Assessment of the Tissue Response to Modification of the Surface of Dental Implants with Carboxyethylphosphonic Acid and Basic Fibroblastic Growth Factor Immobilization (Fgf-2): An Experimental Study on Minipigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Implants and Surface Treatment
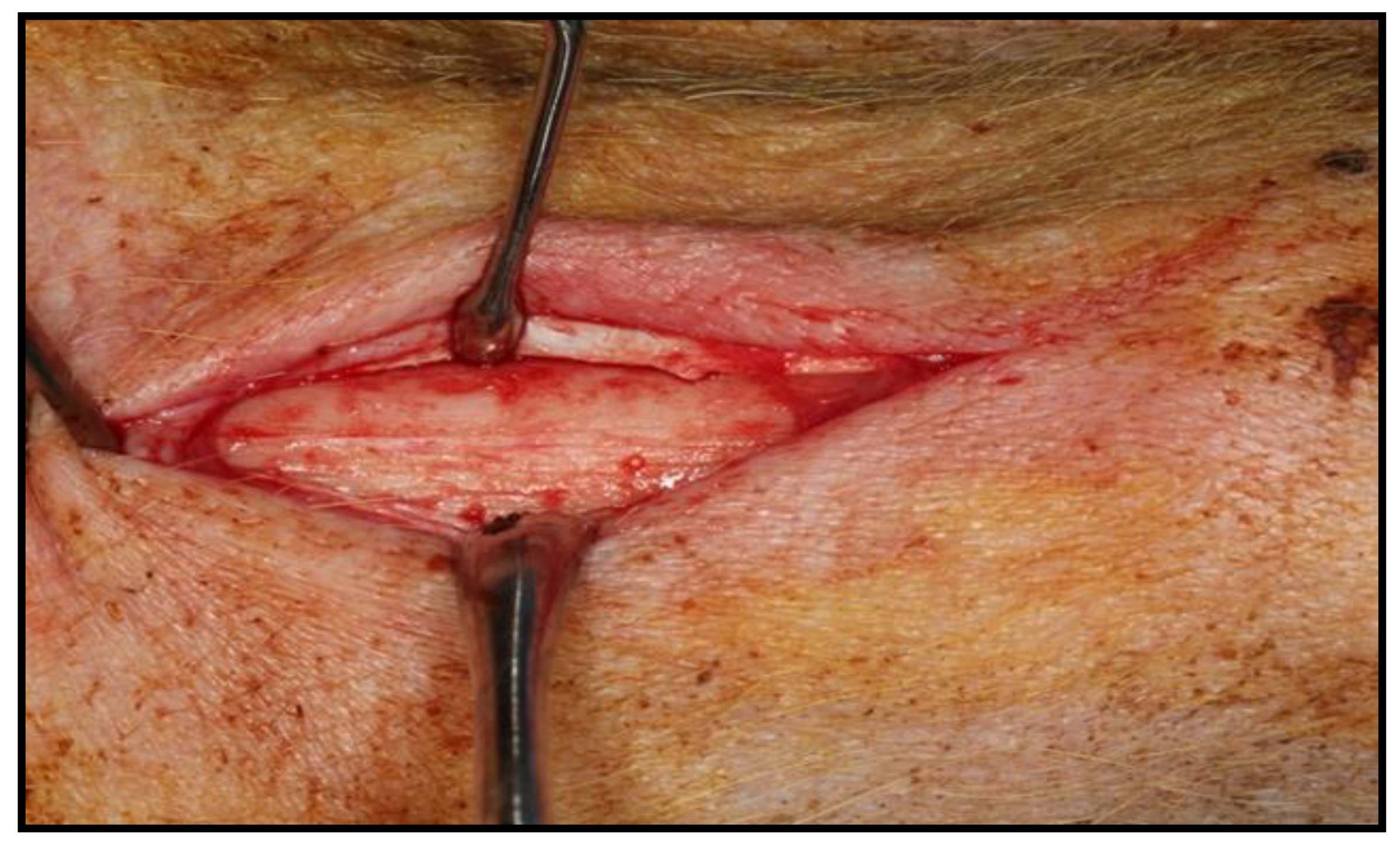
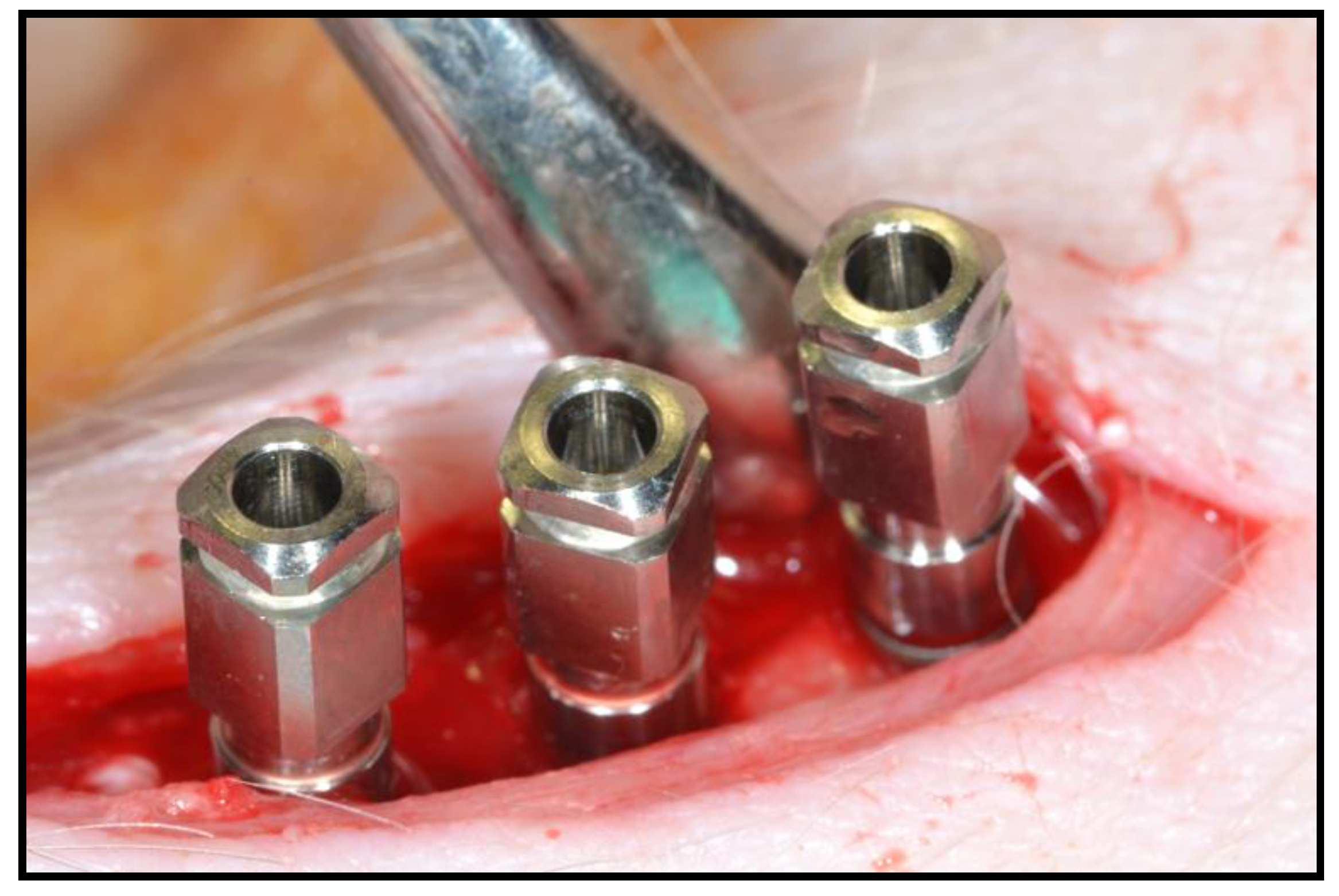
2.3. Surgical Intervention
2.4. Sample Praparation
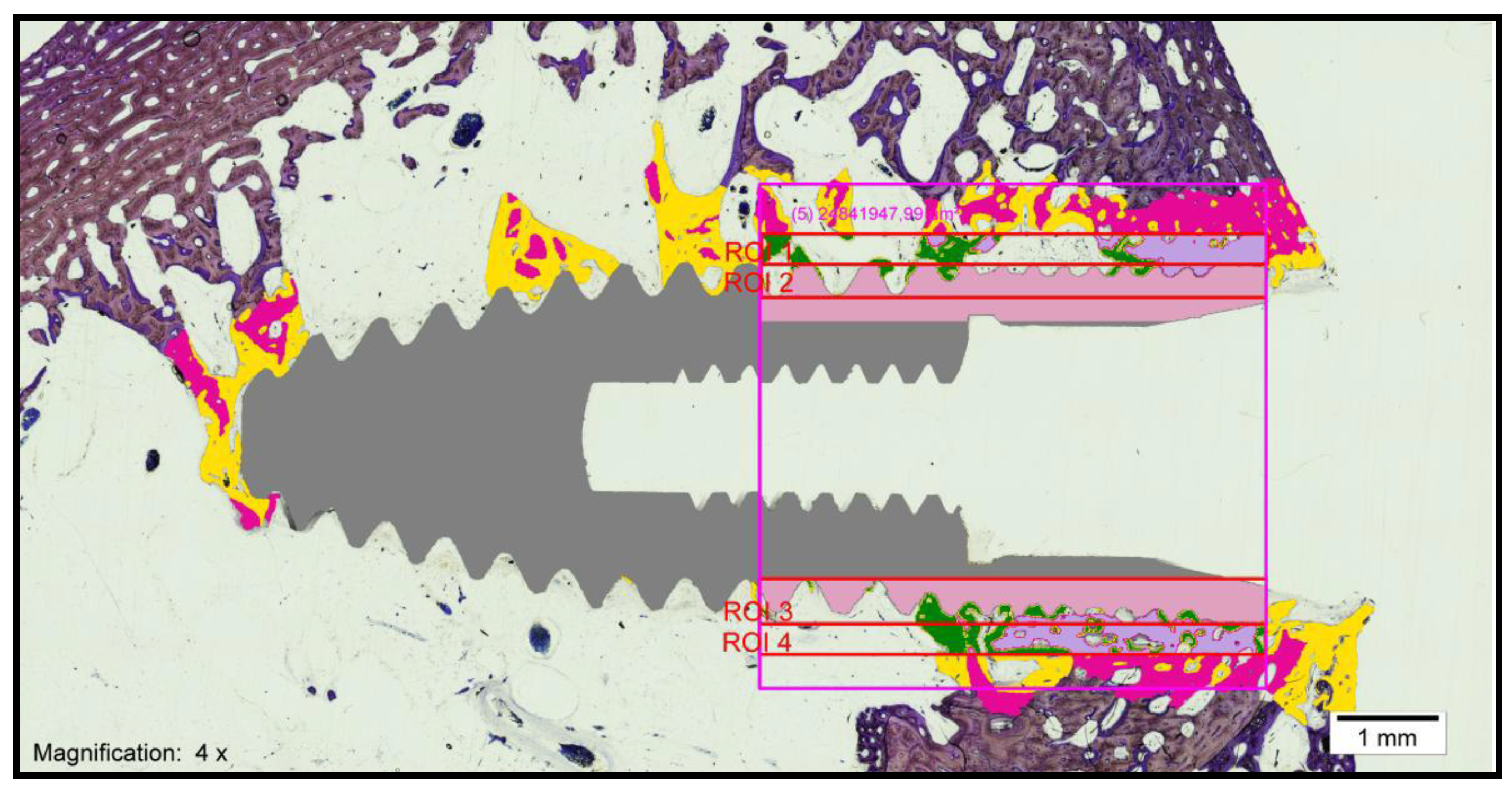
