A Clinical and Histological Study about the Socket Preservation in a Patient under Oral Bisphosphonates Treatment: A Case Report
Abstract
1. Introduction
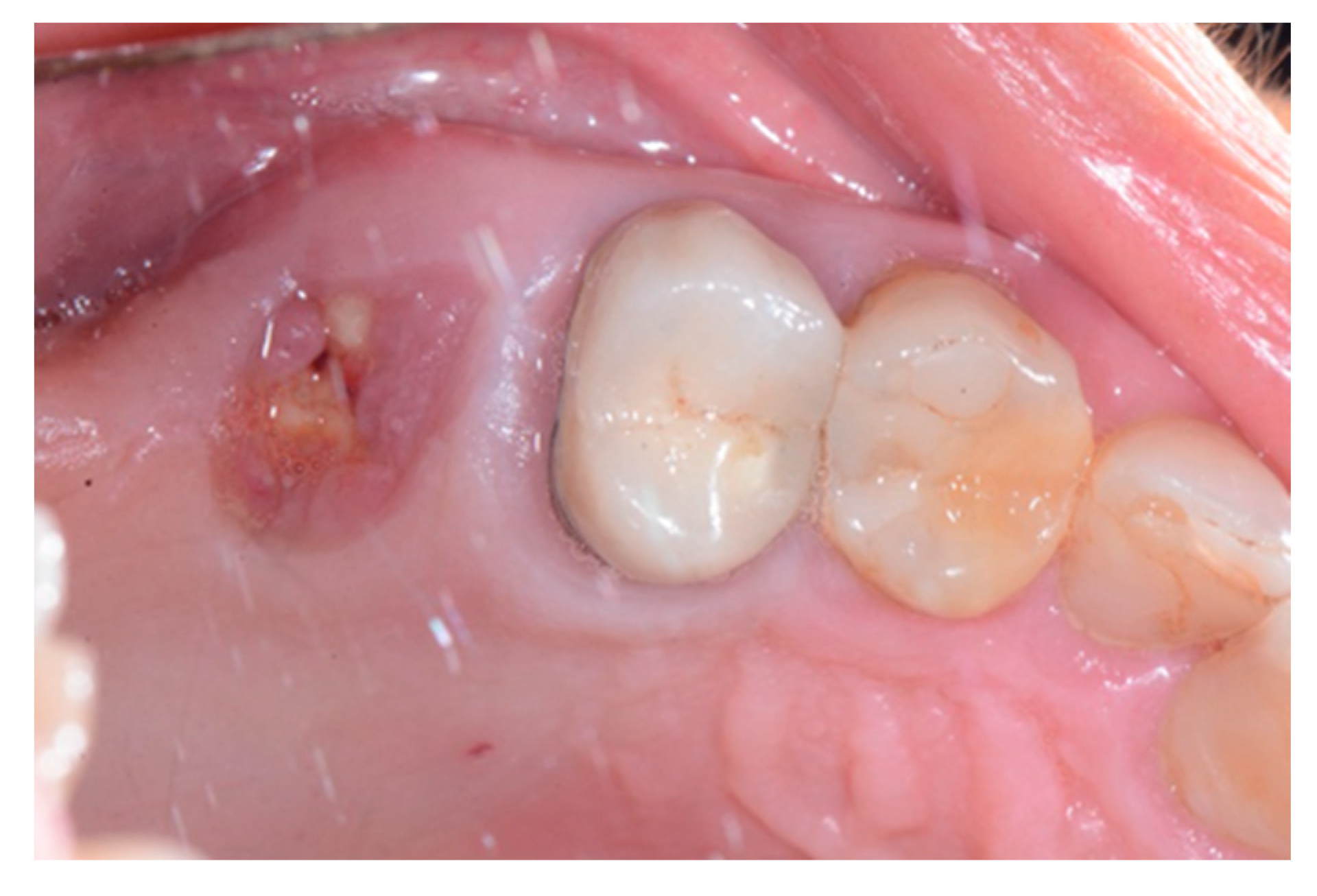
