Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review
Abstract
Simple Summary
Abstract
1. Introduction
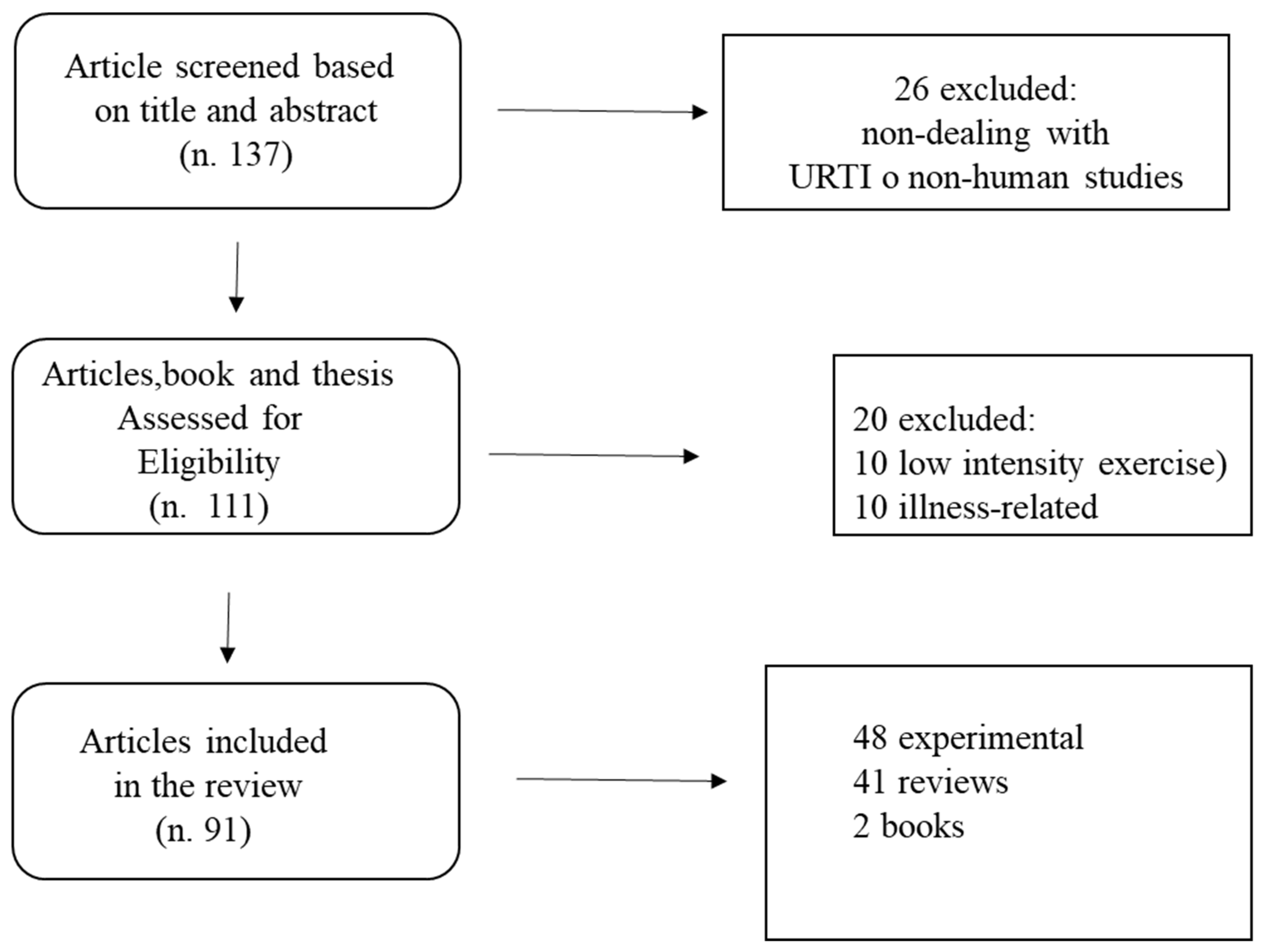
2. Methods
3. Results
3.1. Sport and Upper Respiratory Tract Infections
3.2. Susceptibility to URTI and the “Open Window” Theory
4. Biochemical Correlates of URTI
4.1. Environmental Factors
4.2. Nutrition and URTI
4.3. Carbohydrates
4.4. Fatty Acids
4.5. Vitamin D
4.6. Other Nutrients
5. Strategies to Boost Immune System in Heavily Trained Athletes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Available online: https://www.iedb.org/ (accessed on 23 April 2021).
