PTP opening was detected as a sudden and irreversible fluorescence increase due to Ca
2+ release from mitochondria.
2.13. Murine Experimental Models
p66Shc Knockout Mice. The p66Shc knockout mice, corresponding to the ShcP strain (provided by M. Giorgio, Department of Biomedical Sciences, University of Padova, Padova, Italy), a recognized model of long survival [
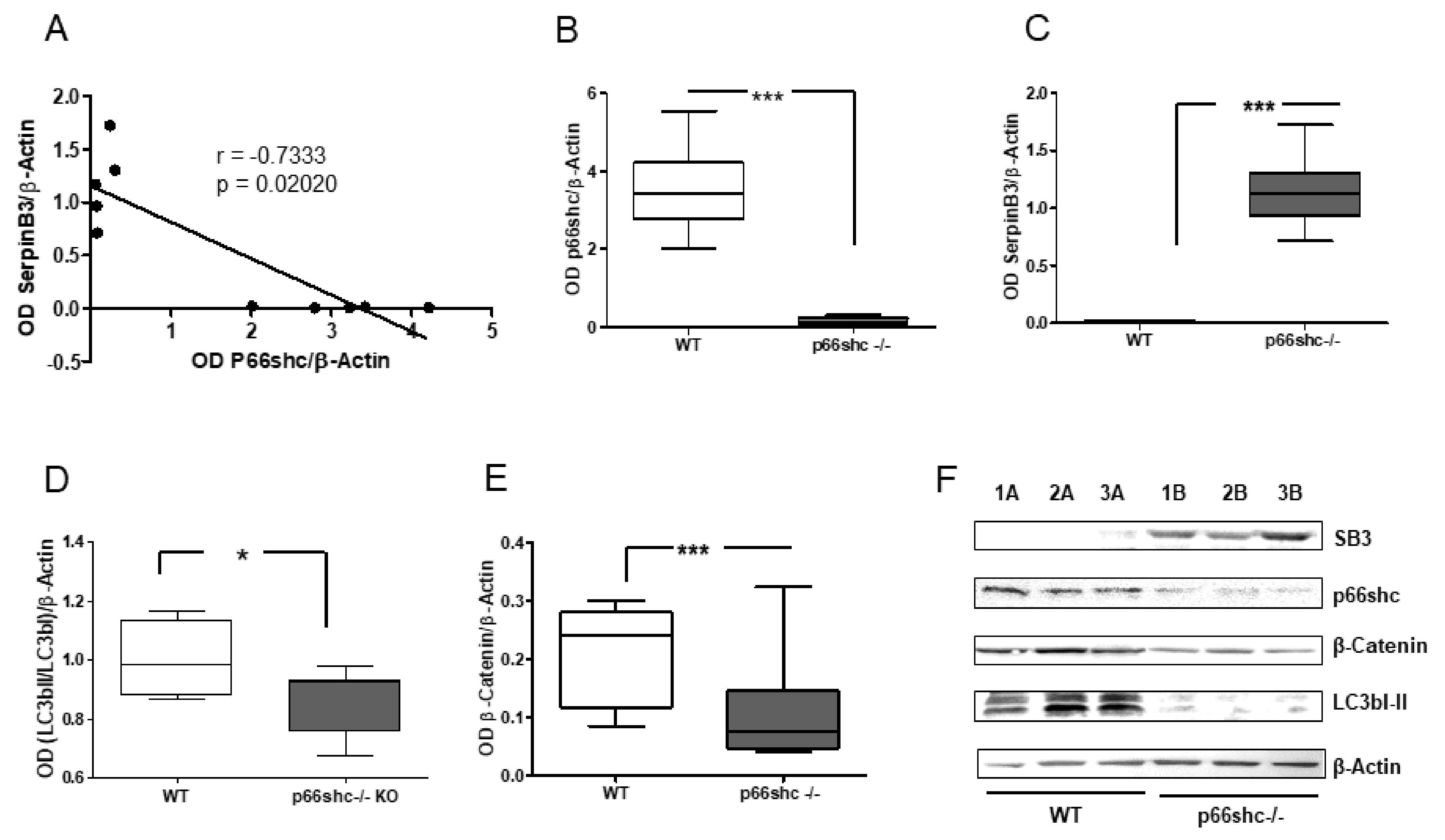
4], and the corresponding wild-type C57BL/6 mice strain were bred at the animal facility of the Venetian Institute of Molecular Medicine (Padua, Italy). The liver of 16 females, 18–24-week-old mice was used to investigate the p66shc extent of expression in relation to different molecules involved in cell death and survival, including SerpinB3, β-catenin and LC3b.