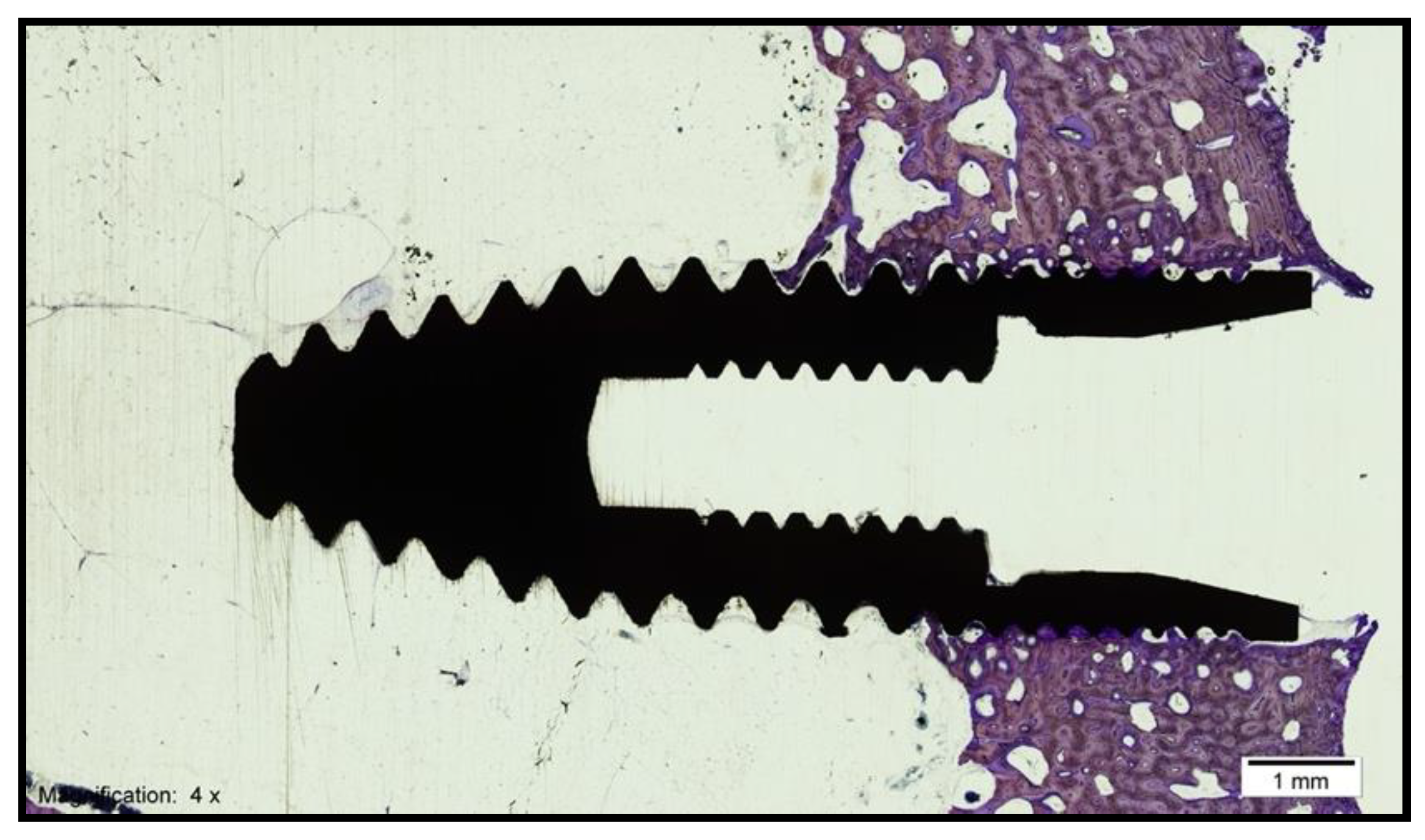
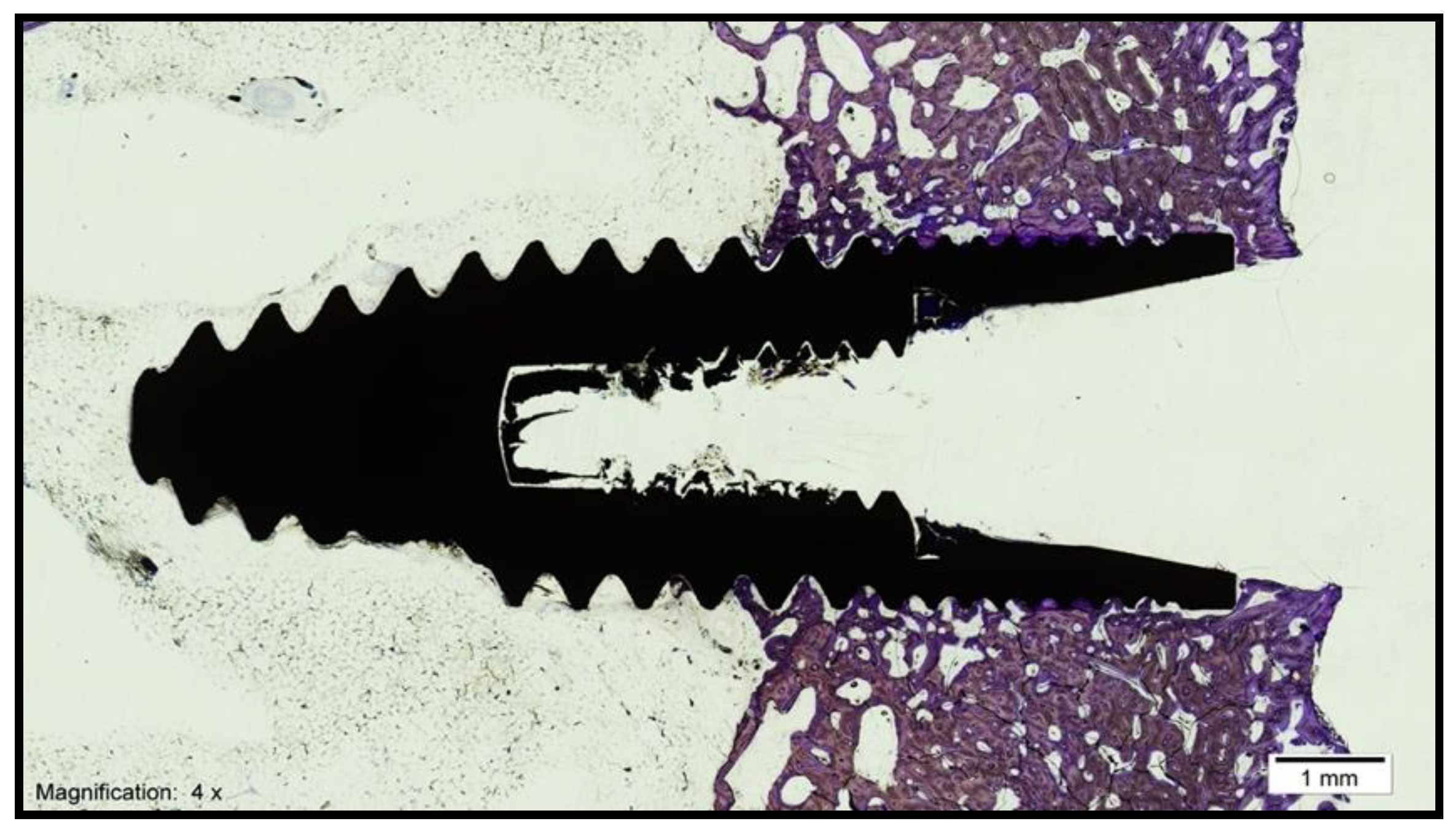
- Bone–implant contact (BIC): percentage of the implant surface in direct contact with the bone (Figure 3).
- Corrected bone–implant contact (BICc): Defined as the length of bone in direct contact with the implant surface concerning the partial perimeter of the implant—that is, eliminating the implant portions not surrounded by bone in the coronal and apical parts of the implant. In this way, implant sections surrounded by tissues other than bone are discarded.
- New bone formation (BV/TV) (bone volume/total volume): Defined as the area of new bone formed after dental implant placement, expressed as a percentage (%). Quantifies the volume of mineralized bone and is generally located between the implant threads and at a distance of up to 300 microns around the implant (peri-implant area).