- Bermon, S. La pratique du sport a-t-elle un impact sur l’état immunitaire ? [Does sport practice have an impact on the immune status?]. Rev. Prat. 2020, 70, 427–431. [Google Scholar]
- Gleeson, M.; Pyne, D.B. Respiratory inflammation and infections in high-performance athletes. Immunol. Cell Biol. 2016, 94, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Dubnov-Raz, G.; Hemilä, H.; Cohen, A.H.; Rinat, B.; Choleva, L.; Constantini, N.W.; Hemilae, H. Vitamin D Supplementation and Upper Respiratory Tract Infections in Adolescent Swimmers: A Randomized Controlled Trial. Pediatr. Exerc. Sci. 2015, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Immune system adaptation in elite athletes. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Atias-Varon, D.; Heled, Y. Strenous and prolonged exercise and upper respiratory tract infection -treatment or threat? Harefuah 2017, 156, 730–734. [Google Scholar] [PubMed]
- Burtscher, J.; Burtscher, M.; Millet, G.P. (Indoor) isolation, stress, and physical inactivity: Vicious circles accelerated by COVID-19? Scand. J. Med. Sci. Sports 2020, 30, 1544–1545. [Google Scholar] [CrossRef]
- Totman, R.; Kiff, J.; Reed, S.E.; Craig, J.W. Predicting experimental colds in volunteers from different measures of recent life stress. J. Psychosom. Res. 1980, 24, 155–163. [Google Scholar] [CrossRef]
- Broadbent, D.E.; Broadbent, M.H.P.; Phillpotts, R.; Wallace, J. Some further studies on the prediction of experimental colds in volunteers by psychological factors. J. Psychosom. Res. 1984, 28, 511–523. [Google Scholar] [CrossRef]
- Tayech, A.; Mejri, M.A.; Makhlouf, I.; Mathlouthi, A.; Behm, D.G.; Chaouachi, A. Second Wave of COVID-19 Global Pandemic and Athletes’ Confinement: Recommendations to Better Manage and Optimize the Modified Lifestyle. Int. J. Environ. Res. Public Heal. 2020, 17, 8385. [Google Scholar] [CrossRef]
- Costa, S.; Santi, G.; Di Fronso, S.; Montesano, C.; Di Gruttola, F.; Ciofi, E.G.; Morgilli, L.; Bertollo, M. Athletes and adversities: Athletic identity and emotional regulation in time of COVID-19. Sport Sci. Heal. 2020, 16, 609–618. [Google Scholar] [CrossRef]
- Valtonen, M.; Waris, M.; Vuorinen, T.; Eerola, E.; Hakanen, A.J.; Mjosund, K.; Grönroos, W.; Heinonen, O.J.; Ruuskanen, O. Common cold in Team Finland during 2018 Winter Olympic Games (PyeongChang): Epidemiology, diagnosis including molecular point-of-care testing (POCT) and treatment. Br. J. Sports Med. 2019, 53, 1093–1098. [Google Scholar] [CrossRef]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fit. 1990, 30, 316–328. [Google Scholar]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; E Turner, J.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W. Infectious episodes in runners before and after a roadrace. J. Sports Med. Phys. Fit. 1989, 29, 289–296. [Google Scholar]
- Peters, E.M.; Bateman, E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. South Afr. Med. J. 1983, 64, 582–584. [Google Scholar]
- Van Tonder, A.; Schwellnus, M.P.; Swanevelder, S.; Jordaan, E.; Derman, W.; Van Rensburg, D.C.J. A prospective cohort study of 7031 distance runners shows that 1 in 13 report systemic symptoms of an acute illness in the 8–12 day period before a race, increasing their risk of not finishing the race 6 times for those runners who started the race: SAFER study IV. Br. J. Sports Med. 2016, 50, 939–945. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nieman, D.C. Upper respiratory tract infections and exercise. Thorax 1995, 50, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J.; Shek, P.N. Exercise, immunity, and susceptibility to infection-A J-shaped relationship? Phys. Sportsmed. 1999, 27, 47–71. [Google Scholar] [CrossRef]
