Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications
Abstract
Simple Summary
Abstract
1. Introduction
1.1. CAM as an Experimental Model
1.2. Advantages and Limitations of the CAM Model
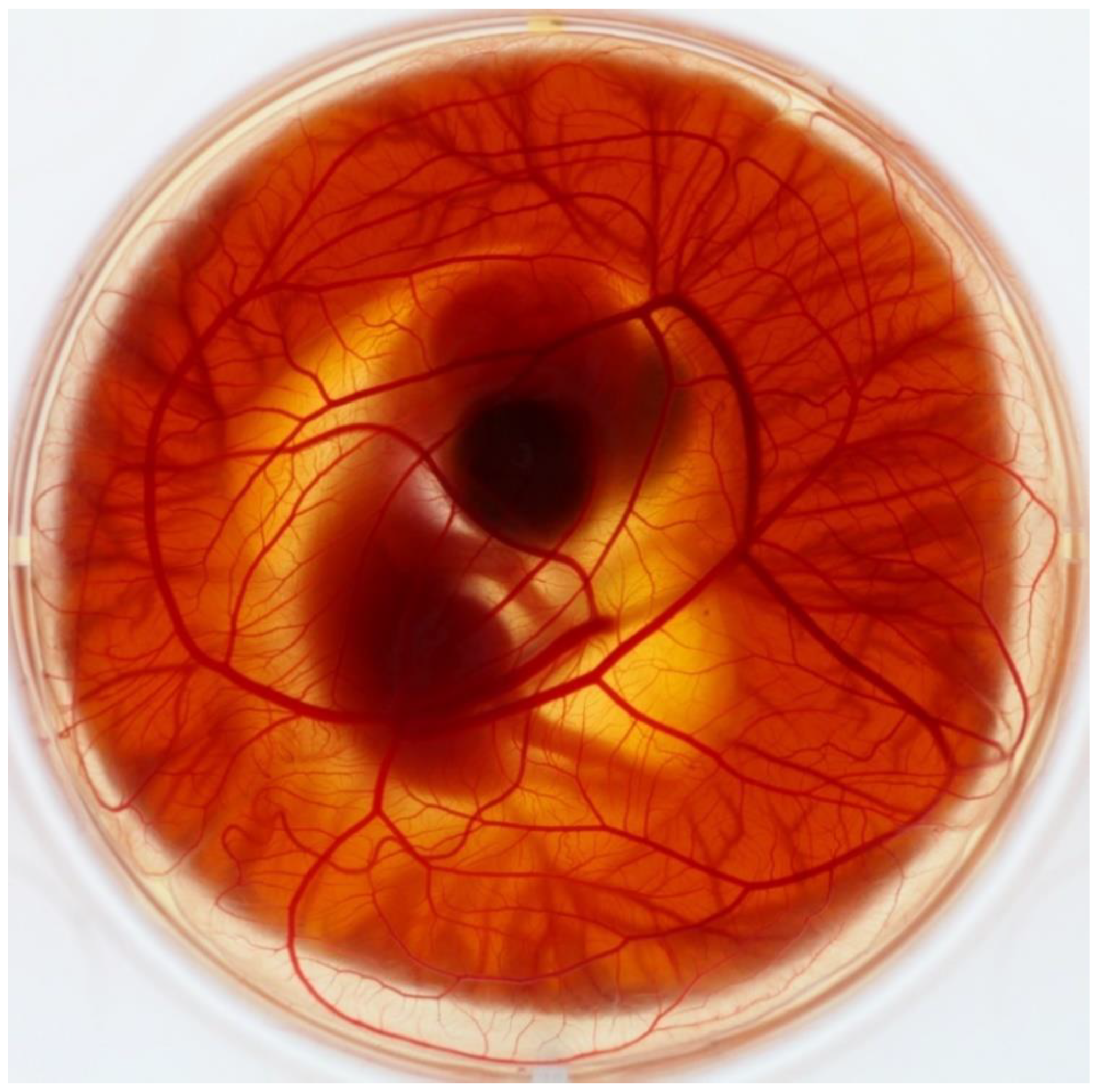
2. CAM Development and Morphology
2.1. Differences in the Embryo Development between Various Avian Species
2.2. Vascular System Development
3. Application
3.1. CAM Model Application in Angiogenesis Research
3.2. CAM Model Application in Transplantations and Tumour Grafting Research
3.3. CAM Model Application in Microbiology and Other Areas
3.4. Quail CAM Model Application
3.4.1. Angiogenesis Research
3.4.2. Xenotransplantations and Angiogenesis Research
3.4.3. Photodynamic Diagnosis and Therapy
3.5. Turkey CAM Model Application
3.6. Duck CAM Model Application
4. Methods and Protocols of CAM Assay Application
4.1. In Ovo and Ex Ovo Incubation Method
4.2. Substance Application on the CAM
4.3. Differences in CAM Assays of Various Avian Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The Chicken Chorioallantoic Membrane Model in Biology, Medicine and Bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Elaroussi, M.A.; Uhland-Smith, A.; Hellwig, W.; DeLuca, H.F. The Role of Vitamin D in Chorioallantoic Membrane Calcium Transport. Biochim. Biophys. Acta Biomembr. 1994, 1192, 1–6. [Google Scholar] [CrossRef]
- Lusimbo, W.S.; Leighton, F.A.; Wobeser, G.A. Histology and Ultrastructure of the Chorioallantoic Membrane of the Mallard Duck (Anas platyrhynchos). Anat. Rec. 2000, 259, 25–34. [Google Scholar] [CrossRef]
- Valdes, T.I.; Klueh, U.; Kreutzer, D.; Moussy, F. Ex Ova Chick Chorioallantoic Membrane as a Novel in Vivo Model for Testing Biosensors. J. Biomed. Mater. Res. A 2003, 67, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.G.; Accili, D. The Chick Chorioallantoic Membrane: A Model of Molecular, Structural, and Functional Adaptation to Transepithelial Ion Transport and Barrier Function during Embryonic Development. J. Biomed. Biotechnol. 2010, 2010, 940741. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Metheuen & Co. Ltd.: London, UK, 1959. [Google Scholar]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick Embryo Chorioallantoic Membrane (CAM): An Alternative Predictive Model in Acute Toxicological Studies for Anti-Cancer Drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rous, P.; Murphy, J.B. Tumor Implantations in the Developing Embryo. J. Am. Med. Assoc. 1911, 56, 741–742. [Google Scholar] [CrossRef]
- Goodpasture, E.W.; Woodruff, A.M.; Buddingh, G.J. The Cultivation of Vaccine and Other Viruses in the Chorioallantoic Membrane of Chick Embryos. Science 1931, 74, 371–372. [Google Scholar] [CrossRef]
- Morrow, G.; Syverton, J.T.; Stiles, W.W.; Berry, G.P. The Growth of Leptospira Icterohemorrhagiae on the Chorioallantoic Membrane of the Chick Embryo. Science 1938, 88, 384–385. [Google Scholar] [CrossRef]
- Isachenko, V.; Mallmann, P.; Petrunkina, A.M.; Rahimi, G.; Nawroth, F.; Hancke, K.; Felberbaum, R.; Genze, F.; Damjanoski, I.; Isachenko, E. Comparison of In Vitro- and Chorioallantoic Membrane (CAM)-Culture Systems for Cryopreserved Medulla-Contained Human Ovarian Tissue. PLoS ONE 2012, 7, e32549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cimpean, A.M.; Ribatti, D.; Raica, M. The Chick Embryo Chorioallantoic Membrane as a Model to Study Tumor Metastasis. Angiogenesis 2008, 11, 311–319. [Google Scholar] [CrossRef]
- Leene, W.; Duyzings, M.J.; van Steeg, C. Lymphoid Stem Cell Identification in the Developing Thymus and Bursa of Fabricius of the Chick. Z. Zellforsch. Mikrosk. Anat. 1973, 136, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Janković, B.D.; Isaković, K.; Lukić, M.L.; Vujanović, N.L.; Petrović, S.; Marković, B.M. Immunological Capacity of the Chicken Embryo. I. Relationship between the Maturation of Lymphoid Tissues and the Occurrence of Cell-Mediated Immunity in the Developing Chicken Embryo. Immunology 1975, 29, 497–508. [Google Scholar]
- Janse, E.M.; Jeurissen, S.H.M. Ontogeny and Function of Two Non-Lymphoid Cell Populations in the Chicken Embryo. Immunobiology 1991, 182, 472–481. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Ranieri, G.; Sorino, S.; Roncali, L. The Chick Embryo Chorioallantoic Membrane as an In Vivo Wound Healing Model. Pathol. Res. Pract. 1996, 192, 1068–1076. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM) Assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Knox, Y.; Munro, C.A.; Thompson, W.D. Infection of Chick Chorioallantoic Membrane (CAM) as a Model for Invasive Hyphal Growth and Pathogenesis of Candida albicans. Med. Mycol. 2003, 41, 331–338. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Grosse, K.; Berndt, A.; Hube, B. Pathogenesis of Candida albicans Infections in the Alternative Chorio-Allantoic Membrane Chicken Embryo Model Resembles Systemic Murine Infections. PLoS ONE 2011, 6, e19741. [Google Scholar] [CrossRef]