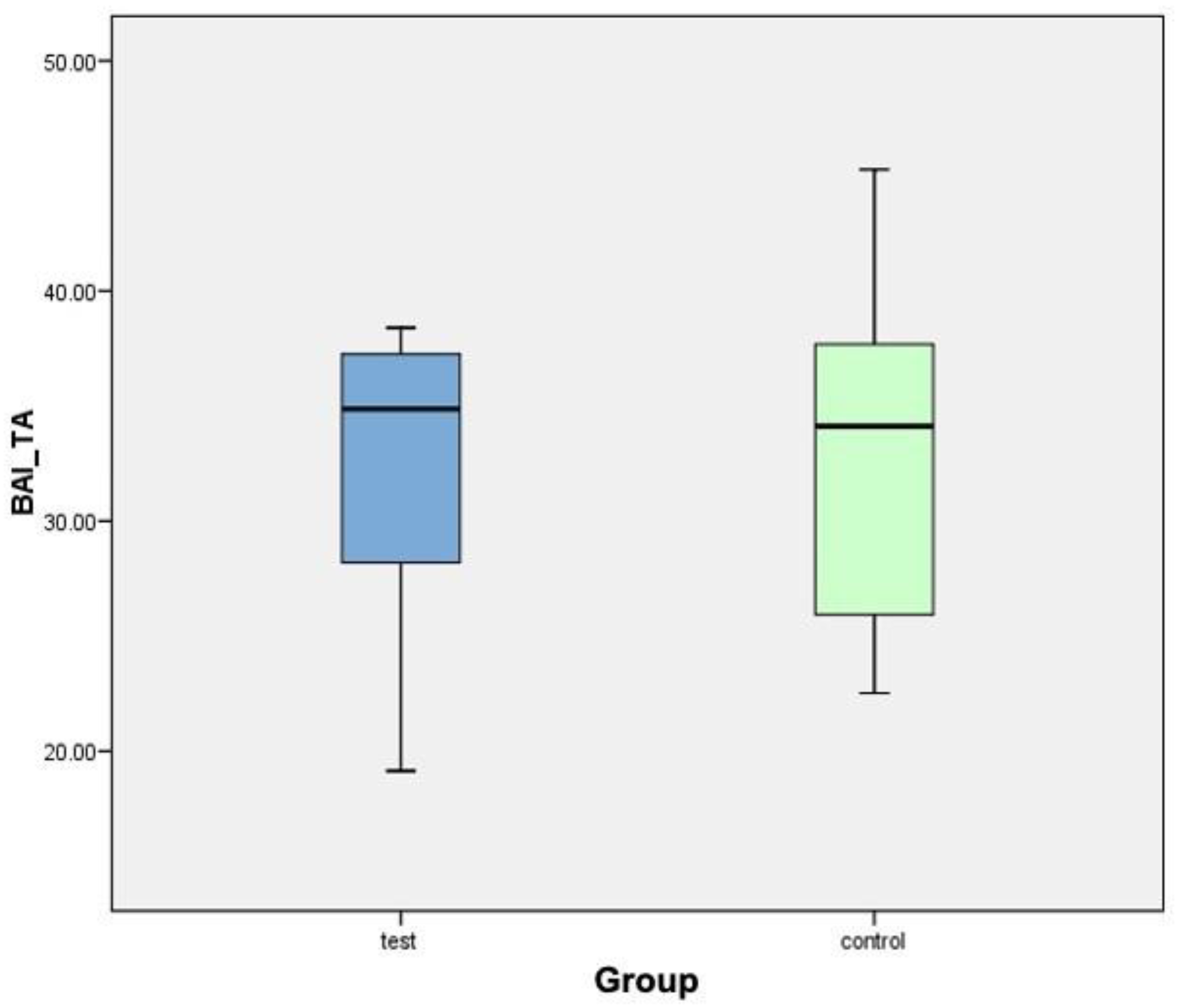
- Interthread bone density (BAI/TA): Defined as the area of bone within the threads of the implant divided by the total area of tissue within them. The final result is multiplied by 100 and expressed as a percentage. In summary, this is the percentage of bone within the interthread portion.
- Peri-implant bone density (BAP/TA): Defined as the bone surface that grows along the length of the implant within the thread in relation to the total available space and the amount of bone in relation to the total surface to a distance of 0.3 mm from the implant. A rectangle, 5 mm long and 300 microns wide, is used in histomorphometry, formed by a line that joins the peaks of the implant threads (parallel to the peak implant thread). Therefore, the peri-implant bone is defined as the area of bone within the rectangle divided by the total area of tissue within the same.
2.5. Statistical Analysis
3. Results
3.1. Histomorphometric Analysis of the Samples
3.1.1. Control Group
3.1.2. Test Group
3.2. Descriptive Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huang, H.-L.; Chang, C.-H.; Hsu, J.-T.; Fallgatter, A.M.; Ko, C.-C. Comparison of implant body designs and threaded designs of dental implants: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2007, 22, 551–562. [Google Scholar]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.-L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implant. Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Strong, J.T.; Bidez, M.W. Fundamentos científicos para el diseño de los implantes dentales. In Implantología Contemporánea; Misch, C.E., Ed.; Elsevier Mosby: Barcelona, Spain, 2009; pp. 200–229. [Google Scholar]
- Baggi, L.; Cappelloni, I.; Di Girolamo, M.; Maceri, F.; Vairo, G. The influence of implant diameter and lenght on stress distribu-tion of osseointegrated implants related to crestal bone geometry: A three-dimensional finite elements analysis. J. Prosthet. Dent. 2008, 100, 422–431. [Google Scholar] [CrossRef]
- Smith, D.C.; Pilliar, R.M.; Chernecky, R. Dental implant materials I: Some effects of preparative procedures on surface topog-raphy. J. Biomed. Mater. Res. 1991, 25, 1045–1068. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Dean, D.; Boyan, B. The Role of Implant Surface Characteristics in the Healing of Bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1. Review focusing on topographic and chemical property sur-faces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Lazzara, R.; Testori, T.; Trisi, P.; Porter, S.; Weinstein, R. A human histologic análisis of Osseotite and machined surfaces using implants with 2 opposing surfaces. Int. J. Periodontics Restor. Dent. 1999, 19, 117–129. [Google Scholar]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Ramazanoglu, M.; Oshida, Y. Osseointegration and bioscience of implant surface. Current concepts at bone-implant interface. Implant Dent. 2011, 3, 57–82. [Google Scholar]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Chappuis, V.; Buser, R.; Brägger, U.; Bornstein, M.M.; Salvi, G.E.; Buser, D. Long-Term Outcomes of Dental Implants with a Titanium Plasma-Sprayed Surface: A 20-Year Prospective Case Series Study in Partially Edentulous Patients. Clin. Implant Dent. Relat. Res. 2013, 15, 780–790. [Google Scholar] [CrossRef]