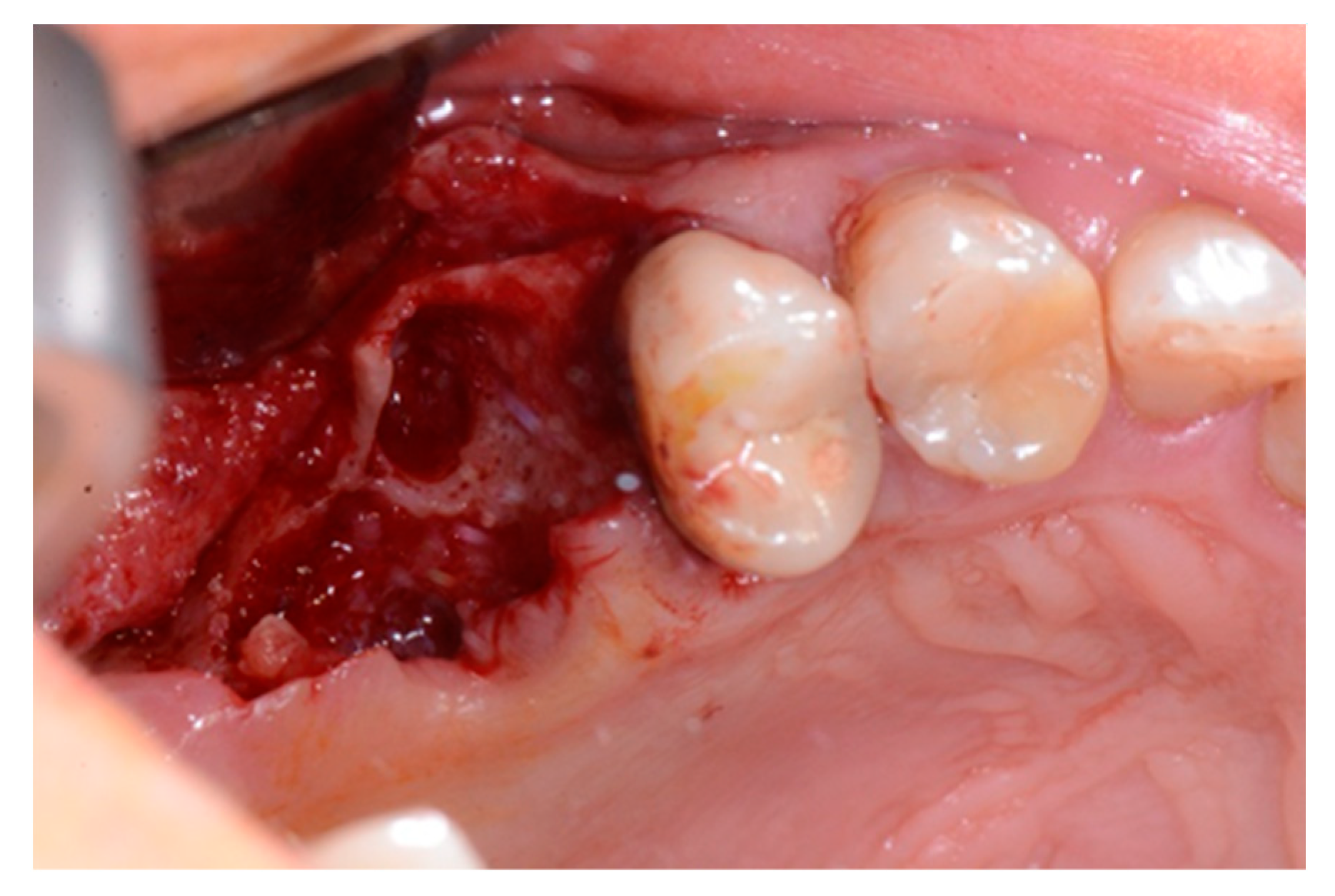