- National Institute of Health. The Public Health Service Responds to Commonly Asked Questions. ILAR J. 1991, 33, 68–70. [Google Scholar] [CrossRef]
- Dünker, N.; Jendrossek, V. Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research. Cancers 2019, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Dupertuis, Y.M.; Delie, F.; Cohen, M.; Pichard, C. In Ovo Method for Evaluating the Effect of Nutritional Therapies on Tumor Development, Growth and Vascularization. Clin. Nutr. Exp. 2015, 2, 9–17. [Google Scholar] [CrossRef]
- U.K. Home Office. Guidance on the Operation of the Animals (Scientific Procedures) Act 1986; H M Government: London, UK, 2014; pp. 1–148.
- Marshall, K.M.; Kanczler, J.M.; Oreffo, R.O.C. Evolving Applications of the Egg: Chorioallantoic Membrane Assay and Ex Vivo Organotypic Culture of Materials for Bone Tissue Engineering. J. Tissue Eng. 2020, 11, 2041731420942734. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, I.; Reis, A.; Ohana, A.; Taizi, M.; Cipok, M.; Tavor, S.; Rund, D.; Deutsch, V.R.; Goldstein, R.S. Engraftment of Human Blood Malignancies to the Turkey Embryo: A Robust New in Vivo Model. Leuk. Res. 2009, 33, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, R.; Akhtar, N.; Lewis, R.L.; Shinners, B.L. Angiogenesis Assays: Problems and Pitfalls. Cancer Metastasis Rev. 2000, 19, 167–172. [Google Scholar] [CrossRef]
- Romanoff, A.L. The Avian Embryo: Structural and Functional Development; McMillan: New York, NY, USA, 1960. [Google Scholar]
- Makanya, A.N.; Dimova, I.; Koller, T.; Styp-Rekowska, B.; Djonov, V. Dynamics of the Developing Chick Chorioallantoic Membrane Assessed by Stereology, Allometry, Immunohistochemistry and Molecular Analysis. PLoS ONE 2016, 11, e0152821. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Ainsworth, S.J.; Stanley, R.L.; Evans, D.J.R. Developmental Stages of the Japanese Quail. J. Anat. 2010, 216, 3–15. [Google Scholar] [CrossRef]
- Capua, I.; Mutinelli, F.; Hablovarid, M.H. Avian Embryo Susceptibility to Italian H7N1 Avian Influenza Viruses Belonging to Different Genetic Lineages. Arch. Virol. 2002, 147, 1611–1621. [Google Scholar] [CrossRef]
- Annas, A.; Brunström, B.; Brittebo, E.B. CYP1A-Dependent Activation of Xenobiotics in Endothelial Linings of the Chorioallantoic Membrane (CAM) in Birds. Arch. Toxicol. 2000, 74, 335–342. [Google Scholar] [CrossRef]
- Ausprunk, D.H.; Knighton, D.R.; Folkman, J. Differentiation of Vascular Endothelium in the Chick Chorioallantois: A Structural and Autoradiographic Study. Dev. Biol. 1974, 38, 237–248. [Google Scholar] [CrossRef]
- Missirlis, E.; Karakiulakis, G.; Maragoudakis, M.E. Angiogenesis Is Associated with Collagenous Protein Synthesis and Degradation in the Chick Chorioallantoic Membrane. Tissue Cell 1990, 22, 419–426. [Google Scholar] [CrossRef]
- Olivo, M.; Bhardwaj, R.; Schulze-Osthoff, K.; Sorg, C.; Jacob, H.J.; Flamme, I. A Comparative Study on the Effects of Tumor Necrosis Factor-α (TNF-α), Human Angiogenic Factor (h-AF) and Basic Fibroblast Growth Factor (BFGF) on the Chorioallantoic Membrane of the Chick Embryo. Anat. Rec. 1992, 234, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Parsons-Wingerter, P.; Lwai, B.; Yang, M.C.; Elliott, K.E.; Milaninia, A.; Redlitz, A.; Clark, J.I.; Sage, E.H. A Novel Assay of Angiogenesis in the Quail Chorioallantoic Membrane: Stimulation by BFGF and Inhibition by Angiostatin According to Fractal Dimension and Grid Intersection. Microvasc. Res. 1998, 55, 201–214. [Google Scholar] [CrossRef]
- Parsons-Wingerter, P.; Chandrasekharan, U.M.; McKay, T.L.; Radhakrishnan, K.; DiCorleto, P.E.; Albarran, B.; Farr, A.G. A VEGF165-Induced Phenotypic Switch from Increased Vessel Density to Increased Vessel Diameter and Increased Endothelial NOS Activity. Microvasc. Res. 2006, 72, 91–100. [Google Scholar] [CrossRef]
- Parsons-Wingerter, P.; Elliott, K.E.; Farr, A.G.; Radhakrishnan, K.; Clark, J.I.; Sage, E.H. Generational Analysis Reveals That TGF-Β1 Inhibits the Rate of Angiogenesis in Vivo by Selective Decrease in the Number of New Vessels. Microvasc. Res. 2000, 59, 221–232. [Google Scholar] [CrossRef]
- Schlatter, P.; König, M.F.; Karlsson, L.M.; Burri, P.H. Quantitative Study of Intussusceptive Capillary Growth in the Chorioallantoic Membrane (CAM) of the Chicken Embryo. Microvasc. Res. 1997, 54, 65–73. [Google Scholar] [CrossRef]