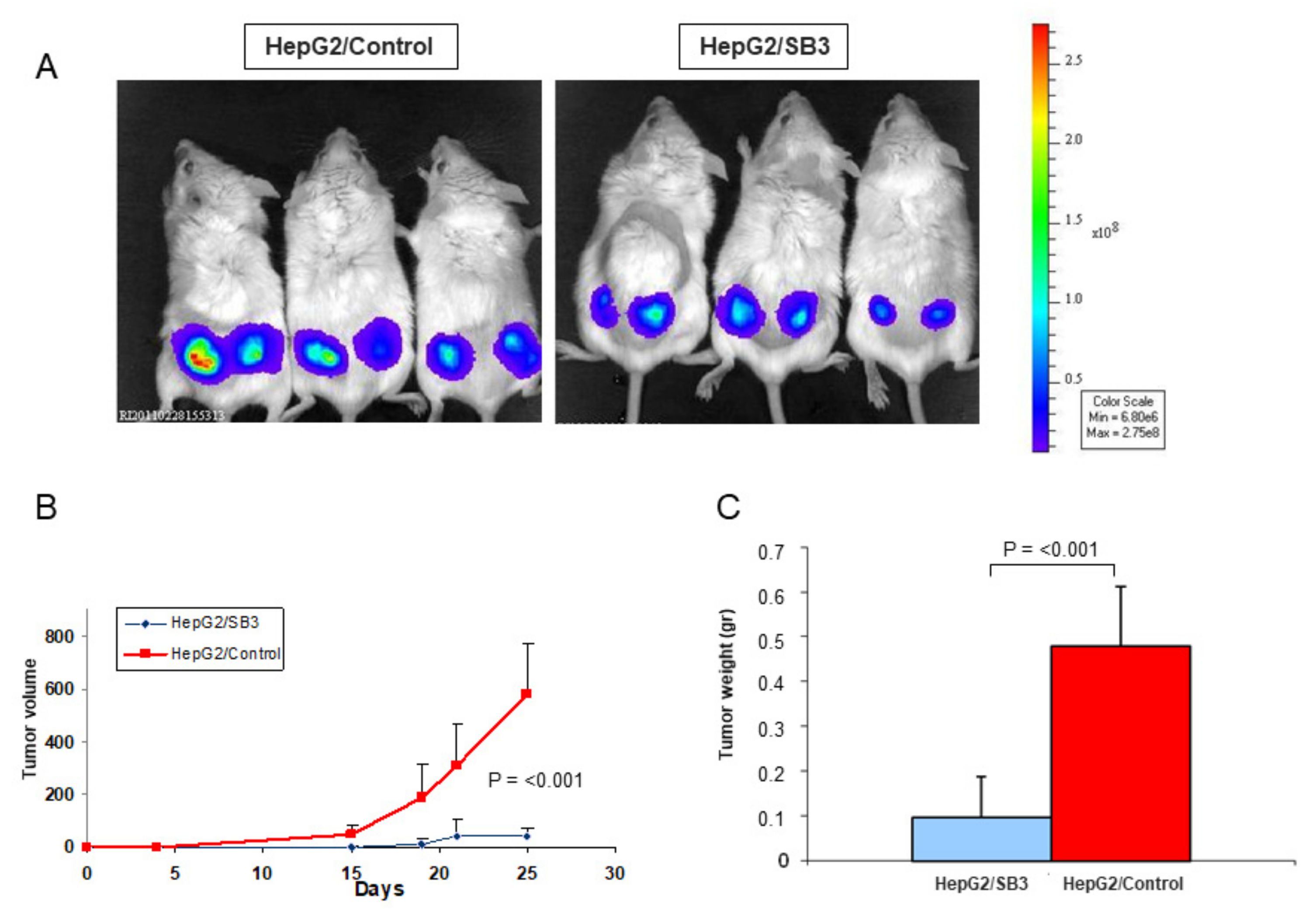
Xenograft Models. To assess the effect of SerpinB3 overexpression on tumor growth, 26 Scid mice (carrying a genetic immune deficiency that affects B and T cells) and 12 Rag-c57 mice (depleted of NK cells) were injected subcutaneously with HepG2/SB3 cells or HepG2/control cells, previously transfected with luciferase, at 1 × 106 concentration (right- and left-hand flanks). Tumor growth was monitored weekly using a BioLuminescent Imaging instrument. Mice were sacrificed at day 25after injection, tumors were removed and morphological and molecular analyses were carried out.