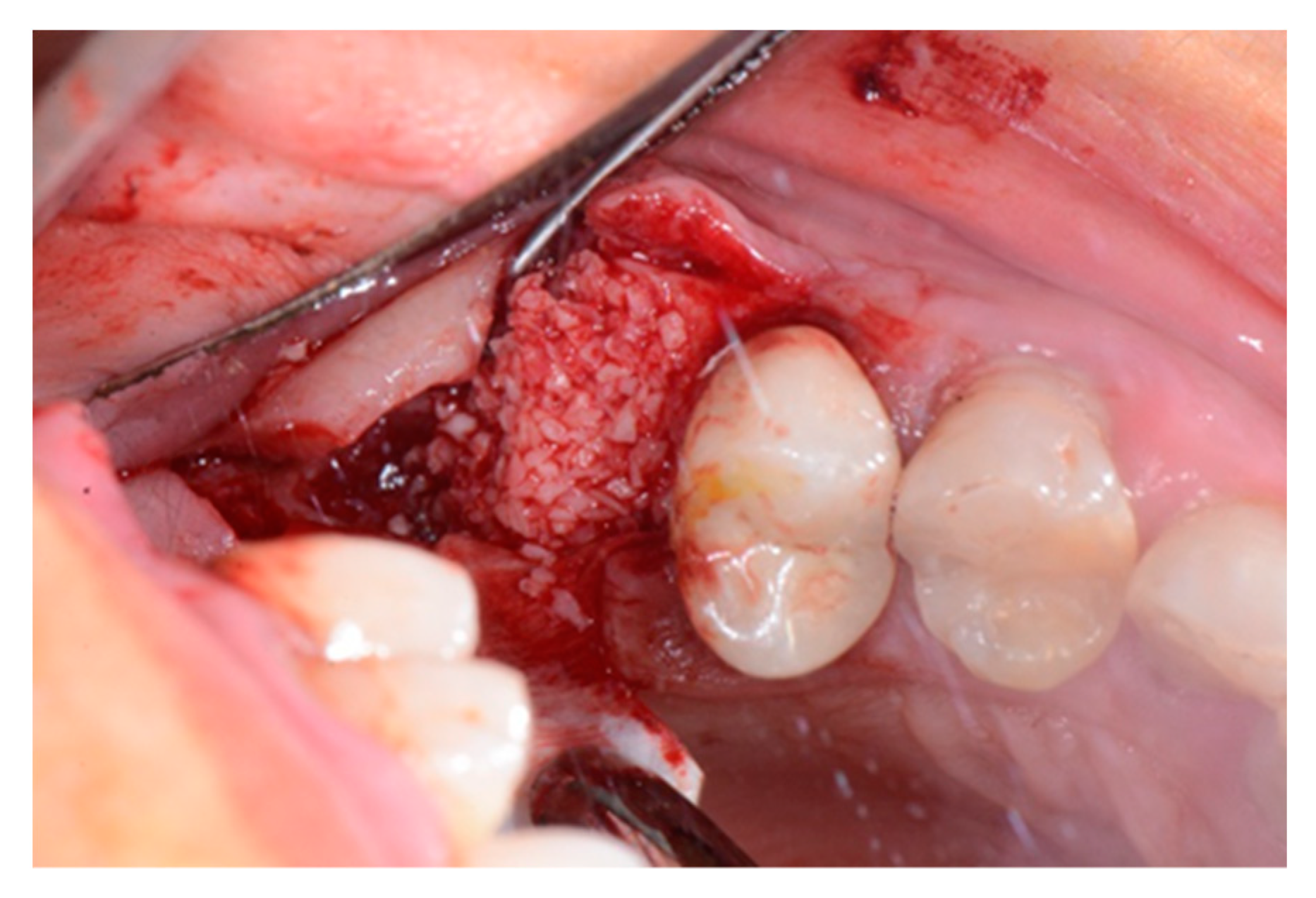
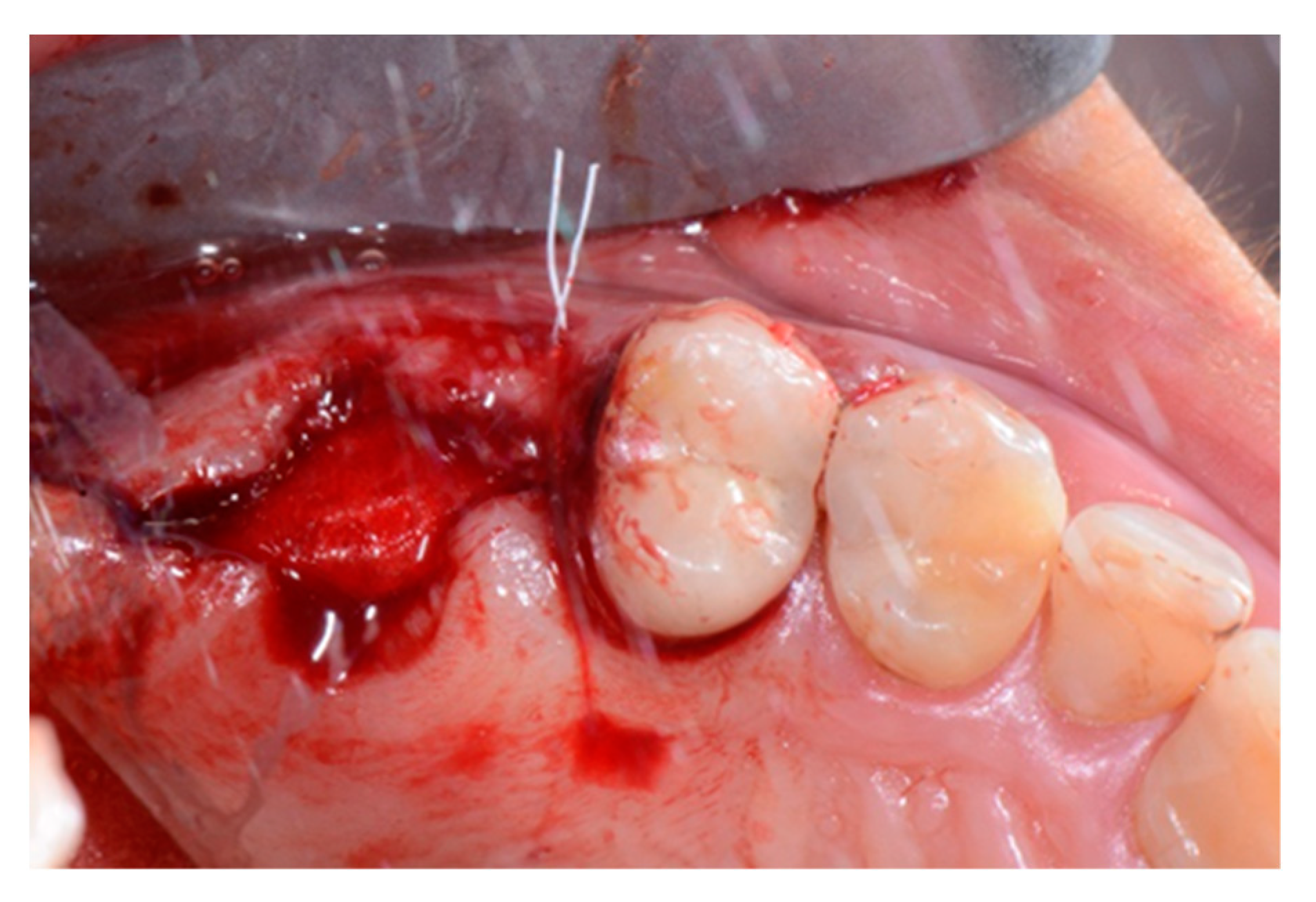