- Malm, C. Susceptibility to infections in elite athletes: The S-curve. Scand. J. Med. Sci. Sports 2006, 16, 4–6. [Google Scholar] [CrossRef]
- Spence, L.; Brown, W.J.; Pyne, D.B.; Nissen, M.D.; Sloots, T.P.; Mccormack, J.G.; Locke, A.S.; Fricker, P.A. Incidence, Etiology, and Symptomatology of Upper Respiratory Illness in Elite Athletes. Med. Sci. Sports Exerc. 2007, 39, 577–586. [Google Scholar] [CrossRef]
- Martin, S.A.; Pence, B.D.; Woods, J.A. Exercise and Respiratory Tract Viral Infections. Exerc. Sport Sci. Rev. 2009, 37, 157–164. [Google Scholar] [CrossRef]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Callister, R.; Gleeson, M. Cytokine Responses to Treadmill Running in Healthy and Illness-Prone Athletes. Med. Sci. Sports Exerc. 2007, 39, 1918–1926. [Google Scholar] [CrossRef]
- Kurowski, M.; Jurczyk, J.; Moskwa, S.; Jarzebska, M.; Krysztofiak, H.; Kowalski, M.L. Winter ambient training conditions are associated with increased bronchial hyperreactivity and with shifts in serum innate immunity proteins in young competitive speed skaters. Arch. Med. Sci. 2018, 14, 60–68. [Google Scholar] [CrossRef]
- Batatinha, H.A.; Biondo, L.A.; Lira, F.S.; Castell, L.M.; Rosa-Neto, J.C. Nutrients, immune system, and exercise: Where will it take us? Nutrition 2019, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Hinojosa, A.; Knight, A.; Compton, C.; Gleeson, M.; Travers, P.J. Reduced thymic output in elite athletes. Brainbehav. Immun. 2014, 39, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Ader, R.; Green, N.; Bovbjerg, D. Conditioned Suppression of a Thymus-Independent Antibody Response*. Psychosom. Med. 1979, 41, 487–491. [Google Scholar] [CrossRef]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine 2018, 104, 136–142. [Google Scholar] [CrossRef]
- Shubin, N.J.; Clauson, M.; Niino, K.; Kasprzak, V.; Tsuha, A.; Guga, E.; Bhise, G.; Acharya, M.; Snyder, J.M.; Debley, J.S.; et al. Thymic stromal lymphopoietin protects in a model of airway damage and inflammation via regulation of caspase-1 activity and apoptosis inhibition. Mucosal Immunol. 2020, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Cicchella, A.; Zini, M.; Paolini, M.; Tiberini, P.; Stefanelli, C. Cycling training effects on fat metabolism blood parameters. Gazz. Med. Ital. Arch. Per Le Sci. Med. 2020, 179, 104–109. [Google Scholar] [CrossRef]
- Nieman, D.C. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports Exerc. 1994, 26, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xu, M.-X.; Wang, Z.-W.; Han, Z.-N.; Dong, Y.-N.; Zhao, J.-X. Effect of aerobic exercise and different levels of fine particulate matter (PM2.5) on pulmonary response in Wistar rats. Life Sci. 2020, 254, 117355. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrition 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef]
- Burke, L.M. Practical Sports Nutrition; Human Kinetics Publishers: Champaign, IL, USA, 2007. [Google Scholar]
- Deldicque, L.; Francaux, M. Potential harmful effects of dietary supplements in sports medicine. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Ardawi, M.M. Glutamine and glucose metabolism in human peripheral lymphocytes. Metabolism 1988, 37, 99–103. [Google Scholar] [CrossRef]
- Graham, J.E.; Christian, L.M.; Kiecolt-Glaser, J.K. Stress, Age, and Immune Function: Toward a Lifespan Approach. J. Behav. Med. 2006, 29, 389–400. [Google Scholar] [CrossRef]
- Bishop, N.C.; Walsh, N.P.; Haines, D.L.; Richards, E.E.; Gleeson, M. Pre-Exercise Carbohydrate Status and Immune Responses to Prolonged Cycling: II. Effect on Plasma Cytokine Concentration. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 503–512. [Google Scholar] [CrossRef]