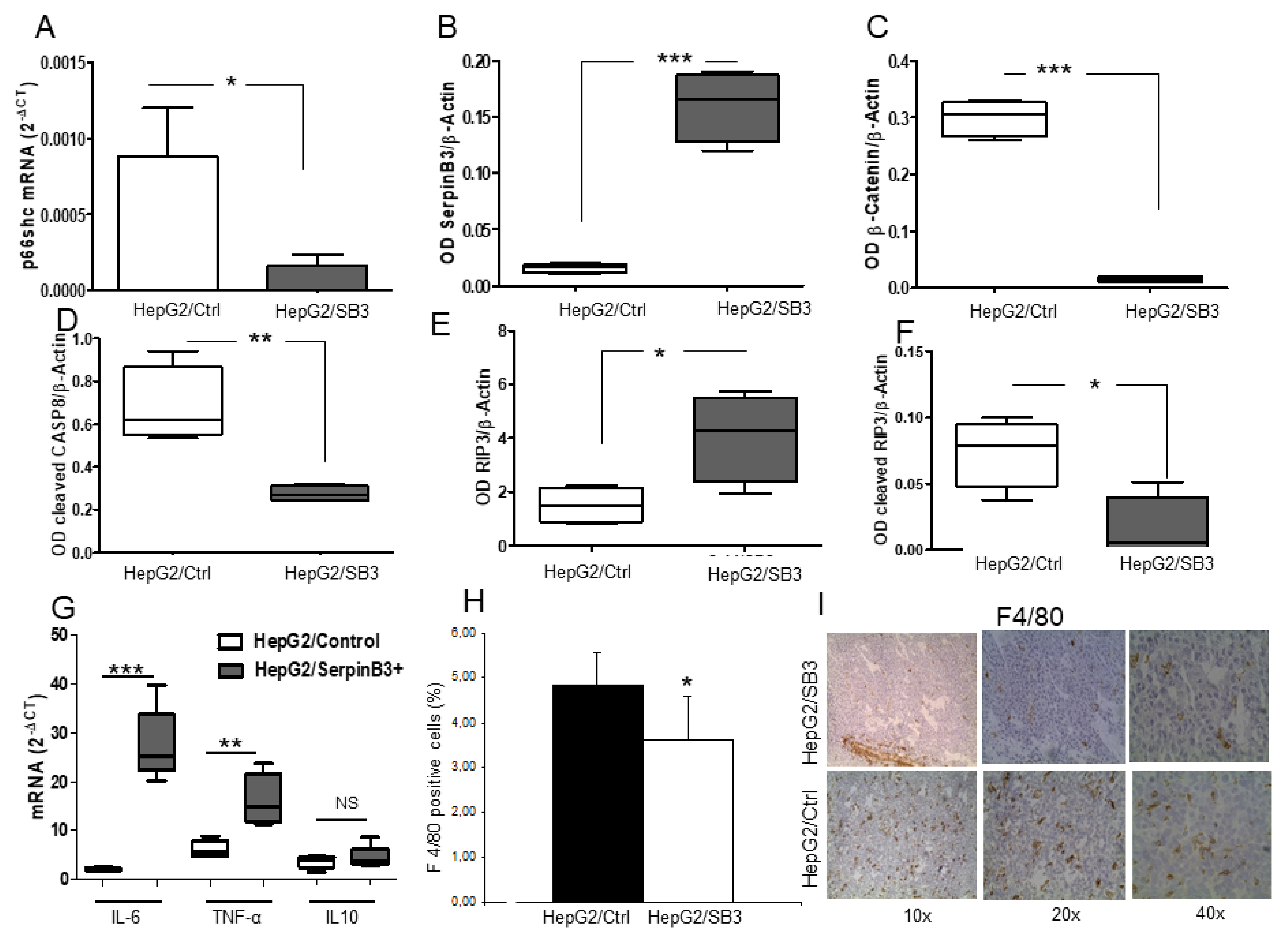
Expression of p66shc, SerpinB3, pro-inflammatory (IL-6 and TNF-α) and anti-inflammatory (IL-10) cytokines were analyzed by real-time PCR. The macrophage marker F4/80 was evaluated by immunohistochemistry. Protein expression of p66shc, SerpinB3, β-Catenin, activated Caspase-8, whole and cleaved RIP3K were investigated by Western blot.
