Phytochemicals, Antioxidant Attributes and Larvicidal Activity of Mercurialis annua L. (Euphorbiaceae) Leaf Extracts against Tribolium confusum (Du Val) Larvae (Coleoptera; Tenebrionidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Insect Larvae
2.3. Chemicals Used
2.4. Preparation of Extracts
2.4.1. Quantification of Total Phenolic Contents
2.4.2. Quantification of Total Flavonoids Contents
2.4.3. Evaluation of Antioxidant Activity
2.5. LC-MS Analysis
2.6. Evaluation of Larvicidal Activity
2.6.1. Toxicity Tests
2.6.2. Statistical Analysis
3. Results and Discussion
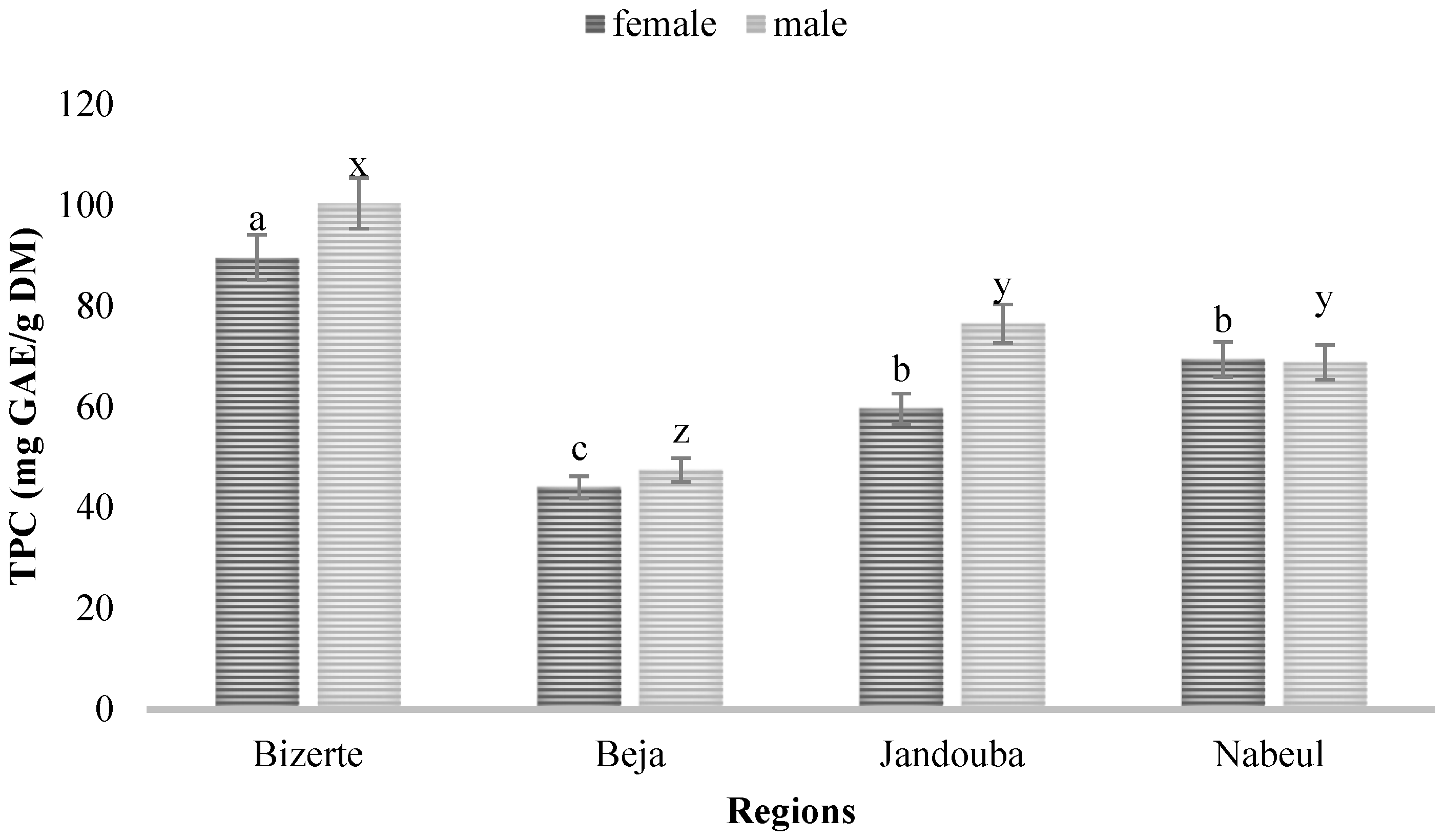
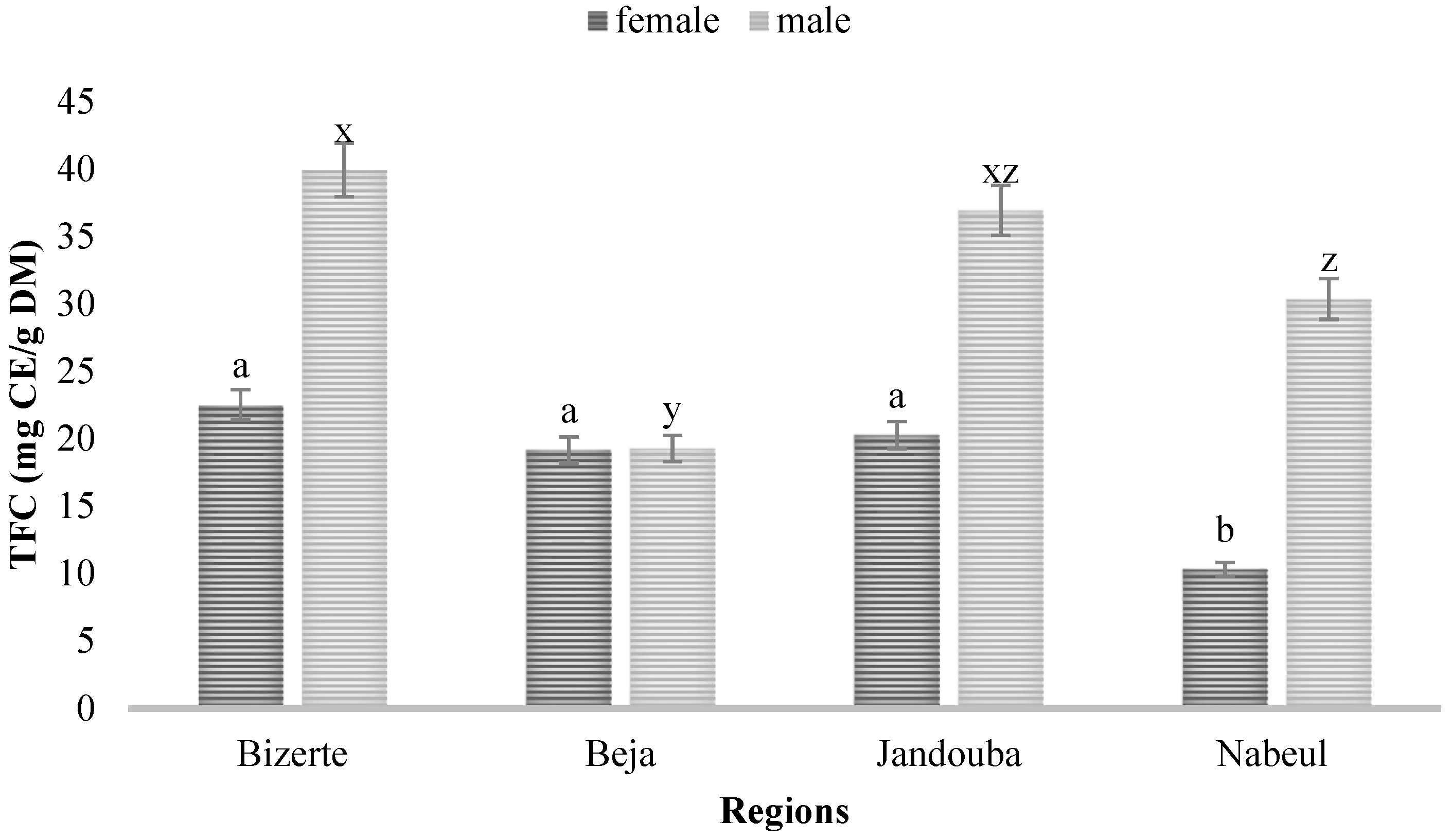
3.1. Quantification of TPC and TFC
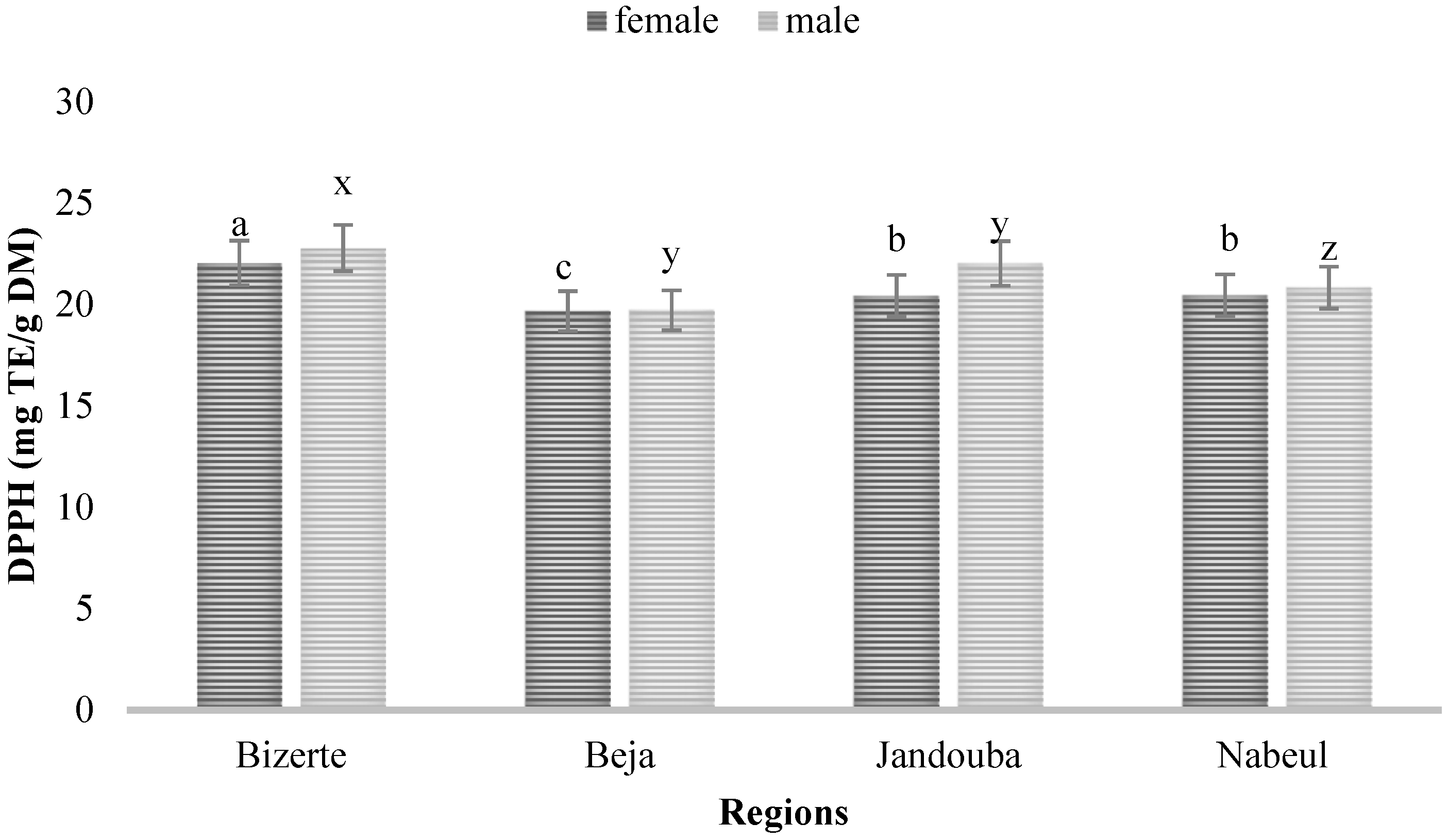
3.2. Antioxidant Activity (AOA)
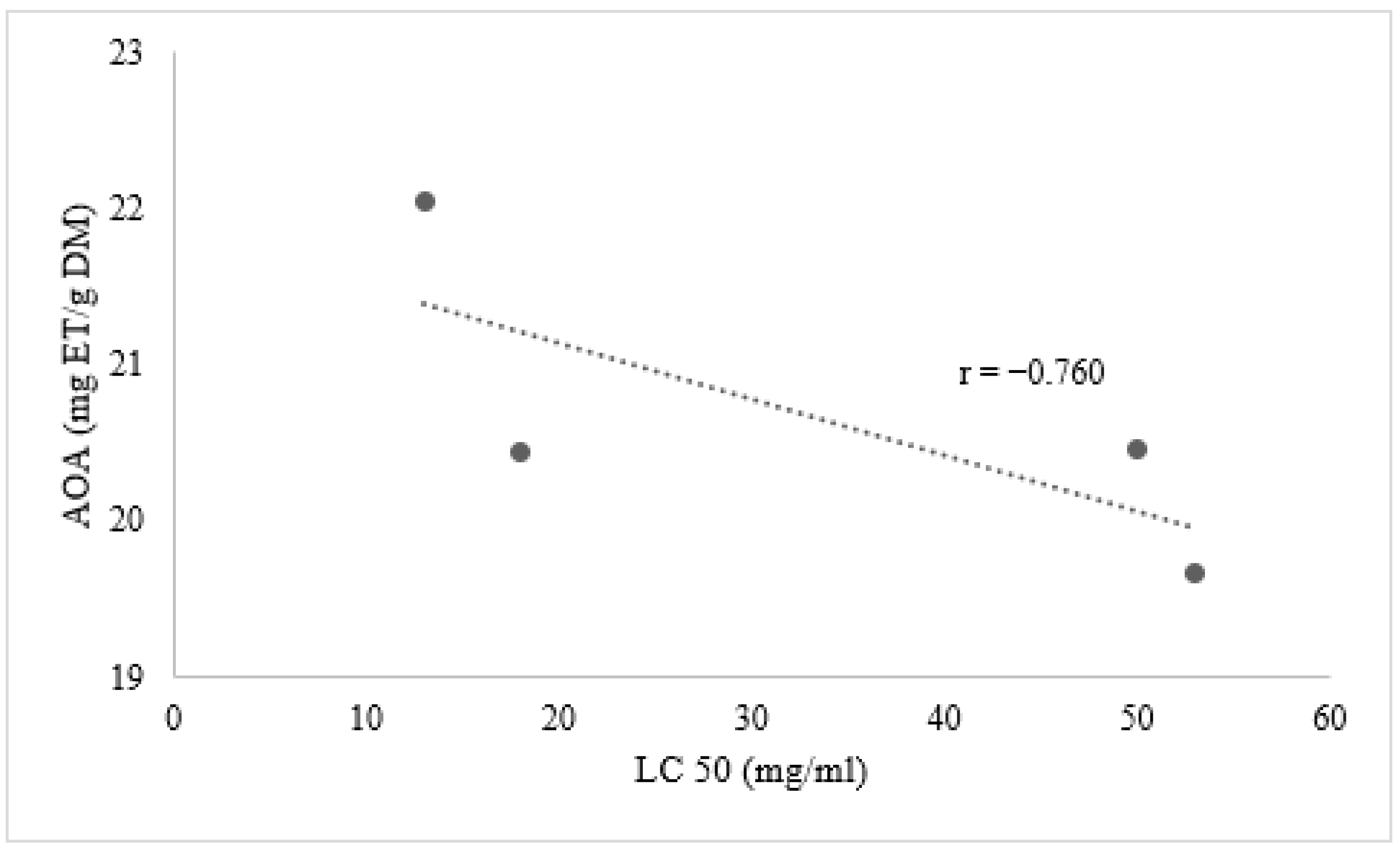