- Vallecillo, M.; Romero, M.N.; Olmedo, M.V.; Reyes, C.; Zorrilla, C. Cylindrical dental implants with Hydroxyapatite- and Tita-nium Plasma Spray–coated surfaces: 5-year results. J. Oral Implantol. 2007, 33, 59–68. [Google Scholar]
- Brunski, J.B.; A Puleo, D.; Nanci, A. Biomaterials and biomechanics of oral and maxillofacial implants: Current status and future developments. Int. J. Oral Maxillofac. Implant. 2000, 15, 15–46. [Google Scholar]
- Klement, J.D.; Poschel, D.B.; Lu, C.; Merting, A.D.; Yang, D.; Redd, P.S.; Liu, K. Osteopontin Blockade Immunotherapy In-creases Cytotoxic T Lymphocyte Lytic Activity and Suppresses Colon Tumor Progression. Cancers 2021, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Przepiórka-Kosińska, J.; Sarecka-Hujar, B.; Raczkiewicz, D.; Kowal, M.; Chyl-Surdacka, K.; Bartosiński, J.; Kosiński, J.; Krasowska, D.; Chodorowska, G. Osteopontin Serum Concentration and Metabolic Syndrome in Male Psoriatic Patients. J. Clin. Med. 2021, 10, 755. [Google Scholar] [CrossRef]
- Salafutdinov, I.; Gazizov, I.; Gatina, D.; Mullin, R.; Bogov, A.; Islamov, R.; Kiassov, A.; Masgutov, R.; Rizvanov, A. Influence of Recombinant Codon-Optimized Plasmid DNA Encoding VEGF and FGF2 on Co-Induction of Angiogenesis. Cells 2021, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Savarino, L.; Aldini, N.; Martín, L.; Giavaresi, G.; Rizzi, G.; Martini, D.; Ruggeri, A.; Giunti, A.; Giardino, R. Biomechanical and histomorphometric investigations on two morphologically differing titanium surfaces with and without fluorohy-droxyapatite coating: An experimental study in sheep tibiae. Biomaterials 2003, 24, 3183–3192. [Google Scholar] [CrossRef]
- Lupi, S.M.; Rodriguez y Baena, A.; Cassinelli, C.; Iviglia, G.; Tallarico, M.; Morra, M.; Rodriguez Y Baena, R. Covalent-ly-Linked Hyaluronan versus Acid Etched Titanium Dental Implants: A Crossover RCT in Humans. Int. J. Mol. Sci. 2019, 20, 763. [Google Scholar] [CrossRef]
- McCracken, M.; E Lemons, J.; Zinn, K. Analysis of Ti-6Al-4V implants placed with fibroblast growth factor 1 in rat tibiae. Int. J. Oral Maxillofac. Implant. 2001, 16, 495–502. [Google Scholar]
- Xiao, L.; Sobue, T.; Esliger, A.; Kronenberg, M.S.; Coffin, J.D.; Doetschman, T.; Hurley, M.M. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 2010, 47, 360–370. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, P.; Li, X.; Doetschman, T.; Coffin, J.D.; Drissi, H.; Hurley, M.M. Exported 18-kDa Isoform of Fibroblast Growth Factor-2 Is a Critical Determinant of Bone Mass in Mice. J. Biol. Chem. 2009, 284, 3170–3182. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues*. The Sage-Schliff (sawing and grinding) Technique. J. Oral Pathol. Med. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Luvizuto, E.R.; Muñoz, F.; Hofbauer, J.; Watzek, G.; Gruber, R. Bone healing around titanium implants in two rat coli-tis models. Clin. Oral Implant. Res. 2013, 24, 224–229. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfac-es: A flexible toolbox. J. R. Soc. Interface 2010, 7, 93–105. [Google Scholar] [CrossRef]