- Bishop, N.C.; Walsh, N.P.; Haines, D.L.; Richards, E.E.; Gleeson, M. Pre-exercise carbohydrate status and immune responses to prolonged cycling: I. Effect on neutrophil degranulation. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 490–502. [Google Scholar] [CrossRef]
- Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Nieman, D.C.; Henson, D.A.; Butterworth, D.E.; Schmitt, R.L.; Bailey, E.M.; Warren, B.J.; Utter, A.; Davis, J.M. Carbohydrate and the cytokine response to 2.5 h of running. J. Appl. Physiol. 1997, 82, 1662–1667. [Google Scholar] [CrossRef]
- Bishop, N.C.; Walsh, N.P.; Scanlon, G.A. Effect of Prolonged Exercise and Carbohydrate on Total Neutrophil Elastase Content. Med. Sci. Sports Exerc. 2003, 35, 1326–1332. [Google Scholar] [CrossRef]
- Scharhag, J.; Meyer, T.; Gabriel, H.; Auracher, M.; Kindermann, W. Mobilization and oxidative burst of neutrophils are influenced by carbohydrate supplementation during prolonged cycling in humans. Eur. J. Appl. Physiol. 2002, 87, 584–587. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Khan, Q.; Drysdale, P.T.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J. Appl. Physiol. 2005, 98, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.C.; Walker, G.J.; Bowley, L.A.; Evans, K.F.; Molyneux, K.; Wallace, F.A.; Smith, A.C. Lymphocyte responses to influenza and tetanus toxoid in vitro following intensive exercise and carbohydrate ingestion on consecutive days. J. Appl. Physiol. 2005, 99, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Fagoaga, O.R.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Nehlsen-Cannarella, S.L. Change in Salivary IgA Following a Competitive Marathon Race. Int. J. Sports Med. 2001, 23, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Calabriso, N.; Carluccio, M.A.; Hugenholtz, P.; De Caterina, R. Chapter 6 -Nutrients and Gene Expression in Cardiovascular Disease. In Principles of Nutrigenetics and Nutrigenomics; Caterina, R.D.E., Martinez, J.A., Kohlmeier, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 469–481. [Google Scholar]
- De Caterina, R. n–3 Fatty Acids in Cardiovascular Disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta (Bba)-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Bannenberg, G.; Serhan, C.N. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys. Acta 2010, 1801, 1260–1273. [Google Scholar] [CrossRef]
- Massaro, M.; Habib, A.; Lubrano, L.; Turco, S.D.; Lazzerini, G.; Bourcier, T.; Weksler, B.B.; De Caterina, R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKCε inhibition. Proc. Natl. Acad. Sci. USA 2006, 103, 15184–15189. [Google Scholar] [CrossRef]
- Hou, T.Y.; McMurray, D.N.; Chapkin, R.S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. 2016, 785, 2–9. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clin. J. Sport Med. 2009, 19, 115–119. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Corder, K.E.; Newsham, K.R.; McDaniel, J.L.; Ezekiel, U.R.; Weiss, E.P. Effects of Short-Term Docosahexaenoic Acid Supplementation on Markers of Inflammation after Eccentric Strength Exercise in Women. J. Sports Sci. Med. 2016, 15, 176–183. [Google Scholar] [PubMed]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef]
- DiLorenzo, F.M.; Drager, C.J.; Rankin, J.W. Docosahexaenoic acid affects markers of inflammation and muscle damage after eccentric exercise. J. Strength Cond. Res. 2014, 28, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Gabriel, B.M.; Gray, P.; Gray, S.R. The Effect of Fish Oil, Vitamin D and Protein on URTI Incidence in Young Active People. Int. J. Sports Med. 2015, 36, 426–430. [Google Scholar] [CrossRef][Green Version]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Nurminen, V.; Seuter, S.; Carlberg, C. Primary Vitamin D Target Genes of Human Monocytes. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- He, C.S.; Aw Yong, X.H.; Walsh, N.P.; Gleeson, M. Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 2016, 22, 42–64. [Google Scholar]