- Rizzo, V.; David, D. Macromolecular Selectivity of Chick Chorioallantoic Membrane Microvessels during Normal Angiogenesis and Endothelial Differentiation. Tissue Cell 1993, 25, 847–856. [Google Scholar] [CrossRef]
- Burton, G.J.; Palmer, M.E. Development of the Chick Chorioallantoic Capillary Plexus under Normoxic and Normobaric Hypoxic and Hyperoxic Conditions: A Morphometric Study. J. Exp. Zool. 1992, 262, 291–298. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Burri, P.H.; Djonov, V. Chorioallantoic Membrane Capillary Bed: A Useful Target for Studying Angiogenesis and Anti-Angiogenesis In Vivo. Anat. Rec. 2001, 264, 317–324. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane as an In Vivo Assay to Study Antiangiogenesis. Pharmaceuticals 2010, 3, 482–513. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane as a Model for Tumor Biology. Exp. Cell Res. 2014, 328, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R. The Chick Embryo Chorioallantoic Membrane as an in Vivo Experimental Model to Study Human Neuroblastoma. J. Cell. Physiol. 2019, 234, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The Chick Embryo and Its Chorioallantoic Membrane (CAM) for the in Vivo Evaluation of Drug Delivery Systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Victorelli, F.D.; Cardoso, V.M.; Ferreira, N.N.; Calixto, G.M.F.; Fontana, C.R.; Baltazar, F.; Gremião, M.P.D.; Chorilli, M. Chick Embryo Chorioallantoic Membrane as a Suitable in Vivo Model to Evaluate Drug Delivery Systems for Cancer Treatment: A Review. Eur. J. Pharm. Biopharm. 2020, 153, 273–284. [Google Scholar] [CrossRef]
- Mandal, P.S.; Mukhopadhayay, S.K.; Pradhan, S.; Mondal, S.; Jana, C.; Patra, N.C.; Hansda, R.N. Development of Nested Polymerase Chain Reaction-Based Diagnosis of Duck Enteritis Virus and Detection of DNA Polymerase Gene from Non-Descriptive Duck Breeds of West Bengal, India. Vet. World 2017, 10, 336–341. [Google Scholar] [CrossRef]
- Luepke, N.P.; Kemper, F.H. The HET-CAM Test: An Alternative to the Draize Eye Test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Lotz, C. Alternative Methods for the Replacement of Eye Irritation Testing. ALTEX 2016, 33, 55–67. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the Study of Irritation and Toxicity of Substances Applied Topically to the Skin and Mucous Membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Kishore, A.S.; Surekha, P.A.; Sekhar, P.V.R.; Srinivas, A.; Murthy, P.B. Hen Egg Chorioallantoic Membrane Bioassay: An in Vitro Alternative to Draize Eye Irritation Test for Pesticide Screening. Int. J. Toxicol. 2008, 27, 449–453. [Google Scholar] [CrossRef]
- Tay, S.L.M.; Heng, P.W.S.; Chan, L.W. An Investigation of the Chick Chorioallantoic Membrane as an Alternative Model to Various Biological Tissues for Permeation Studies. J. Pharm. Pharmacol. 2011, 63, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Kunzi-Rapp, K.; Rück, A.; Kaufmann, R. Characterization of the Chick Chorioallantoic Membrane Model as a Short-Term in Vivo System for Human Skin. Arch. Dermatol. Res. 1999, 291, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Slodownik, D.; Grinberg, I.; Spira, R.M.; Skornik, Y.; Goldstein, R.S. The Human Skin/Chick Chorioallantoic Membrane Model Accurately Predicts the Potency of Cosmetic Allergens. Exp. Dermatol. 2009, 18, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Monteiro Machado, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Testing Vaginal Irritation with the Hen’s Egg Test-Chorioallantoic Membrane Assay. ALTEX 2018, 35, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mangir, N.; Dikici, S.; Claeyssens, F.; Macneil, S. Using Ex Ovo Chick Chorioallantoic Membrane (CAM) Assay to Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV Crosslinked Biodegradable Hydrogel Containing Adipose Derived Stem Cells to Promote Vascularization for Skin Wounds and Tissue Engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef]
- Ribatti, D. Chick Embryo Chorioallantoic Membrane as a Useful Tool to Study Angiogenesis. Int. Rev. Cell Mol. Biol. 2008, 270, 181–224. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus Guidelines for the Use and Interpretation of Angiogenesis Assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- Kirchner, L.M.; Schmidt, S.P.; Gruber, B.S. Quantitation of Angiogenesis in the Chick Chorioallantoic Membrane Model Using Fractal Analysis. Microvasc. Res. 1996, 51, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Vickerman, M.B.; Keith, P.A.; McKay, T.L.; Gedeon, D.J.; Watanabe, M.; Montano, M.; Karunamuni, G.; Kaiser, P.K.; Sears, J.E.; Ebrahem, Q.; et al. VESGEN 2D: Automated, User-Interactive Software for Quantification and Mapping of Angiogenic and Lymphangiogenic Trees and Networks. Anat. Rec. 2009, 292, 320–332. [Google Scholar] [CrossRef]