- Moon, K.S.; Choi, E.J.; Oh, S.; Kim, S. The Effect of Covalently Immobilized FGF-2 on Biphasic Calcium Phosphate Bone Substi-tute on Enhanced Biological Compatibility and Activity. Biomed. Res. Int. 2015, 2015, 742192. [Google Scholar] [CrossRef] [PubMed]
- Mamalis, A.A.; Markopoulou, C.; Vrotsos, I.; Koutsilirieris, M. Chemical modification of an implant surface increases osteogen-esis and simultaneously reduces osteoclastogenesis: An in vitro study. Clin. Oral Implant. Res. 2011, 22, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Takei, Y.; Minamizaki, T.; Yoshiko, Y. Functional Diversity of Fibroblast Growth Factors in Bone Formation. Int. J. Endocrinol. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Valta, M.P.; Hentunen, T.; Qu, Q.; Valve, E.M.; Harjula, A.; Seppänen, J.A.; Väänänen, H.K.; Härkönen, P.L. Regulation of Osteoblast Differentiation: A Novel Function for Fibroblast Growth Factor 8. Endocrinology 2006, 147, 2171–2182. [Google Scholar] [CrossRef]
- Bosetti, M.; Leigheb, M.; Brooks, R.A.; Boccafoschi, F.; Cannas, M.F. Regulation of osteoblast and osteoclast functions by FGF-6. J. Cell. Physiol. 2010, 225, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Kim, B.; Lin, W.; Cho, M. Melatonin promotes osteoblast diferentiation and bone formation. J. Biol. Chem. 1999, 30, 22041–22047. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Satorres-Nieto, M.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A.; Maté-Sánchez de Val, J.E.; Gargallo-Albiol, J.; Gómez-Moreno, G.; Romanos, G.E. Influence of surface treatment on osseointegration of dental implants: Histological, histo-morphometric and radiological analysis in vivo. Clin. Oral Investig. 2015, 19, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Dundar, S.; Yaman, F.; Saybak, A.; Ozupek, M.F.; Toy, V.E.; Gul, M.; Ozercan, I.H. Evaluation of Effects of Topical Melatonin Application on Osseointegration of Dental Implant: An Experimental Study. J. Oral Implant. 2016, 42, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.B.; A Gerard, D.; E Larsen, P. Histomorphometric analysis of implant anchorage for 3 types of dental implants following 6 months of healing in baboon jaws. Int. J. Oral Maxillofac. Implant. 2001, 15, 785–791. [Google Scholar]
- Slaets, E.; Carmeliet, G.; Naert, I.; Duyck, J. Early cellular responses in cortical bone healing around unloaded titanium im-plants: An animal study. J. Periodontol. 2006, 77, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Kurokawa, T.; Nakamura, K.; Matsushita, T.; Mamada, K.; Kawaguchi, H. Stimulation of bone formation by recombinant fibroblast growth factor-2 in callotasis bone lengthening of rabbits. Calcif. Tissue Int. 1999, 64, 542–546. [Google Scholar] [CrossRef]
- Simion, M.; Benigni, M.; Al-Hezaimi, K.; Kim, D.M. Early bone formation adjacent to oxidized and machined implant surfaces: A histologic study. Int. J. Periodontics Restor. Dent. 2015, 35, 9–17. [Google Scholar] [CrossRef]