- Sabetta, J.R.; DePetrillo, P.; Cipriani, R.J.; Smardin, J.; Burns, L.A.; Landry, M.L. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE 2010, 5, e11088. [Google Scholar] [CrossRef]
- Halliday, T.M.; Peterson, N.J.; Thomas, J.J.; Kleppinger, K.; Hollis, B.W.; Larson-Meyer, D.E. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med. Sci. Sports Exerc. 2011, 43, 335–343. [Google Scholar] [CrossRef]
- He, C.S.; Handzlik, M.; Fraser, W.D.; Muhamad, A.; Preston, H.; Richardson, A.; Gleeson, M. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 2013, 19, 86–101. [Google Scholar] [PubMed]
- He, C.S.; Fraser, W.D.; Tang, J.; Brown, K.; Renwick, S.; Rudland-Thomas, J.; Teah, J.; Tanqueray, E.; Gleeson, M. The effect of 14 weeks of vitamin D3 supplementation on antimicrobial peptides and proteins in athletes. J. Sports Sci. 2016, 34, 67–74. [Google Scholar] [CrossRef]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neal, R.E. Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef] [PubMed]
- Scullion, L.; Baker, D.; Healey, P.; Edwards, A.; Love, T.; Black, K. No Association between Vitamin D and Acute Respiratory Tract Infections Amongst Elite New Zealand Rugby Players and Rowers. Int. J. Vitam. Nutr. Res. Suppl. 2018, 88, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cannon, G.; Meydani, S.A.; Fielding, R.A.; Fiatarone, M.A.; Meydani, M.; Farhangmehr, M.; Orencole, S.F.; Blumberg, J.B.; Evans, W.J. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am. J. Physiol. 1991, 260, R1235–R1240. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Henson, D.A.; Sha, W.; Shanely, R.A.; Knab, A.M.; Cialdella-Kam, L.; Jin, F. Bananas as an energy source during exercise: A metabolomics approach. PLoS ONE 2012, 7, e37479. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S.; Petriz, B.; Kajėnienė, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar] [PubMed]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.; Nieman, D.C.; Bermon, S.; Peeling, P. Exercise-Induced Illness and Inflammation: Can Immunonutrition and Iron Help? Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 181–188. [Google Scholar] [CrossRef]
- Brodersen, K. Philostratos: Sport in der Antike (Peri Gymnastikes/Über das Training); Marix: Wiesbaden, Germany, 2015. [Google Scholar]
- Buguet, A.; Cespuglio, R.; Radomski, M.W. Sleep and stress in man: An approach through exercise and exposure to extreme environments. Can. J. Physiol. Pharm. 1998, 76, 553–561. [Google Scholar] [CrossRef]
- Kakanis, M.W.; Peake, J.; Brenu, E.W.; Simmonds, M.; Gray, B.; Hooper, S.L.; Marshall-Gradisnik, S.M. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc. Immunol. Rev. 2010, 16, 119–137. [Google Scholar] [CrossRef]
- Tomiyama, C.; Watanabe, M.; Honma, T.; Inada, A.; Hayakawa, T.; Ryufuku, M.; Abo, T. The effect of repetitive mild hyperthermia on body temperature, the autonomic nervous system, and innate and adaptive immunity. Biomed. Res. 2015, 36, 135–142. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Laukkanen, T.; Kunutsor, S.K. Cardiovascular and Other Health Benefits of Sauna Bathing: A Review of the Evidence. Mayo Clin. Proc. 2018, 93, 1111–1121. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef]
- Major, B.; Rattazzi, L.; Brod, S.; Pilipović, I.; Leposavić, G.; D’Acquisto, F. Massage-like stroking boosts the immune system in mice. Sci. Rep. 2015, 5, 109–113. [Google Scholar] [CrossRef]