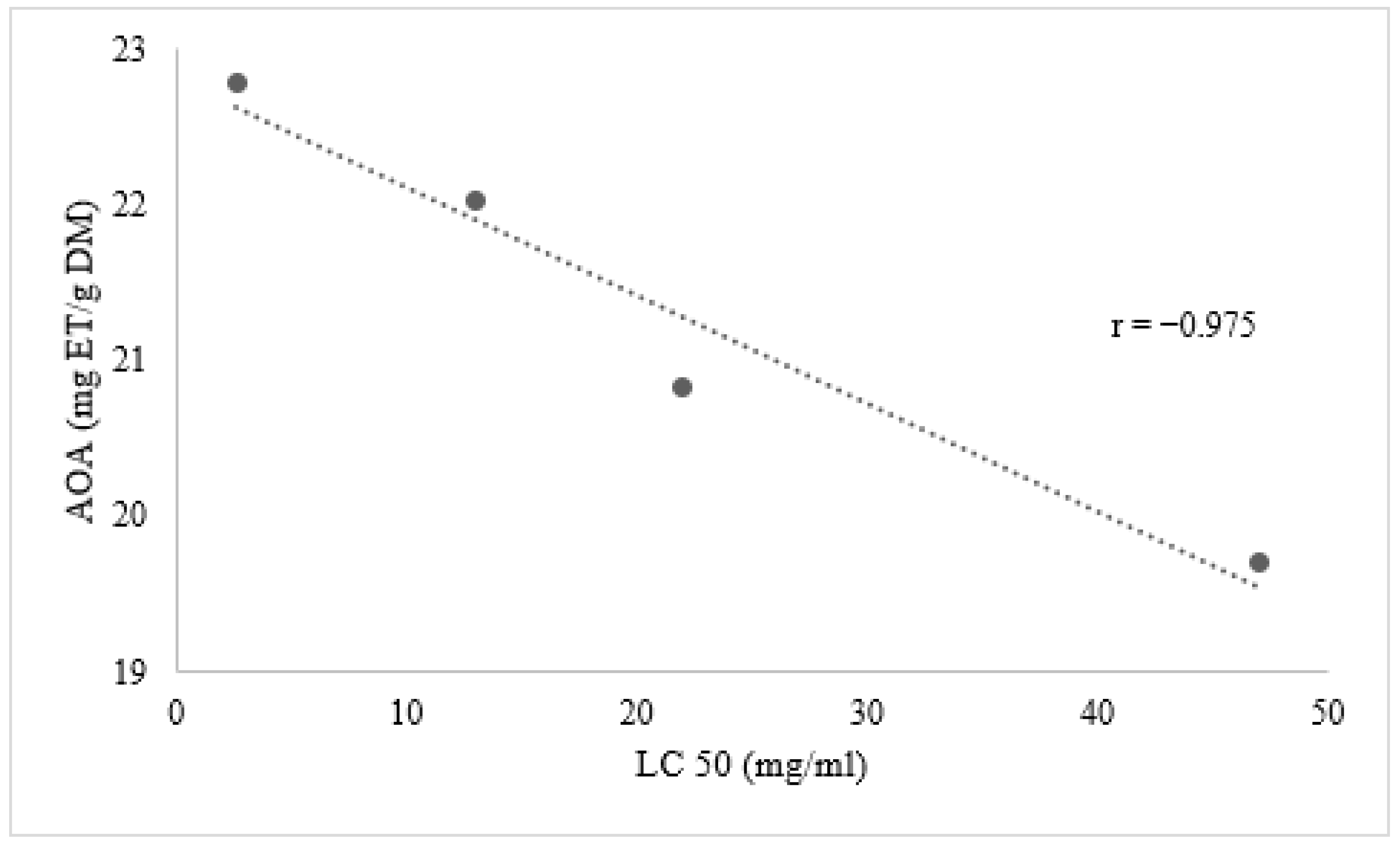
3.3. Relationship between TPC, TFC and AOA
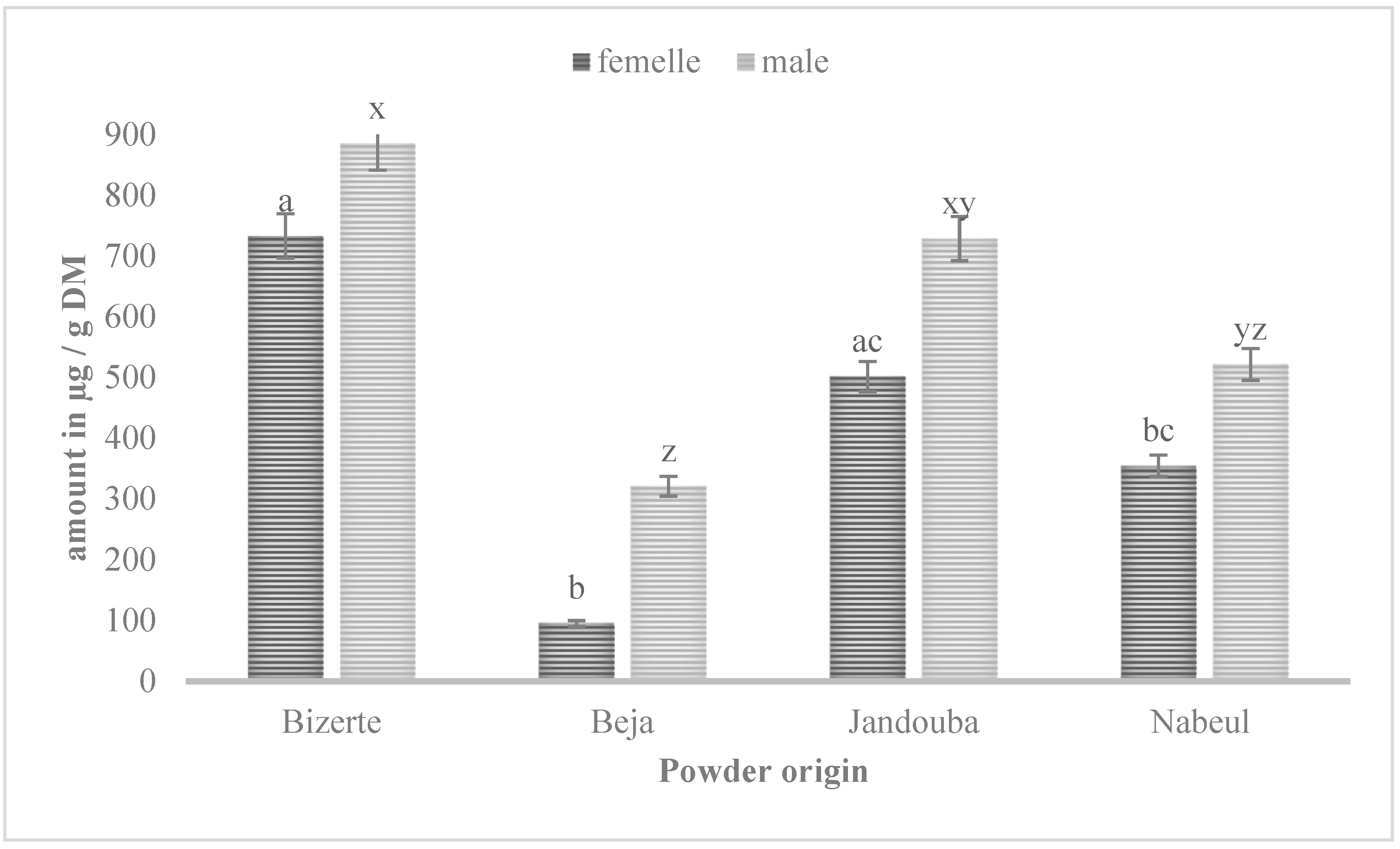
3.4. LC-MS
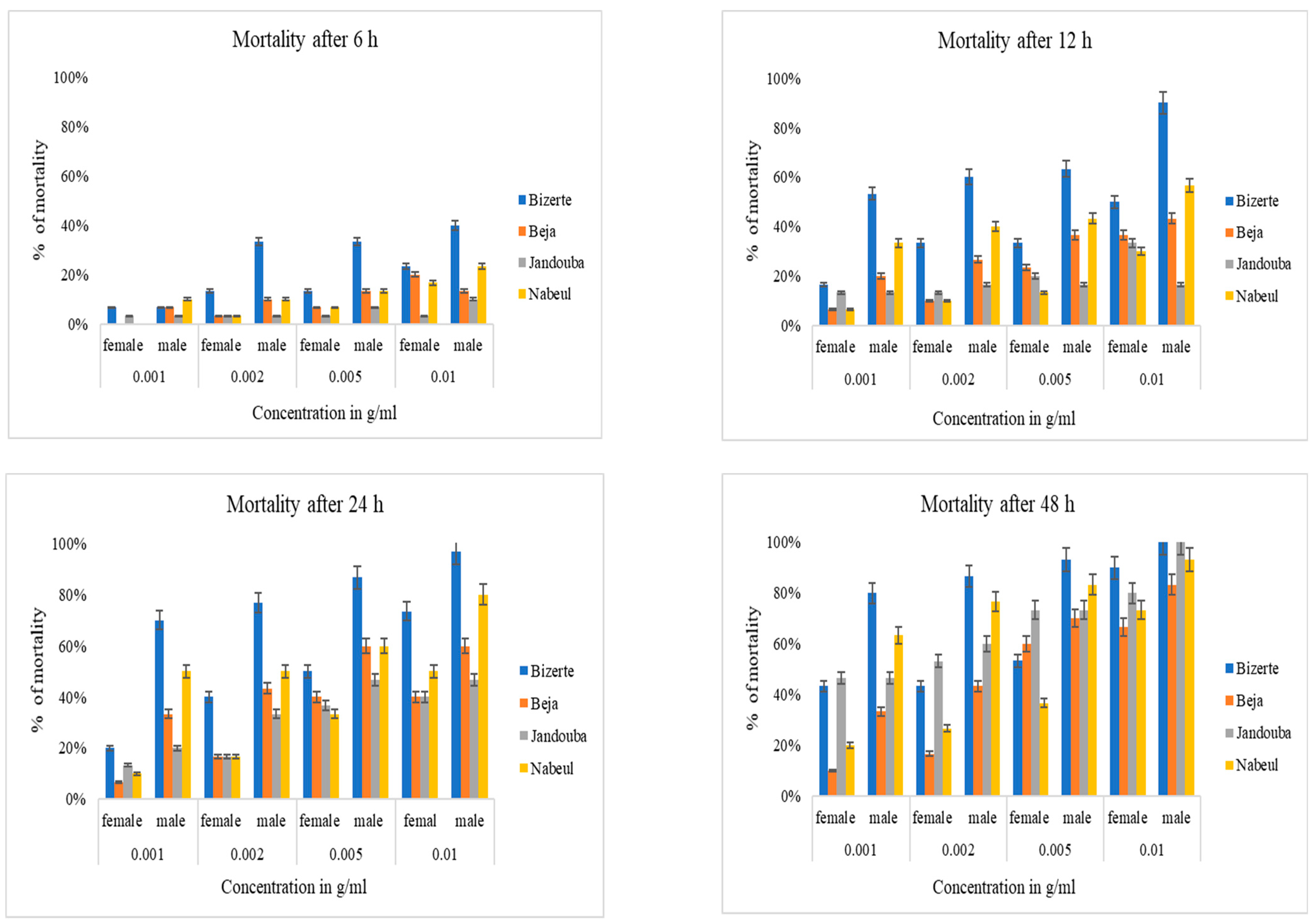
4. Larvicidal Activity
Relation between Phenolic Compounds and Larvicidal Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shafiei, B.; Akbarinia, M.; Jalali, S.G.; Hosseini, S.M.; Azizi, P. Effect of Fire on Herbal Layer Biodiversity in a Temperate Forest of Northern Iran. Pak. J. Biol. Sci. 2006, 9, 2273–2277. [Google Scholar] [CrossRef]
- Oran, S.A.; Al Eisawi, D.M. Check-list of medicinal plants in Jordan. Dirasat 1998, 25, 84–112. [Google Scholar]
- Blanco-Salas, J.; Vazquez, F.M.; Hortigón-Vinagre, M.P.; Ruiz-Tellez, T. Bioactive Phytochemicals from Mercurialis spp. Used in Traditional Spanish Medicine. Plants 