- Lubkin, S.R.; Funk, S.E.; Sage, E.H. Quantifying Vasculature: New Measures Applied to Arterial Trees in the Quail Chorioallantoic Membrane. J. Theor. Med. 2005, 6, 173–180. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; van Berkel, M.; van den Bergh, H.; Griffioen, A.W. Vascular Regrowth Following Photodynamic Therapy in the Chicken Embryo Chorioallantoic Membrane. Angiogenesis 2010, 13, 281–292. [Google Scholar] [CrossRef][Green Version]
- Giannopoulou, E.; Katsoris, P.; Hatziapostolou, M.; Kardamakis, D.; Kotsaki, E.; Polytarchou, C.; Parthymou, A.; Papaioannou, S.; Papadimitriou, E. X-Rays Modulate Extracellular Matrixin Vivo. Int. J. Cancer 2001, 94, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an In Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.; Schenker, A.; Sähr, H.; Lehner, B.; Fellenberg, J. Optimization of the Chicken Chorioallantoic Membrane Assay as Reliable in Vivo Model for the Analysis of Osteosarcoma. PLoS ONE 2019, 14, e0215312. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Javerzat, S.; Gilges, D.; Meyre, A.; de Lafarge, B.; Eichmann, A.; Bikfalvi, A. Accessing Key Steps of Human Tumor Progression in Vivo by Using an Avian Embryo Model. Proc. Natl. Acad. Sci. USA 2005, 102, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, T.; Kavalar, R.; Barone, T.A.; Plunkett, R.J. Experimental Model and Immunohistochemical Comparison of U87 Human Glioblastoma Cell Xenografts on the Chicken Chorioallantoic Membrane and in Rat Brains. Anticancer Res. 2010, 30, 4851–4860. [Google Scholar] [PubMed]
- Buríková, M.; Bilčík, B.; Máčajová, M.; Výboh, P.; Bizik, J.; Mateašík, A.; Miškovský, P.; Čavarga, I. Hypericin Fluorescence Kinetics in the Presence of Low Density Lipoproteins: Study on Quail CAM Assay for Topical Delivery. Gen. Physiol. Biophys. 2016, 35, 459–468. [Google Scholar] [CrossRef]
- Huntosova, V.; Gay, S.; Nowak-Sliwinska, P.; Rajendran, S.K.; Zellweger, M.; van den Bergh, H.; Wagnières, G. In Vivo Measurement of Tissue Oxygenation by Time-Resolved Luminescence Spectroscopy: Advantageous Properties of Dichlorotris(1, 10-Phenanthroline)-Ruthenium(II) Hydrate. J. Biomed. Opt. 2014, 19, 077004. [Google Scholar] [CrossRef]
- Rajkumar, K.; Mvs, S.; Koganti, S.; Burgula, S. Selenium Nanoparticles Synthesized Using Pseudomonas Stutzeri (MH191156) Show Antiproliferative and Anti-Angiogenic Activity against Cervical Cancer Cells. Int. J. Nanomed. 2020, 15, 4523–4540. [Google Scholar] [CrossRef]
- Lenkavska, L.; Blascakova, L.; Jurasekova, Z.; Macajova, M.; Bilcik, B.; Cavarga, I.; Miskovsky, P.; Huntosova, V. Benefits of Hypericin Transport and Delivery by Low- and High-Density Lipoproteins to Cancer Cells: From In Vitro to Ex Ovo. Photodiagn. Photodyn. Ther. 2019, 25, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Majerník, M.; Jendželovský, R.; Babinčák, M.; Košuth, J.; Ševc, J.; Tonelli Gombalová, Z.; Jendželovská, Z.; Buríková, M.; Fedoročko, P. Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane. Int. J. Mol. Sci. 2019, 20, 3004. [Google Scholar] [CrossRef] [PubMed]
- Olek, M.; Kasperski, J.; Skaba, D.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A. Photodynamic Therapy for the Treatment of Oral Squamous Carcinoma-Clinical Implications Resulting from in Vitro Research. Photodiagn. Photodyn. Ther. 2019, 27, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Liu, M.; Scanlon, C.S.; Banerjee, R.; D’Silva, N.J. The Chick Chorioallantoic Membrane In Vivo Model to Assess Perineural Invasion in Head and Neck Cancer. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Knighton, D.; Ausprunk, D.; Tapper, D.; Folkman, J. Avascular and Vascular Phases of Tumour Growth in the Chick Embryo. Br. J. Cancer 1977, 35, 347–356. [Google Scholar] [CrossRef]
- Hecht, M.; Schulte, J.H.; Eggert, A.; Wilting, J.; Schweigerer, L. The Neurotrophin Receptor TrkB Cooperates with C-Met in Enhancing Neuroblastoma Invasiveness. Carcinogenesis 2005, 26, 2105–2115. [Google Scholar] [CrossRef]
- Komatsu, A.; Matsumoto, K.; Saito, T.; Muto, M.; Tamanoi, F. Patient Derived Chicken Egg Tumor Model (PDcE Model): Current Status and Critical Issues. Cells 2019, 8, 440. [Google Scholar] [CrossRef]
- Chambers, A.F.; Shafir, R.; Ling, V. A Model System for Studying Metastasis Using the Embryonic Chick. Cancer Res. 1982, 42, 4018–4025. [Google Scholar]
- Bobek, V.; Plachy, J.; Pinterova, D.; Kolostova, K.; Boubelik, M.; Jiang, P.; Yang, M.; Hoffman, R.M. Development of a Green Fluorescent Protein Metastatic-Cancer Chick-Embryo Drug-Screen Model. Clin. Exp. Metastasis 2004, 21, 347–352. [Google Scholar] [CrossRef]