All the experiments, conducted in accordance with the “Principles of laboratory animal care” (NIH publication no. 85–23, revised 1985;
http://grants1.nih.gov/grants/olaw/references/phspol.htm (accessed on 10 January 2011)), were approved by the local ethical committee and the Italian Ministry of Health.
2.14. Statistical Analysis
Statistical analysis was performed by Student’s t-test or ANOVA for analysis of variance when appropriate (p < 0.05 was considered significant). Spearman’s rank correlation was used to measure statistical dependence between two variables. For molecular variables a cut-off value ≥ median value was defined as “high-mRNA expression” to be considered as a dichotomous categorical variable, while cases with values < median value, were defined as “low-mRNA expression”. The overall time of survival curves was calculated using the Kaplan–Meier method and compared using the log-rank test. Data in bar graphs represent means ± SEM and were obtained from at least three independent experiments. Western-blot and morphological images are representative of at least three experiments with similar results.
4. Discussion
In this study we have analyzed the relationship between p66shc and SerpinB3 expression in hepatocellular carcinoma in relation to clinical outcome and its potential involvement in cell death. Both molecules have been shown to control cell death with opposite effects, although p66shc, known for its pro-apoptotic function due to ROS production [
4,
5], has been recently identified as an important factor implicated in the regulation of the autophagic process, which may rather lead to lymphocyte survival [
12]. In the absence of p66shc the autophagic flux is impaired and lower levels of LC3II are observed, as also detected in our
p66shc−/− mice, where SerpinB3 was found to be overexpressed. SerpinB3 is an anti-apoptotic molecule commonly detected in tumors with worse survival, including HCC [
20,
21,
22].
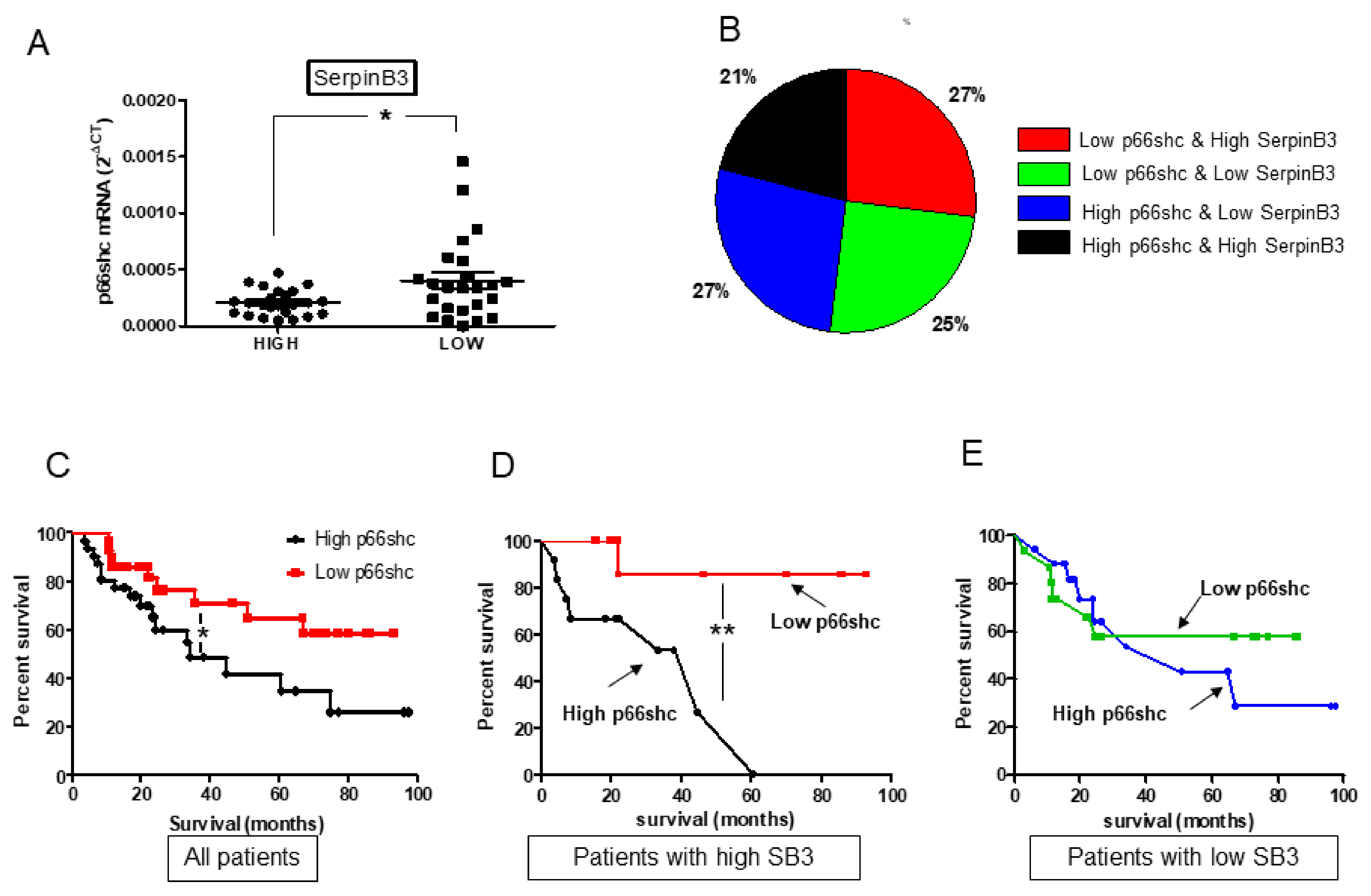