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Aldouri, N.; Shakya, A. Fatty Acids Analysis and Antioxidant Activity of a Lipid Extract obtained from Mercurialis annua L. grown wildly in Jordan. Acta Pol. Pharm. 2018, 75. [Google Scholar] [CrossRef]
- Doukkali, Z.; Kamal, R. Anti-Anxiety Effects of Mercurialis annua Aqueous Extract in the Elevated Plus Maze Test. J. Pharmacol. Rep. 2016, 1, 1–5. [Google Scholar]
- Al-Bakri, G.; Afifi, F.U. Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. J. Microbiol. Methods 2007, 68, 19–25. [Google Scholar] [CrossRef]
- Bustanji, Y.; AlDouri, N.; Issa, A.; Mashallah, S.; Assaf, A.; Aburjai, T.; Mohammad, M. Cytotoxic effect of Mercurialis annua L. methanolic extract on six human solid cancer cell lines. SRE 2012, 7, 3218–3222. [Google Scholar] [CrossRef]
- Ostrozhenkova, E.; Eylert, E.; Schramek, N.; Golan-Goldhirsh, A.; Bacher, A.; Eisenreich, W. Biosynthesis of the chromogen hermidin from Mercurialis annua L. Phytochemistry 2007, 68, 2816–2824. [Google Scholar] [CrossRef]
- Wafa, G.; Amadou, D.; Larbi, K.M.; Héla, E.F.O. Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis L. Ind. Crop. Prod. 2014, 56, 43–51. [Google Scholar] [CrossRef]
- Pottier-Alapetite, G. Flore de la Tunisie. Angiospermes-dicotyledones… Apétales-Dialypétales; Première Partie; Imprimerie Officielle de la République Tunisienne; Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l’Agriculture:: Tunis, Tunisia, 1979; p. 466.
- Benoît, H.P.; McCauley, E.; Post, J.R. Testing the Demographic Consequences of Cannibalism in Tribolium confusum. Ecology 1998, 79, 2839–2851. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.C.; Baudelaire, É.; Scher, J.; Dicko, A. Antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L.) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Mousavi, M.; Zaiter, A.; Modarressi, A.; Baudelaire, E.; Dicko, A. The positive impact of a new parting process on antioxidant activity, malic acid and phenolic content of Prunus avium L., Prunus persica L. and Prunus domestica subsp. Insititia L. powders. Microchem. J. 2019, 149, 103962. [Google Scholar] [CrossRef]
- Chen, Y.; Rai, R.; Zhou, Z.R.; Kanoh, J.; Ribeyre, C.; Yang, Y.; Zheng, H.; Damay, P.; Wang, F.; Lei, M.; et al. A conserved motif within RAP1 has diversified roles in telomere protection and regulation in different organisms. Nat. Struct. Mol. Biol. 2011, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, E.; Tournier, H.A.; Mordujovich de Buschiazzo, P.; Saavedra, G.; Schinella, G.R. Antioxidant activity of Paraguayan plant extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant. Songklanakarin J. Sci. Technol. (SJST) 2004, 26, 211–219. [Google Scholar]
- Aouinty, B.; Oufara, S.; Mellouki, F.; Mahari, S. Evaluation préliminaire de l’activité larvicide des extraits aqueux des feuilles du ricin (Ricinus communis L.) et du bois de thuya (Tetraclinis articulata (Vahl) Mast.) sur les larves de quatre moustiques culicidés: Culex pipiens (Linné), Aedes caspius (Pallas), Culiseta longiareolata (Aitken) et Anopheles maculipennis (Meigen). Biotechnol. Agron. Soc. Environ. 2006, 10. Available online: https://doaj.org/article/64680bf90616411192fd766d5898df91 (accessed on 2 February 2021).