- Palmer, T.D.; Lewis, J.; Zijlstra, A. Quantitative Analysis of Cancer Metastasis Using an Avian Embryo Model. J. Vis. Exp. 2011, 30, e2815. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.S.; Li, M.; Sanchez, E.; Li, J.; Berenson, A.; Wirtschafter, E.; Wang, J.; Shen, J.; Li, Z.; et al. A Novel Angiogenesis Model for Screening Anti-Angiogenic Compounds: The Chorioallantoic Membrane/Feather Bud Assay. Int. J. Oncol. 2010, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Li, M.; Wang, C.; Nichols, C.M.; Li, J.; Chen, H.; Berenson, J.R. Anti-Myeloma Effects of the Novel Anthracycline Derivative INNO-206. Clin. Cancer Res. 2012, 18, 3856–3867. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Mussa, S.; Lindemann, D.; Oelschlaeger, T.A.; Deadman, M.; Ferguson, D.J.P.; Moxon, R.; Schroten, H. The Avian Chorioallantoic Membrane in Ovo—A Useful Model for Bacterial Invasion Assays. Int. J. Med. Microbiol. 2002, 292, 267–275. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Binkowska, J.; Kohli, N.; Sharma, V. Towards the Development of a Novel Ex Ovo Model of Infection to Pre-Screen Biomaterials Intended for Treating Chronic Wounds. J. Funct. Biomater. 2020, 11, 37. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Three—Photosensitizer Pharmacokinetics, Biodistribution, Tumor Localization and Modes of Tumor Destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One—Photosensitizers, Photochemistry and Cellular Localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Henry, M.K.; Unsworth, B.R.; Sychev, V.; Guryeva, T.S.; Dadasheva, O.A.; Piert, S.J.; Lagel, K.E.; Dubrovin, L.C.; Jahns, G.C.; Boda, K.; et al. Launch Conditions Might Affect the Formation of Blood Vessels in the Quail Chorioallantoic Membrane. Folia Vet. 1998, 42, S25–S31. [Google Scholar]
- Parsons-Wingerter, P.; Elliott, K.E.; Clark, J.I.; Farr, A.G. Fibroblast Growth Factor-2 Selectively Stimulates Angiogenesis of Small Vessels in Arterial Tree. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1250–1256. [Google Scholar] [CrossRef]
- Roethy, W.; Fiehn, E.; Suehiro, K.; Gu, A.; Yi, G.H.; Shimizu, J.; Wang, J.; Zhang, G.; Ranieri, J.; Akella, R.; et al. A Growth Factor Mixture That Significantly Enhances Angiogenesis in Vivo. J. Pharmacol. Exp. Ther. 2001, 299, 494–500. [Google Scholar]
- McKay, T.L.; Gedeon, D.J.; Vickerman, M.B.; Hylton, A.G.; Ribita, D.; Olar, H.H.; Kaiser, P.K.; Parsons-Wingerter, P. Selective Inhibition of Angiogenesis in Small Blood Vessels and Decrease in Vessel Diameter throughout the Vascular Tree by Triamcinolone Acetonide. Investig. Opthalmol. Vis. Sci. 2008, 49, 1184. [Google Scholar] [CrossRef]
- Zakraoui, O.; Marcinkiewicz, C.; Aloui, Z.; Othman, H.; Grépin, R.; Haoues, M.; Essafi, M.; Srairi-Abid, N.; Gasmi, A.; Karoui, H.; et al. Lebein, a Snake Venom Disintegrin, Suppresses Human Colon Cancer Cells Proliferation and Tumor-Induced Angiogenesis through Cell Cycle Arrest, Apoptosis Induction and Inhibition of VEGF Expression. Mol. Carcinog. 2017, 56, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Abiuso, A.M.B.; Varela, M.L.; Haro Durand, L.; Besio Moreno, M.; Marcos, A.; Ponzio, R.; Rivarola, M.A.; Belgorosky, A.; Pignataro, O.P.; Berensztein, E.; et al. Histamine H4 Receptor as a Novel Therapeutic Target for the Treatment of Leydig-Cell Tumours in Prepubertal Boys. Eur. J. Cancer 2018, 91, 125–135. [Google Scholar] [CrossRef]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef]
- Výboh, P.; Zeman, M.; Bilčík, B.; Šárniková, B.; Košťál, Ľ. Angiogenic Effect of Leptin in the Quail Chorioallantoic Membrane. Acta Vet. Brno 2010, 79, 13–17. [Google Scholar] [CrossRef][Green Version]
- Macajova, M.; Cavarga, I.; Sykorova, M.; Valachovic, M.; Novotna, V.; Bilcik, B. Modulation of Angiogenesis by Topical Application of Leptin and High and Low Molecular Heparin Using the Japanese Quail Chorioallantoic Membrane Model. Saudi J. Biol. Sci. 2020, 27, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Gazit, A.; Staniszewska, I.; Marcinkiewicz, C.; Lelkes, P.I. Nerve Growth Factor (NGF) Promotes Angiogenesis in the Quail Chorioallantoic Membrane. Endothelium 2006, 13, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Petrovova, E.; Giretova, M.; Kvasilova, A.; Benada, O.; Danko, J.; Medvecky, L.; Sedmera, D. Preclinical Alternative Model for Analysis of Porous Scaffold Biocompatibility in Bone Tissue Engineering. ALTEX 2019, 36, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Wabo, H.K.; Eyong, K.O.; Feussi, M.T.; Wiench, B.; Krusche, B.; Tane, P.; Folefoc, G.N.; Efferth, T. Anticancer Activities of Six Selected Natural Compounds of Some Cameroonian Medicinal Plants. PLoS ONE 2011, 6, e21762. [Google Scholar] [CrossRef]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of Some Cameroonian Spices and Selected Medicinal Plant Extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Eichhorn, T.; Wiench, B.; Krusche, B.; Efferth, T. Cytotoxicity, Anti-Angiogenic, Apoptotic Effects and Transcript Profiling of a Naturally Occurring Naphthyl Butenone, Guieranone A. Cell Div. 2012, 7, 16. [Google Scholar] [CrossRef]