In our cohort of patients with HCC, p66shc has been found to be associated with the clinical outcome. The worst overall survival was observed when p66shc was highly expressed, as already shown in previous studies [
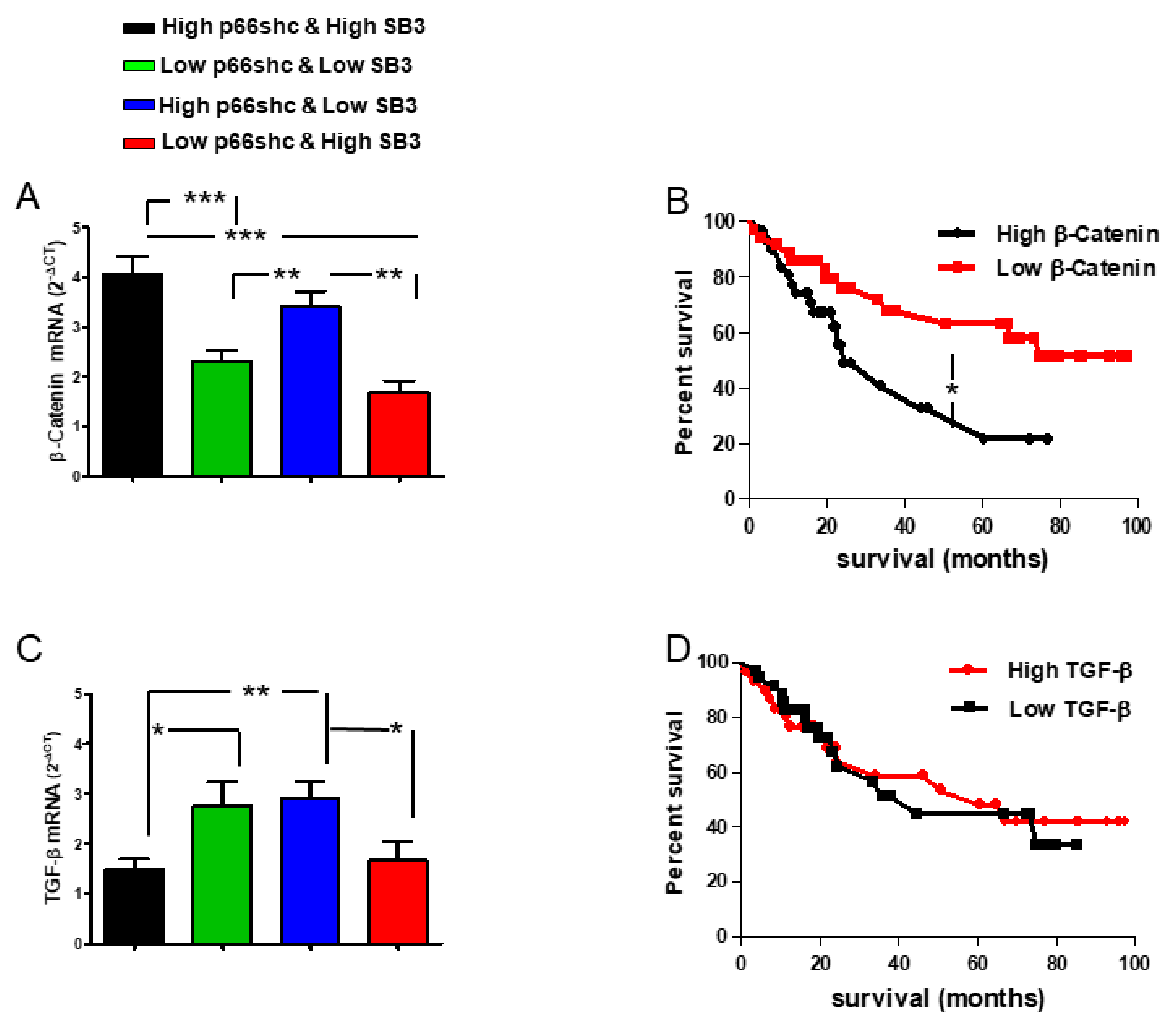
12]. Matching different levels of expression of p66shc and SerpinB3, we identified two subgroups of patients characterized by a clearly distinct behavior in terms of survival rate. The worst survival rate was observed when both p66shc and SerpinB3 were highly expressed and this pattern was associated with the concomitant presence of high levels of β-catenin, notoriously involved in malignant transformation, often in parallel with SerpinB3 [
20,
22,
30]. This subgroup was also characterized by low levels of TGF-β1 that were similar to those observed in the subgroup of patients with the best prognosis where, however, the high levels of SerpinB3 were associated with low p66shc expression. These findings are in keeping with the fact that β-catenin, but not TGF-β1, was differently expressed in relation to patients’ survival.
The sharp difference in clinical outcome observed in surgically resected patients with high levels of SerpinB3, but different extent of p66shc expression in tumoral tissue, has potentially interesting implications for management of patients with HCC, since different therapeutic approaches could be proposed based on the expression pattern of the two molecules. For example, liver transplantation could be considered especially for those patients with high levels of both SerpinB3 and p66shc.
On the basis of these premises, we have investigated how the p66shc/SerpinB3 relationship could affect cell death. In cultured cell lines, as also observed in
p66shc−/− mice, an inverse relationship between the presence of SerpinB3 and p66shc was documented. In HepG2 cells constitutively expressing p66shc, this molecule actually was markedly reduced when enforced SerpinB3 expression was induced. When cells overexpressing SerpinB3 and low levels of p66shc were exposed to different extents of oxidative stress conditions, an opposite behavior was observed in relation to ROS concentration. Indeed, they were more resistant to apoptosis at low H