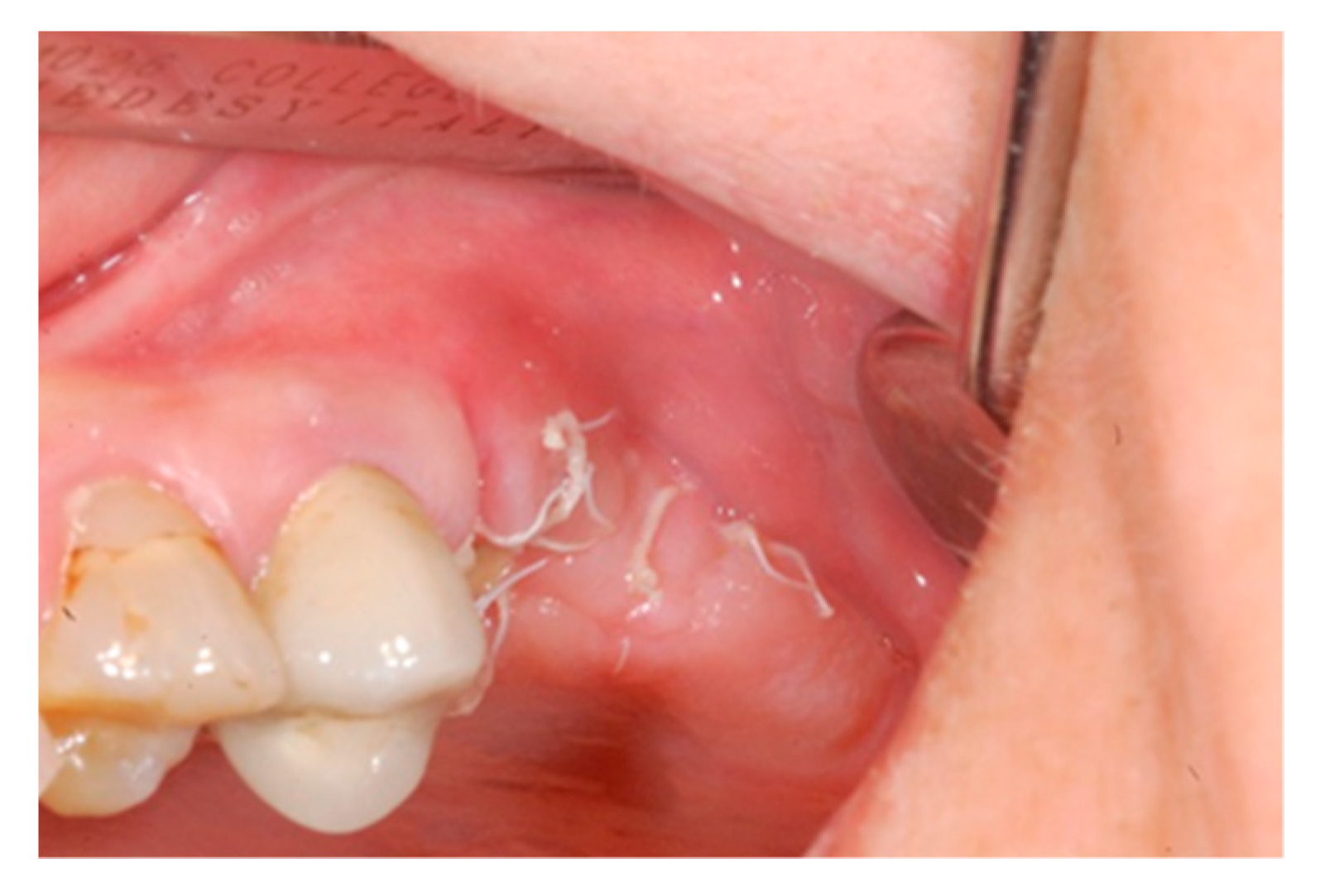