- Cui, H.; Guo, W.; Zhang, B.; Li, G.; Li, T.; Yuan, Y.; Zhang, N.; Yang, Y.; Feng, W.; Chu, F.; et al. BA-12 Inhibits Angiogenesis via Glutathione Metabolism Activation. Int. J. Mol. Sci. 2019, 20, 4062. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Wang, Z.; Li, G.; Li, L.; Huo, S.; Zhang, B.; He, R.; Chen, K.; Xu, B.; et al. Tetrahydropalmatine Triggers Angiogenesis via Regulation of Arginine Biosynthesis. Pharmacol. Res. 2021, 163, 105242. [Google Scholar] [CrossRef]
- Seo, E.-J.; Kuete, V.; Kadioglu, O.; Krusche, B.; Schröder, S.; Greten, H.J.; Arend, J.; Lee, I.-S.; Efferth, T. Antiangiogenic Activity and Pharmacogenomics of Medicinal Plants from Traditional Korean Medicine. Evid. Based Complement. Altern. Med. 2013, 2013, 131306. [Google Scholar] [CrossRef]
- Staniszewska, I.; Zaveri, S.; Del Valle, L.; Oliva, I.; Rothman, V.L.; Croul, S.E.; Roberts, D.D.; Mosher, D.F.; Tuszynski, G.P.; Marcinkiewicz, C. Interaction of Alpha9beta1 Integrin with Thrombospondin-1 Promotes Angiogenesis. Circ. Res. 2007, 100, 1308–1316. [Google Scholar] [CrossRef]
- Scotti, L.; Di Pietro, M.; Pascuali, N.; Irusta, G.; de Zúñiga, I.; Gomez Peña, M.; Pomilio, C.; Saravia, F.; Tesone, M.; Abramovich, D.; et al. Sphingosine-1-Phosphate Restores Endothelial Barrier Integrity in Ovarian Hyperstimulation Syndrome. Mol. Hum. Reprod. 2016, 22, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Abramovich, D.; Pascuali, N.; Durand, L.H.; Irusta, G.; de Zúñiga, I.; Tesone, M.; Parborell, F. Inhibition of Angiopoietin-1 (ANGPT1) Affects Vascular Integrity in Ovarian Hyperstimulation Syndrome (OHSS). Reprod. Fertil. Dev. 2016, 28, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Vasse, J. Transplantation of Turtle Embryonic Thymus into Quail Embryo: Colonization by Quail Cells. J. Embryol. Exp. Morphol. 1983, 77, 309–322. [Google Scholar] [PubMed]
- Talavera-Adame, D.; Dafoe, D.C.; Ng, T.T.; Wachsmann-Hogiu, S.; Castillo-Henkel, C.; Farkas, D.L. Enhancement of Embryonic Stem Cell Differentiation Promoted by Avian Chorioallantoic Membranes. Tissue Eng. Part A 2009, 15, 3193–3200. [Google Scholar] [CrossRef]
- Brachvogel, B.; Pausch, F.; Farlie, P.; Gaipl, U.; Etich, J.; Zhou, Z.; Cameron, T.; von der Mark, K.; Bateman, J.F.; Pöschl, E. Isolated Anxa5+/Sca-1+ Perivascular Cells from Mouse Meningeal Vasculature Retain Their Perivascular Phenotype In Vitro and In Vivo. Exp. Cell Res. 2007, 313, 2730–2743. [Google Scholar] [CrossRef]
- Papoutsi, M.; Sleeman, J.P.; Wilting, J. Interaction of Rat Tumor Cells with Blood Vessels and Lymphatics of the Avian Chorioallantoic Membrane. Microsc. Res. Tech. 2001, 55, 100–107. [Google Scholar] [CrossRef]
- Papoutsi, M.; Siemeister, G.; Weindel, K.; Tomarev, S.I.; Kurz, H.; Schächtele, C.; Martiny-Baron, G.; Christ, B.; Marmé, D.; Wilting, J. Active Interaction of Human A375 Melanoma Cells with the Lymphatics in Vivo. Histochem. Cell Biol. 2000, 114, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Schmidtova, S.; Dorssers, L.C.J.; Kalavska, K.; Gillis, A.J.M.; Oosterhuis, J.W.; Stoop, H.; Miklikova, S.; Kozovska, Z.; Burikova, M.; Gercakova, K.; et al. Napabucasin Overcomes Cisplatin Resistance in Ovarian Germ Cell Tumor-Derived Cell Line by Inhibiting Cancer Stemness. Cancer Cell Int. 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Staniszewska, I.; Lazarovici, P.; Tuszynski, G.P.; Del Valle, L.; Marcinkiewicz, C. Regulatory Effect of Nerve Growth Factor in 9 1 Integrin-Dependent Progression of Glioblastoma. Neuro Oncol. 2008, 10, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Staniszewska, I.; Del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic Activity of Obtustatin as Alpha1beta1 Integrin Inhibitor in Experimental Melanoma Growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Momic, T.; Cohen, G.; Reich, R.; Arlinghaus, F.T.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vixapatin (VP12), a c-Type Lectin-Protein from Vipera Xantina Palestinae Venom: Characterization as a Novel Anti-Angiogenic Compound. Toxins 2012, 4, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Debreova, M.; Csaderova, L.; Burikova, M.; Lukacikova, L.; Kajanova, I.; Sedlakova, O.; Kery, M.; Kopacek, J.; Zatovicova, M.; Bizik, J.; et al. CAIX Regulates Invadopodia Formation through Both a PH-Dependent Mechanism and Interplay with Actin Regulatory Proteins. Int. J. Mol. Sci. 2019, 20, 2745. [Google Scholar] [CrossRef]