- Ang, J.Y.; Lua, J.L.; Mathur, A.; Thomas, R.; Asmar, B.I.; Savasan, S.; Buck, S.; Long, M.; Shankaran, S.A. Randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics 2012, 130, e1549–e1558. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P. Recommendations to maintain immune health in athletes. Eur. J. Sport Sci. 2018, 18, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thapliyal, A.; Chandra, T.; Singh, S.; Baduni, H.; Waheed, S.M. Rhythmic breathing: Immunological, biochemical, and physiological effects on health. Adv. Mind Body Med. 2015, 29, 18–25. [Google Scholar] [PubMed]
- Carter, K.S.; Carter, R., 3rd. Breath-based meditation: A mechanism to restore the physiological and cognitive reserves for optimal human performance. World J. Clin. Cases 2016, 16, 99–102. [Google Scholar] [CrossRef]
- Maund, P.R.; Irvine, K.N.; Reeves, J.; Strong, E.; Cromie, R.; Dallimer, M.; Davies, Z.G. Wetlands for Wellbeing: Piloting a Nature-Based Health Intervention for the Management of Anxiety and Depression. Int. J. Environ. Res. Public. Health 2019, 16, 4413. [Google Scholar] [CrossRef]
- Slavich, G.M. Social Safety Theory: A Biologically Based Evolutionary Perspective on Life Stress, Health, and Behavior. Annu. Rev. Clin. Psychol. 2020, 7. [Google Scholar] [CrossRef]
| Author | Biochemical Parameter Measured | Population and or Treatment | Major Findings |
|---|---|---|---|
| Cox et al. 2007 [23] | IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, and IL-1-RA | Illness prone vs. non illness prone athletes | IL-8, IL-10, IL-1RA lower at rest in illness-prone runners IL-10, 1-RA lower post exercise IL-6 strongly elevated post exercise |
| Kurowski et al. 2018 [24] | HSPA1,IL-1RA sCD14 | Speed skaters | sCD14 and IL-1RA elevated in skaters vs. control. HSPA1 elevated in winter. |
| Prieto et al. 2014 [26] | TREC, CDT4, CDT3, CDT8, T-cells | Triathletes | Reduced compared to controls. CDT3 unvaried. |
| Bishop et al. 2001 [41] | Cortisol, IL-6, TNFα | Heavy cycling (CHO) | CHO ingestion reduced levels of cortisol and IL-6 but not TNFα |
| Nehlsen et al. 1997 [43] | IL-6, Ll-10, IL-1RA | Marathon (CHO) | CHO ingestion attenuates cytokine levels |
| Cannarella et al. 1997 [44] | IL-6, IL-1RA | Marathoners (CHO) | Lower IL-1RA after run. No increase in IL-1beta and IL-6. |
| Bishop et al. 2003 [45] | Neutrophil elastase content | Cyclists (CHO) | Blood levels Preserved by CHO ingestion |
| Scharhag et al. 2002 [46] | rhodamine(123), neutrophils | Cyclists (CH0) | Dimished after exercise with CHS |
| Lancaster et al. 2004 [47] | IFN-gamma positive CD4 + and CD8+ T lymphocytes | Cyclists (CHO) | CHO prevented the decrease of IFN-γ + CD4 and CD8+ T lymphocytes and the suppression of IFN- γ production |
| Nieman et al. 2002 [48] | Salivary IgA | Marathon (CHO) | The sIgA decreased in runners following a competitive marathon was not influenced by carbohydrate ingestion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicchella, A.; Stefanelli, C.; Massaro, M. Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review. Biology 2021, 10, 362. https://doi.org/10.3390/biology10050362
Cicchella A, Stefanelli C, Massaro M. Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review. Biology. 2021; 10(5):362. https://doi.org/10.3390/biology10050362
Chicago/Turabian StyleCicchella, Antonio, Claudio Stefanelli, and Marika Massaro. 2021. "Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review" Biology 10, no. 5: 362. https://doi.org/10.3390/biology10050362
APA StyleCicchella, A., Stefanelli, C., & Massaro, M. (2021). Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review. Biology, 10(5), 362. https://doi.org/10.3390/biology10050362