- Soha, S.A.S.; Dougnon, T.V.; Ohouko, O.F.; Asouko, S.J.; Dougnon, T.J.; Youssao, A.K.; Farougou, S.; Kpodekon, T.T.M. Larval cytotoxicity and acute oral toxicity of aqueous extracts of elaeis guineensis leaves and khaya senegalensis stem bark in wistar rats. Int. J. Adv. Res. 2019. [Google Scholar] [CrossRef]
- Mohapatra, C.; Rengarajan, K. Manual on bioassays in the laboratory and their techniques. Cmfri Spec. Publ. 1995, 64, 1–75. [Google Scholar]
- Caridade, T.N.S.; Araújo, R.D.; Oliveira, A.N.A.; Souza, T.S.A.; Ferreira, N.C.F.; Avelar, D.S.; Teles, Y.C.F.; Silvelra, E.R.; Araújo, R.M. Chemical composition of four different species of the Waltheria genus. Biochem. Syst. Ecol. 2018, 80, 81–83. [Google Scholar] [CrossRef]
- Singh, P.A.; Brindavanam, N.B.; Kimothi, G.P.; Aeri, V. Evaluation of in vivo anti-inflammatory and analgesic activity of Dillenia indica f. elongata (Miq.) Miq. and Shorea robusta stem bark extracts. Asian Pac. J. Trop. Dis. 2016, 6, 75–81. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, e162750. [Google Scholar] [CrossRef]
- Aquino, R.; Behar, I.; D’agostino, M.; De Simone, F.; Schettino, O.; Pizza, C. Phytochemical investigation on Mercurialis annua. Biochem. Syst. Ecol. 1987, 15, 667–669. [Google Scholar] [CrossRef]
- Bachchu, M.A.A.; Ara, K.; Uddin, M.N.; Ara, R. Larvicidal Efficacies of Four Indigenous Plant Extracts Against Red FlourBeetle, Tribolium Castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Asiat. Soc. Bangladesh Sci. 2017, 43, 2. [Google Scholar] [CrossRef]
- An, N.T.G.; Huong, L.T.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Ngoc, N.T.B.; Setzer, W.N. Mosquito Larvicidal Activity, Antimicrobial Activity, and Chemical Compositions of Essential Oils from Four Species of Myrtaceae from Central Vietnam. Plants 2020, 9, 544. [Google Scholar] [CrossRef]
- Swargiary, A.; Daimari, M.; Roy, M.; Haloi, D.; Ramchiary, B. Evaluation of phytochemical properties and larvicidal activities of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula against Aedes aegypti. Asian Pac. J. Trop. Med. 2019, 12, 224. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Andrade, E.H.A.; Kato, M.J.; Carreira, L.M.M.; Guimarães, E.F.; Maia, J.G.S. Antioxidant capacity and larvicidal and antifungal activities of essential oils and extracts from Piper krukoffii. Nat. Prod. Commun. 2011, 6, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Beatovic, D.; Krstic-Milosevic, D.; Trifunovic, S.; Siljegovic, J.; Glamoclija, J.; Ristic, M.; Jelacic, S. Chemical Composition, Antioxidant and Antimicrobial Activities of the Essential Oils of Twelve Ocimum basilicum L. Cultivars Grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Huang, J.L.; Fu, S.T.; Jiang, Y.Y.; Cao, Y.B.; Guo, M.L.; Wang, Y.; Xu, Z. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacol. Biochem. Behav. 2007, 86, 741–748. [Google Scholar] [CrossRef]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg. Med. Chem. Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Experimental studies on bioactive potential of rutin. Chron. Young Sci. 2013. [Google Scholar] [CrossRef]
- Sogan, N.; Kapoor, N.; Singh, H.; Kala, S.; Nayak, A.; Nagpal, B.N. Larvicidal activity of Ricinus communis extract against mosquitoes. J. Vector Borne Dis. 2018, 55, 282. [Google Scholar] [CrossRef] [PubMed]
- Edziri, H.; Jaziri, R.; Chehab, H.; Verschaeve, L.; Flamini, G.; Boujnah, D.; Hammami, M.; Aouni, M.; Mastouri, M. A comparative study on chemical composition, antibiofilm and biological activities of leaves extracts of four Tunisian olive cultivars. Heliyon 2019, 5, e01604. [Google Scholar] [CrossRef] [PubMed]