- Bardet, S.M.; Carr, L.; Soueid, M.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Multiphoton Imaging Reveals That Nanosecond Pulsed Electric Fields Collapse Tumor and Normal Vascular Perfusion in Human Glioblastoma Xenografts. Sci. Rep. 2016, 6, 34443. [Google Scholar] [CrossRef]
- González-Iriarte, M.; Carmona, R.; Pérez-Pomares, J.M.; Macías, D.; Angel Medina, M.; Quesada, A.R.; Muñoz-Chápuli, R. A Modified Chorioallantoic Membrane Assay Allows for Specific Detection of Endothelial Apoptosis Induced by Antiangiogenic Substances. Angiogenesis 2003, 6, 251–254. [Google Scholar] [CrossRef]
- Cárdenas, C.; Quesada, A.R.; Medina, M.A. Anti-Angiogenic and Anti-Inflammatory Properties of Kahweol, a Coffee Diterpene. PLoS ONE 2011, 6, e23407. [Google Scholar] [CrossRef]
- Čavarga, I.; Bilčík, B.; Výboh, P.; Záškvarová, M.; Chorvát, D.; Kasák, P.; Mlkvý, P.; Mateašík, A.; Chorvátová, A.; Miškovský, P. Photodynamic Effect of Hypericin after Topical Application in the Ex Ovo Quail Chorioallantoic Membrane Model. Planta Med. 2014, 80, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Langguth, P.; Spicher, K.; Enzmann, H. Comparative Toxicity and Cell-Tissue Distribution Study on Nanoparticular Iron Complexes Using Avian Embryos and HepG2-Cells. Transl. Res. 2008, 151, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Borges, M.; Kadyrov, M. Forskolin-Induced Differentiation of BeWo Cells Stimulates Increased Tumor Growth in the Chorioallantoic Membrane (CAM) of the Turkey (Meleagris gallopavo) Egg. Ann. Anat. 2011, 193, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Witt, A.; Ahr, H.; Brendler-Schwaab, S.; Enzmann, H.; Steinke, W. Carcinogen-Induced Mitochondrial DNA Damage in the In Ovo Model. Toxicol. In Vitro 1998, 12, 329–333. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Almeda, R.A.; Vidallon, M.L.P.; Reyes, C.T. Enhanced Bioactivity and Efficient Delivery of Quercetin through Nanoliposomal Encapsulation Using Rice Bran Phospholipids. J. Sci. Food Agric. 2019, 99, 1980–1989. [Google Scholar] [CrossRef]
- Canoy, J.L.; Bitacura, J.G. Cytotoxicity and Antiangiogenic Activity of Turbinaria ornata Agardh and Padina australis Hauck Ethanolic Extracts. Anal. Cell. Pathol. 2018, 2018, 3709491. [Google Scholar] [CrossRef] [PubMed]
- Yahya, A.L.L. Angiosuppressive and Teratogenic Influence of an Edible Medicinal Herb, Insulin Plant (Costus igneus) in Mallard Duck (Anas platyrhynchos) Embryos. In Proceedings of the Multi-Disciplinary Manila (Philippines) Conferences, Cebu, Philippines, 26–27 January 2017. [Google Scholar]
- Paul, J.; Gamallo, M.; Gamallo, J.P.M.; Espere, G.; Carillo, D.M.C.; Dianne, N. Evaluation of Antiangiogenic Property of Ocimum basilica Ethanolic Leaf Extract by Using Duck Embryo Chorioallantoic Membrane (Cam) Assay and Its Morphometric Analysis Evaluation of Antiangiogenic Property of Ocimum Morphometric Analysis. Int. J. Herb. Med. 2016, 4, 22–26. [Google Scholar]
- Herrera, A.A.; Amor, E.C. Antiangiogenic Activity of Extracts and Fractions from an Endemic Plant Ardisia pyramidalis (CAV.) Pers. from Bataan, Philippines Using Duck in Ovo Chorioallantoic Membrane Assay. J. Med. Plants Res. 2011, 5, 2637–2646. [Google Scholar]
- Camposano, J.E.; Dela Torre, G.L.T.; Laxamana, J.G.; Larcia II, L.L. Screening for the Anti-Angiogenic Activity of Selected Philippine Medicinal Plants Using Chorioallantoic Membrane Assay. Mahidol. Univ. J. Pharm. Sci. 2016, 43, 173–182. [Google Scholar]
- Acedo, J.Z.; Reyes, C.T.; Rodriguez, E.B. Antimicrobial Peptide Structure, Function, and Mode of Action View Project Health-Promoting Lipids from Purslane (Portulaca Oleracea L.): Isolation, Characterization, Quantification and In Vivo Assay of Angiogenic Activity. Philipp Agric. Sci. 2012, 95, 327–334. [Google Scholar]
- Maniago, K.G.N.; Mari, C.G.S.; Pareja, M.C.; Grace, K.; Maniago, N. Angiogenic Effect of Curcuma longa Linn. (Turmeric) Tea Powder on the Chorioallantoic Membrane of 10-Day Old Annas Luzonica (Duck) Eggs. Sch. Res. Libr. Ann. Biol. Res. 2014, 5, 32–37. [Google Scholar]
- Deocaris, C.C.; Castro, M.C.P.; Oabel, A.T.; Co, E.L.; Mojica, E.-R.E. Screening for Anti-Angiogenic Activity in Shiitake Mushroom (Lentinus edodes Berk) Extracts. J. Med. Sci. 2004, 5, 43–46. [Google Scholar] [CrossRef]