- Nakamura, T.; Hara, Y.; Tagawa, M.; Tamura, M.; Yuge, T.; Fukuda, H.; Nigi, H. Recombinant Human Basic Fibroblast Crowth Factor Accelerates Fracture Healing by Enhancing Callus Remodeling in Experimental Dog Tibial Fracture. J. Bone Miner. Res. 1998, 13, 942–949. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Neufeld, G.; Schweigerer, L. Fibroblast growth factor: Structural and biological properties. J. Cell. Physiol. Suppl. 1987, 5, 15–26. [Google Scholar] [CrossRef]
- Keiichi, K.; Mitsunobu, K.; Masafumi, S.; Yutaka, D.; Toshiaki, S. Induction of new bone by basic FGF-loaded porous carbonate apatite implants in femur defects in rats. Clin. Oral Implant. Res. 2009, 20, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Stenport, V.F.; Johansson, C.B.; Sawase, T.; Yamasaki, Y.; Oida, S. FGF-4 and titanium implants: A pilot study in rabbit bone. Clin. Oral Implant. Res. 2003, 14, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.; López-Peña, M.; Miño, N.; Gómez-Moreno, G.; Guardia, J.; Cutando, A. Topical Application of Melatonin and Growth Hormone Accelerates Bone Healing around Dental Implants in Dogs. Clin. Implant. Dent. Relat. Res. 2009, 14, 226–235. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Fang, D.; Shi, S. The miniature pig: A useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530–537. [Google Scholar] [CrossRef]
- Mosekilde, L.; Kragstrup, J.; Richards, A. Compressive strength, ash weight, and volume of vertebral trabecular bone in ex-perimental fluorosis in pigs. Calcif. Tissue Int. 1987, 40, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Grosskopf, B.; Omelka, R.; Vondráková, M.; Bauerová, M. Differences among species in compact bone tissue microstructure of mammalian skeleton: Use of a discriminant function analysis for species identification. J. Forensic Sci. 2006, 51, 1235–1239. [Google Scholar] [CrossRef]
- Martínez-González, J.M.; Cano-Sánchez, J.; Campo-Trapero, J.; Gonzalo-Lafuente, J.C.; Díaz-Regañón, J.; Vázquez–Piñeiro, M.T. Evaluation of minipigs as an animal model for alveolar distraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Laiblin, C.; Jaeschke, G. Clinical-chemical investigations of the metabolism of bone and muscle under stress in the Göttiningen miniature pig. An experimental study. Berl. Munch. Tierarztl. Wochenschr. 1979, 92, 124. [Google Scholar]
- Beddoe, H.A. Quantitative study of the structure of trabecular bone in man, rhesus monkey, beagle and miniature pig. Calcif. Tissue Res. 1978, 25, 273–281. [Google Scholar] [CrossRef]
- Kragstrup, J.; Richards, A.; Fejerskov, O. Effects of fluoride on cortical bone remodeling in the growing domestic pig. Bone 1989, 10, 421–424. [Google Scholar] [CrossRef]
- Fuerst, G.; Gruber, R.; Tangl, S.; Sanroman, F.; Watzek, G. Enhanced bone-to-implant contact by platelet-released growth factors in mandibular cortical bone: A histomorphometric study in minipigs. Int. J. Oral Maxillofac. Implant. 2003, 18, 685–690. [Google Scholar]
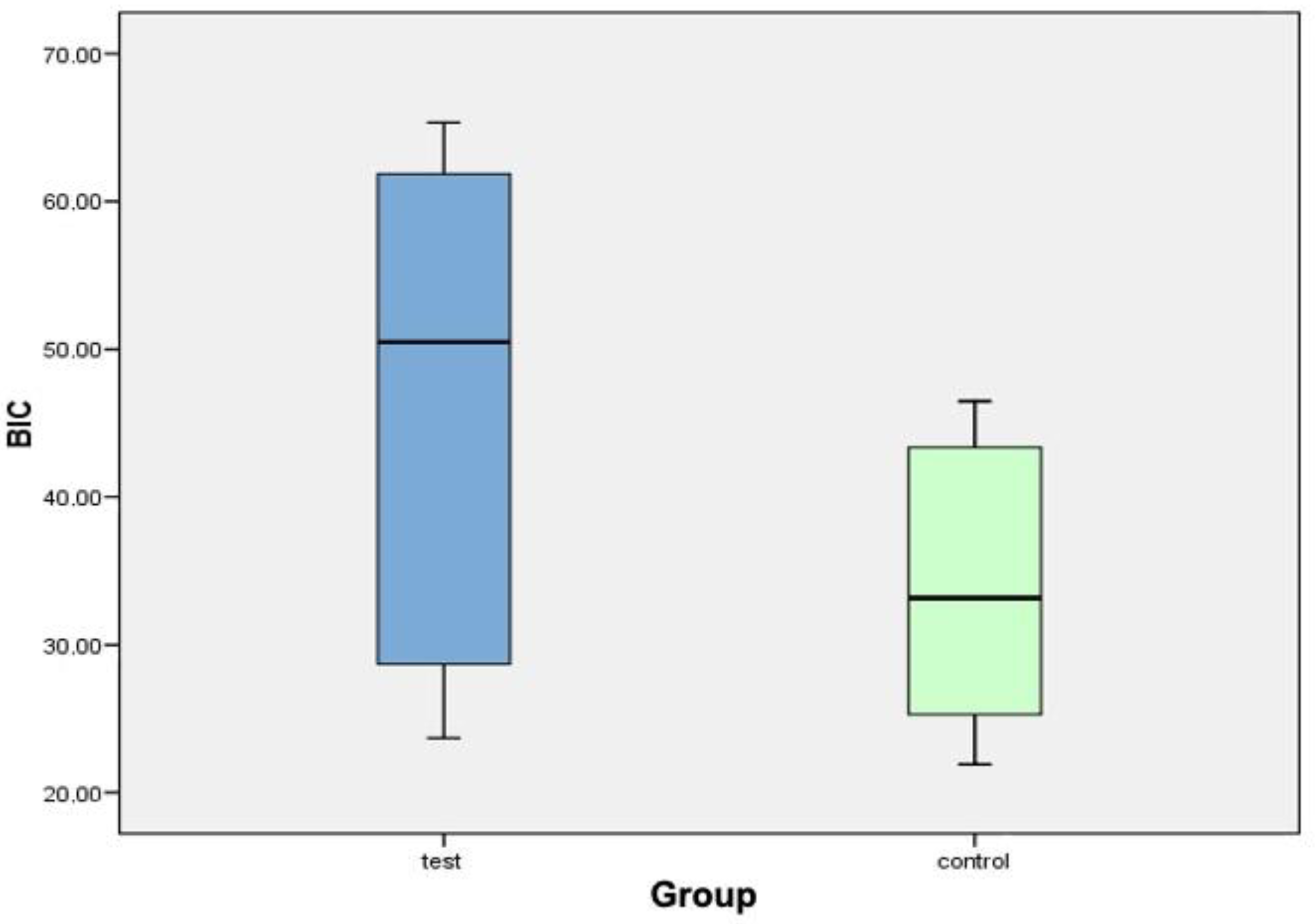
| Implant (Test Group) | Bone-Implant Contact (BIC) | Implant (Control Group) | Bone-Implant Contact (BIC) |
|---|---|---|---|
| C2TIMSP1 | 23.69% | C1TIMSE1 | 21.91% |
| C2TIDSP2 | 28.15% | C1TICSE1 | 27.02% |
| C4TIMSP3 | 42.60% | C1TIDSE3 | 23.55% |
| C4TICSP4 | 58.38% | C3TIMSE4 | 28.82% |
| C4TIDSP5 | 29.21% | C3TIDSE5 | 37.53% |