- Muanda, N.F.; Dicko, A.; Soulimani, R. Chemical composition and biological activities of Ficus capensis leaves extracts. Undefined 2010, 3, 147–160. [Google Scholar]
| RT (min) | Amount of Flavonol in µg/g DM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male Plant | Female Plant | ||||||||
| Bizerte | Beja | Jandouba | Nabeul | Bizerte | Beja | Jandouba | Nabeul | ||
| Narcissin | 17.5 ± 0.3 | 885.4 ± 0.001 | 320.73 ± 0.018 | 728.48 ± 0.04 | 521.31 ± 0.038 | 732.78 ± 0.38 | 95.02 ± 0.1 | 501.38 ± 0.73 | 354.29 ± 0.32 |
| Gallocatechin | 7.3 ± 0.2 | 205.68 ± 0.005 | 57.1 ± 0.043 | 181.36 ± 0.004 | 61.89 ± 0.02 | 155.37 ± 0.001 | 42.67 ± 0.005 | 150.45 | 59.06 ± 0.02 |
| Rutin | 15.4 ± 0.3 | 44.18 ± 0.016 | 8.11 ± 0.0002 | 23.98 ± 0.005 | 10.82 ± 0.061 | 18.38 ± 0.004 | 1.4 ± 0.0003 | 11.57 ± 0.001 | 7.4 ± 0.102 |
| Epigallocatechin | 9.07 ± 0.2 | 35.37 ± 0.0007 | 12.78 ± 0.082 | 23.68 ± 0.004 | 20.68 ± 0.006 | 34.57 ± 0.0008 | 10.11 ± 0.0012 | 17.29 ± 0.005 | 14.63 ± 0.164 |
| Epicatechin | 15.3 ± 0.2 | 27.46 ± 0.04 | 12.35 ± 0.001 | 24.57 ± 0.003 | 18.4 ± 0.0009 | 21.02 ± 0.003 | 8.16 ± 0.0006 | 13.16 ± 0.001 | 11.01 ± 0.001 |
| Provenance | Plant Material | Time (h) | LC50 g/mL± SE | χ2 | 95% Lower Confidence | 95% Upper Confidence |
|---|---|---|---|---|---|---|
| Bizerte | LFP | 24 | 0.0038 ± 0.172 | 2.969 | 0.00312 | 0.00498 |
| LMP | 24 | 0.00039 ± 0.217 | 2.237 | 0.000126 | 0.00069 | |
| LFP | 48 | 0.0020 ± 0.178 | 20.439 | - | - | |
| LMP | 48 | 0.00026 ± 0.282 | 3.406 | 0.00006 | 0.0005 | |
| Nabeul | LFP | 24 | 0.0022 ± 0.134 | 0.126 | - | - |
| LMP | 24 | 0.0014 ± 0.172 | 5.472 | 0.00066 | 0.0022 | |
| LFP | 48 | 0.0049 ± 0.196 | 10.69 | - | - | |
| LMP | 48 | 0.00046 ± 0.201 | 1.21 | 0.00024 | 0.00099 | |
| Jandouba | LFP | 24 | 0.012 ± 0.196 | 5.045 | 0.0085 | 0.0022 |
| LMP | 24 | 0.0034 ± 0.162 | 1.711 | 0.00059 | 0.024 | |
| LFP | 48 | 0.00135 ± 0.176 | 0.795 | 0.00096 | 0.0042 | |
| LMP | 48 | 0.00132 ± 0.207 | 16.234 | - | - | |
| Béja | LFP | 24 | 0.0157 ± 0.186 | 1.985 | 0.0096 | 0.042 |
| LMP | 24 | 0.0092 ± 0.173 | 2.49 | 0.00013 | 0.069 | |
| LFP | 48 | 0.0052 ± 0.181 | 6.859 | 0.00023 | 0.034 | |
| LMP | 48 | 0.0022 ± 0.181 | 0.99 | 0.0018 | 0.0028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Nasr, R.; Baudelaire, E.D.; Dicko, A.; El Ferchichi Ouarda, H. Phytochemicals, Antioxidant Attributes and Larvicidal Activity of Mercurialis annua L. (Euphorbiaceae) Leaf Extracts against Tribolium confusum (Du Val) Larvae (Coleoptera; Tenebrionidae). Biology 2021, 10, 344. https://doi.org/10.3390/biology10040344
Ben Nasr R, Baudelaire ED, Dicko A, El Ferchichi Ouarda H. Phytochemicals, Antioxidant Attributes and Larvicidal Activity of Mercurialis annua L. (Euphorbiaceae) Leaf Extracts against Tribolium confusum (Du Val) Larvae (Coleoptera; Tenebrionidae). Biology. 2021; 10(4):344. https://doi.org/10.3390/biology10040344
Chicago/Turabian StyleBen Nasr, Rania, Elie Djantou Baudelaire, Amadou Dicko, and Hela El Ferchichi Ouarda. 2021. "Phytochemicals, Antioxidant Attributes and Larvicidal Activity of Mercurialis annua L. (Euphorbiaceae) Leaf Extracts against Tribolium confusum (Du Val) Larvae (Coleoptera; Tenebrionidae)" Biology 10, no. 4: 344. https://doi.org/10.3390/biology10040344
APA StyleBen Nasr, R., Baudelaire, E. D., Dicko, A., & El Ferchichi Ouarda, H. (2021). Phytochemicals, Antioxidant Attributes and Larvicidal Activity of Mercurialis annua L. (Euphorbiaceae) Leaf Extracts against Tribolium confusum (Du Val) Larvae (Coleoptera; Tenebrionidae). Biology, 10(4), 344. https://doi.org/10.3390/biology10040344