- Villaflores, O.B.; Ortega, K.M.M.; Empaynado-Porto, A.; Lirio, S.; Yak, H.-K.; Albano, D.R.; Corpuz, M.J.-A.T. Anti-Angiogenic Activity of Gracilaria coronopifolia J.G. Agardh Extract by Lowering the Levels of Trace Metals (Iron, Zinc and Copper) in Duck Chorioallantoic Membrane and in Vitro Activation of AMP-Kinase. Mol. Biol. Rep. 2019, 46, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.B.; Vidallon, M.L.P.; Mendoza, D.J.R.; Reyes, C.T. Health-Promoting Bioactivities of Betalains from Red Dragon Fruit (Hylocereus polyrhizus (Weber) Britton and Rose) Peels as Affected by Carbohydrate Encapsulation. J. Sci. Food Agric. 2016, 96, 4679–4689. [Google Scholar] [CrossRef]
- Raga, D.D.; Herrera, A.A.; Shen, C.-C.; Ragasa, C.Y. Triterpenes from Ardisia Cf. elliptica (Subgenus Tinus) Limit Vascular Density and Promote von Willebrand Factor Expression on Duck Chorioallantoic Membrane. Pharm. Chem. J. 2013, 47, 44–49. [Google Scholar] [CrossRef]
- Raga, D.D.; Herrera, A.A.; Ragasa, C.Y. Angio-Suppressive Triterpenoids from Ardisia Cf. elliptica (Subgenus Tinus) on Duck (Anas Platyrynchos L.) Chorioallantoic Membrane. Chin. J. Nat. Med. 2013, 11, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Boydston, W.R.; Sohal, G.S. Decrease in Acetylcholine Receptor Number Correlated with Increased Naturally Occurring Trochlear Motor Neuron Death during Development. Exp. Neurol. 1983, 79, 801–807. [Google Scholar] [CrossRef]
- Sohal, G.S.; Kumaresan, K.; Hirano, S.; Ali, M.M. Synapse Formation on Trochlear Motor Neurons under Conditions of Increased and Decreased Cell Death during Development. Int. J. Dev. Neurosci. 1991, 9, 563–570. [Google Scholar] [CrossRef]
- Gionti, E.; Jullien, P.; Pontarelli, G.; Sanchez, M. A Continuous Line of Chicken Embryo Cells Derived from a Chondrocyte Culture Infected with RSV. Cell Differ. Dev. 1989, 27, 215–223. [Google Scholar] [CrossRef]
- Sellier, N.; Brillard, J.-P.; Dupuy, V.; Bakst, M.R. Comparative Staging of Embryo Development in Chicken, Turkey, Duck, Goose, Guinea Fowl, and Japanese Quail Assessed from Five Hours After Fertilization Through Seventy-Two Hours of Incubation. J. Appl. Poult. Res. 2006, 15, 219–228. [Google Scholar] [CrossRef]
- Li, S.; Bai, S.; Qin, X.; Zhang, J.; Irwin, D.M.; Zhang, S.; Wang, Z. Comparison of Whole Embryonic Development in the Duck (Anas platyrhynchos) and Goose (Anser cygnoides) with the Chicken (Gallus gallus). Poult. Sci. 2019, 98, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.; Mikawa, T. Avians as a Model System of Vascular Development. Methods Mol. Biol. 2015, 1214, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dünker, N. Chick Ex Ovo Culture and Ex Ovo CAM Assay: How It Really Works. J. Vis. Exp. 2009, 30, e1620. [Google Scholar] [CrossRef]
- Meta, M.; Kundekova, B.; Bilcik, B.; Macajova, M. The Effect of Silicone Ring Application on CAM Vasculature in Japanese Quail (Coturnix japonica). In Proceedings of the 17th Student Scientific Conference 2019, Wrocław, Poland, 18–21 September 2019. [Google Scholar]
- Huss, D.; Poynter, G.; Lansford, R. Japanese Quail (Coturnix japonica) as a Laboratory Animal Model. Lab Anim. 2008, 37, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A. Intravascular Drug Delivery with a Pulsed Liquid Microjet. Arch. Ophthalmol. 2002, 120, 1206. [Google Scholar] [CrossRef] [PubMed]



| Species | Development Duration | Experimental Period | Experimental Window | References | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallus gallus | 21 | ED7–ED14 (* ED18) | 8–12 days | [61] | ||||||||||||||||||||||||
| Coturnix japonica | 16 | ED6–ED12 | 7 days | [118] | ||||||||||||||||||||||||
| Meleagris gallopavo | 28–30 | ED10–ED25 | 14–15 days | [26,126] | ||||||||||||||||||||||||
| Anas platyrynchos | 27–28 | ED7–ED24 | 15–18 days | [140,145] | ||||||||||||||||||||||||
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| Gallus gallus | 12 days | |||||||||||||||||||||||||||
| Coturnic japonica | 7 days | |||||||||||||||||||||||||||
| Meleagris gallopavo | 16 days | |||||||||||||||||||||||||||
| Anas platyrynchos | 18 days | |||||||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundeková, B.; Máčajová, M.; Meta, M.; Čavarga, I.; Bilčík, B. Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications. Biology 2021, 10, 301. https://doi.org/10.3390/biology10040301
Kundeková B, Máčajová M, Meta M, Čavarga I, Bilčík B. Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications. Biology. 2021; 10(4):301. https://doi.org/10.3390/biology10040301
Chicago/Turabian StyleKundeková, Barbora, Mariana Máčajová, Majlinda Meta, Ivan Čavarga, and Boris Bilčík. 2021. "Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications" Biology 10, no. 4: 301. https://doi.org/10.3390/biology10040301
APA StyleKundeková, B., Máčajová, M., Meta, M., Čavarga, I., & Bilčík, B. (2021). Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications. Biology, 10(4), 301. https://doi.org/10.3390/biology10040301