| C6TIMSP6 | 61.36% | C5TIMSE6 | 46.47% |
| C6TICSP7 | 65.34% | C5TICSE7 | 40.60% |
| C6TIDSP8 | 62.39% | C5TIDSE8 | 46.11% |
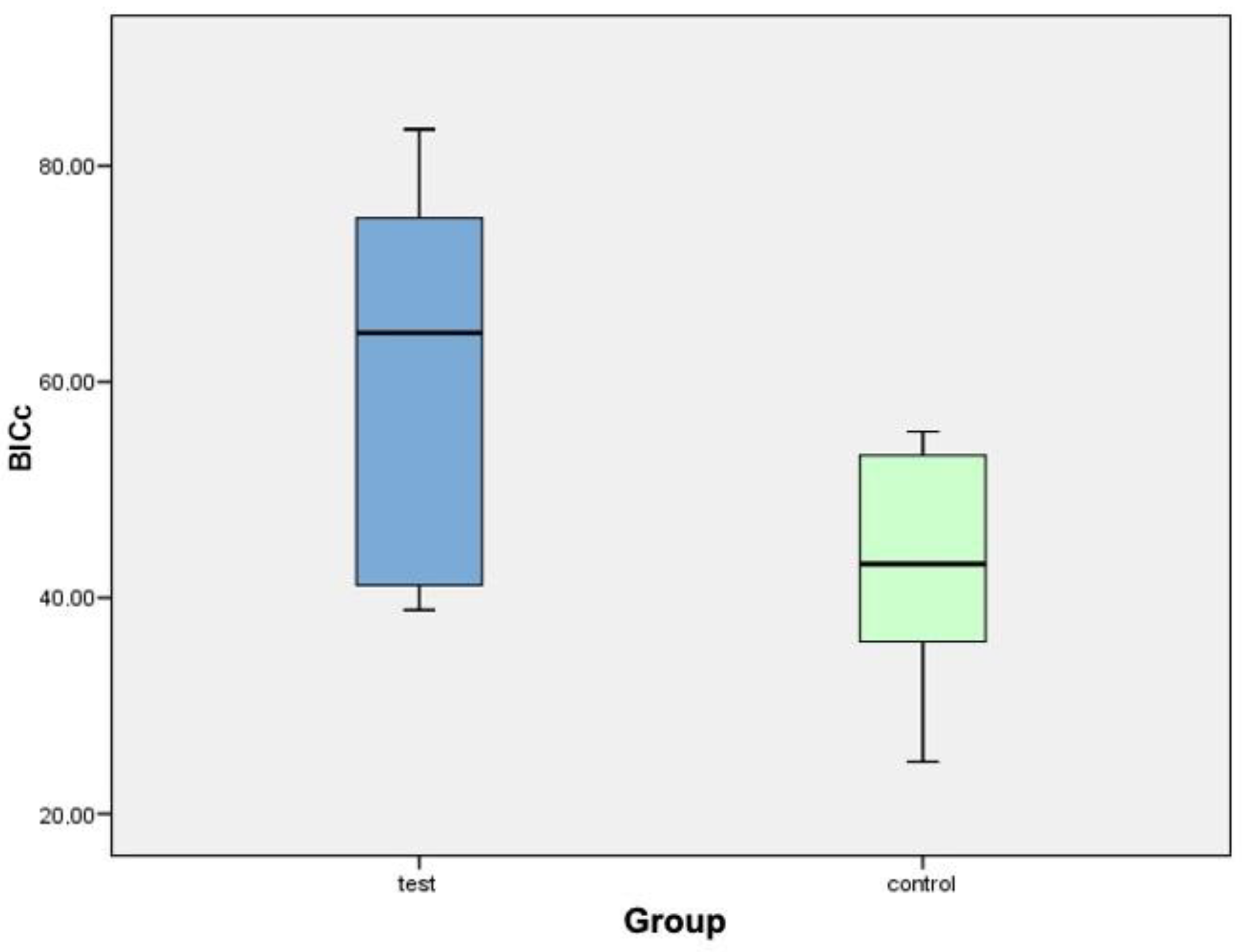
| Implant (Test Group) | Corrected Bone-Implant Contact (BICC) | Implant (Control Group) | Corrected Bone-Implant Contact (BICC) |
|---|---|---|---|
| C2TIMSP1 | 39.56% | C1TIMSE1 | 24.81% |
| C2TIDSP2 | 42.74% | C1TICSE2 | 40.94% |
| C4TIMSP3 | 57.85% | C1TIDSE3 | 37.46% |
| C4TICSP4 | 73.91% | C3TIMSE4 | 34.42% |
| C4TIDSP5 | 38.87% | C3TIDSE5 | 45.31% |
| C6TIMSP6 | 76.38% | C5TIMSE6 | 51.39% |
| C6TICSP7 | 71.15% | C5TICSE7 | 54.97% |
| C6TIDSP8 | 83.36% | C5TIDSE8 | 55.37% |
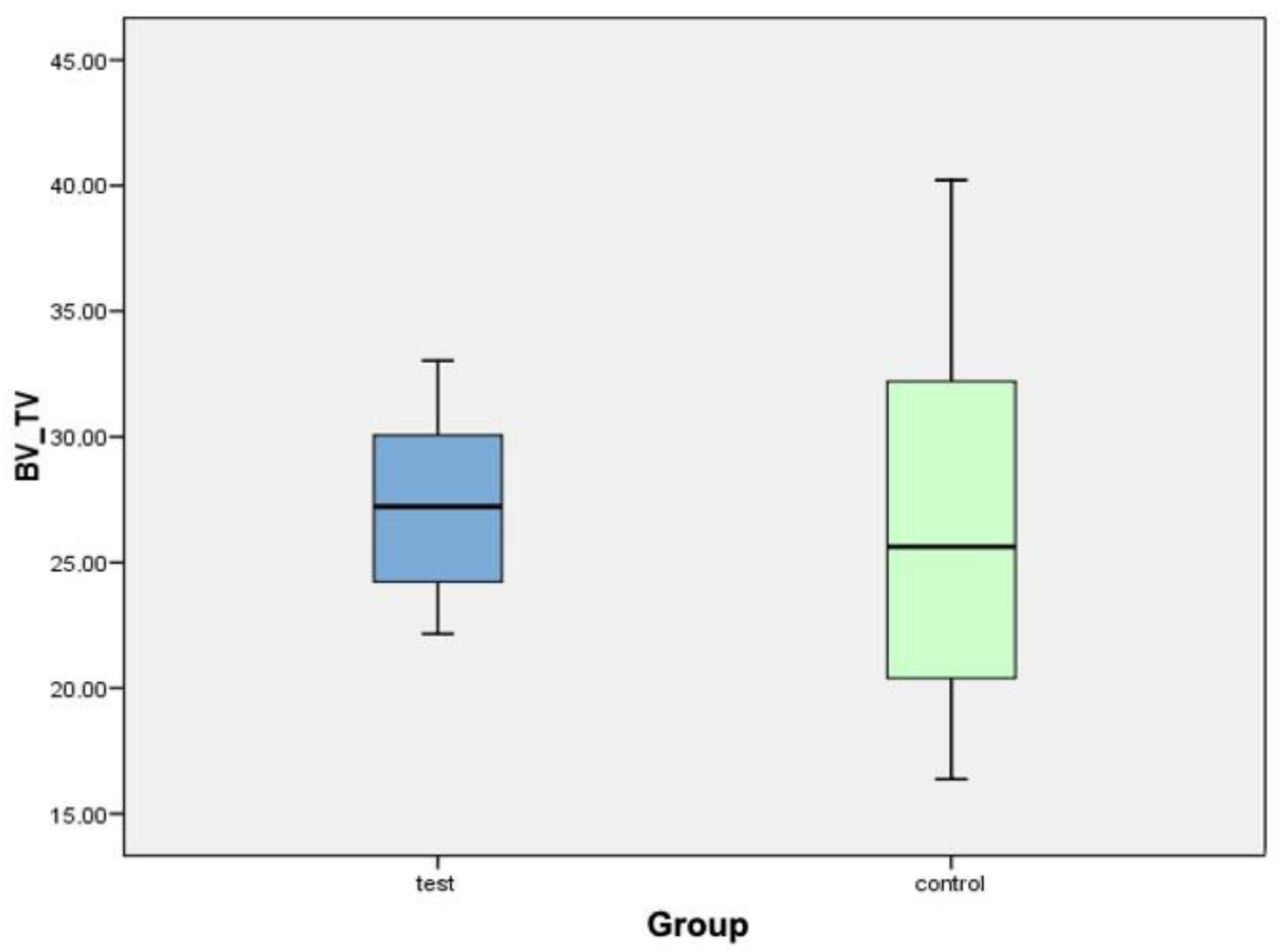
| Implant (Test Group) | New Bone Formation (BV/TV) | Implant (Control Group) | New Bone Formation (BV/TV) |
|---|---|---|---|
| C2TIMSP1 | 27.37% | C1TIMSE1 | 26.79% |
| C2TIDSP2 | 28.58% | C1TICSE1 | 20.71% |
| C4TIMSP3 | 33.03% | C1TIDSE3 | 24.48% |
| C4TICSP4 | 22.16% | C3TIMSE4 | 16.38% |
| C4TIDSP5 | 27.07% | C3TIDSE5 | 40.22% |
| C6TIMSP6 | 22.28% | C5TIMSE6 | 33.38% |
| C6TICSP7 | 31.55% | C5TICSE7 | 31.03% |
| C6TIDSP8 | 26.18% | C5TIDSE8 | 20.08% |
| Implant (Test Group) | Interthread Bone (BAI/TA) | Implant (Control Group) | Interthread Bone (BAI/TA) |
|---|---|---|---|
| C2TIMSP1 | 19.14% | C1TIMSE1 | 39.15% |
| C2TIDSP2 | 26.44% | C1TICSE1 | 27.72% |
| C4TIMSP3 | 38.39% | C1TIDSE3 | 24.14% |
| C4TICSP4 | 35.88% | C3TIMSE4 | 22.52% |
| C4TIDSP5 | 37.16% | C3TIDSE5 | 36.22% |
| C6TIMSP6 | 37.35% | C5TIMSE6 | 45.28% |
| C6TICSP7 | 29.93% | C5TICSE7 | 35.59% |
| C6TIDSP8 | 33.87% | C5TIDSE8 | 32.64% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragoneses, J.; Suárez, A.; López-Valverde, N.; Martínez-Martínez, F.; Aragoneses, J.M. Assessment of the Tissue Response to Modification of the Surface of Dental Implants with Carboxyethylphosphonic Acid and Basic Fibroblastic Growth Factor Immobilization (Fgf-2): An Experimental Study on Minipigs. Biology 2021, 10, 358. https://doi.org/10.3390/biology10050358
Aragoneses J, Suárez A, López-Valverde N, Martínez-Martínez F, Aragoneses JM. Assessment of the Tissue Response to Modification of the Surface of Dental Implants with Carboxyethylphosphonic Acid and Basic Fibroblastic Growth Factor Immobilization (Fgf-2): An Experimental Study on Minipigs. Biology. 2021; 10(5):358. https://doi.org/10.3390/biology10050358
Chicago/Turabian StyleAragoneses, Javier, Ana Suárez, Nansi López-Valverde, Francisco Martínez-Martínez, and Juan Manuel Aragoneses. 2021. "Assessment of the Tissue Response to Modification of the Surface of Dental Implants with Carboxyethylphosphonic Acid and Basic Fibroblastic Growth Factor Immobilization (Fgf-2): An Experimental Study on Minipigs" Biology 10, no. 5: 358. https://doi.org/10.3390/biology10050358
APA StyleAragoneses, J., Suárez, A., López-Valverde, N., Martínez-Martínez, F., & Aragoneses, J. M. (2021). Assessment of the Tissue Response to Modification of the Surface of Dental Implants with Carboxyethylphosphonic Acid and Basic Fibroblastic Growth Factor Immobilization (Fgf-2): An Experimental Study on Minipigs. Biology, 10(5), 358. https://doi.org/10.3390/biology10050358