2O
2 concentration, as previously reported [
16], but, surprisingly, these cells were more prone to die compared to control cells expressing low SerpinB3 and high p66shc levels, in the presence of a high concentration of pro-oxidant stimuli such as H
2O
2 and diamide. Higher Ca
2+ concentrations were detected, which may act as a trigger of the mitochondrial permeability transition pore (mtPTP), resulting in earlier cell death [
29]. In line with this prediction, when subcutaneously injected into the xenograft model, cells overexpressing SerpinB3 and low p66shc induced smaller tumors that also showed trivial levels of β-catenin, supporting the better prognosis observed in the subgroup of patients with a similar pattern to these two molecules. In addition, these tumors were characterized by inhibition of Caspase-8 activity that, in a proinflammatory background, could make cells more sensitive to necroptosis, a recently described TNF-α induced form of programmed cell death [
31]. The high levels of TNF-α, the accumulation of receptor-interacting protein kinase-3 (RIP3K) and its lack of cleavage by Caspase-8 in mice injected with HepG2/SB3 provide evidence of necroptotic death in this experimental model, highlighting its protective function in slowing down tumor growth.
This scenario suggests a novel beneficial function of SerpinB3 through induction of cell death by necroptosis when p66shc is downregulated in conditions of oxidative stress. Further studies are required to better understand the microenvironment and cellular factors responsible for the different expression profiles of these molecules found in tumor specimens. Cancer cells often acquire the ability to evade cell death induced by chemotherapeutic agents, and induction of necroptosis could represent an efficient way to overcome cell-death resistance [
32].