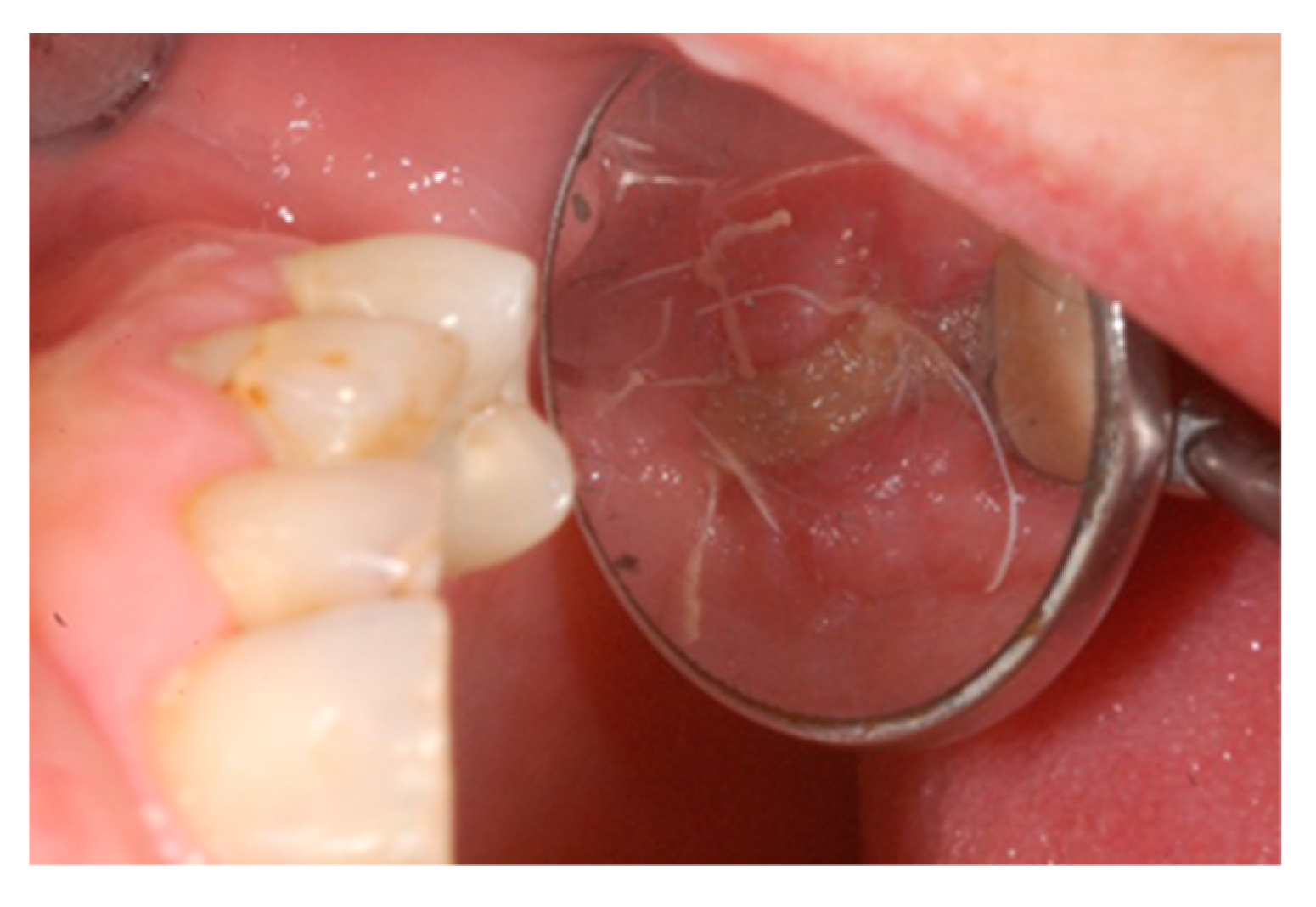
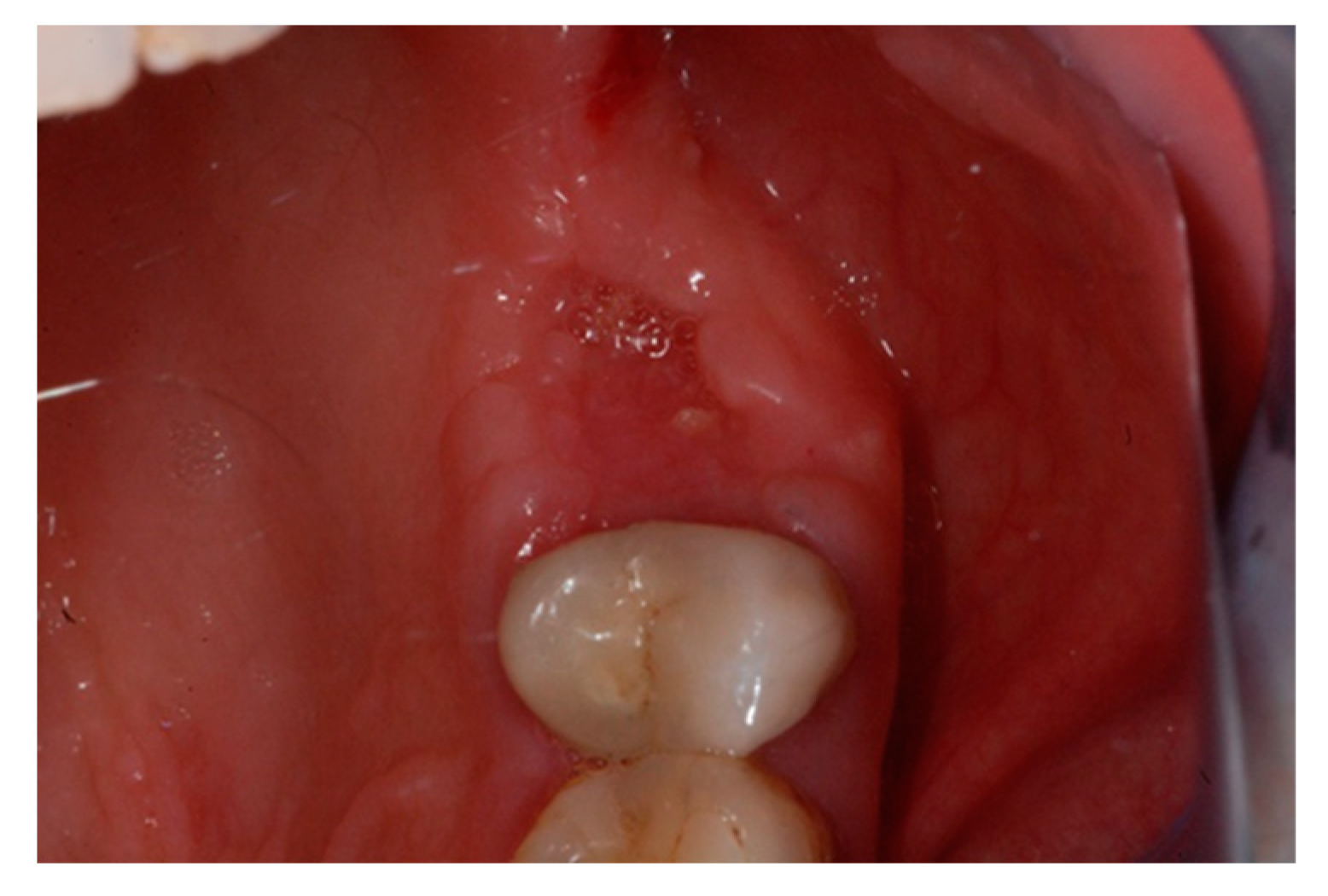
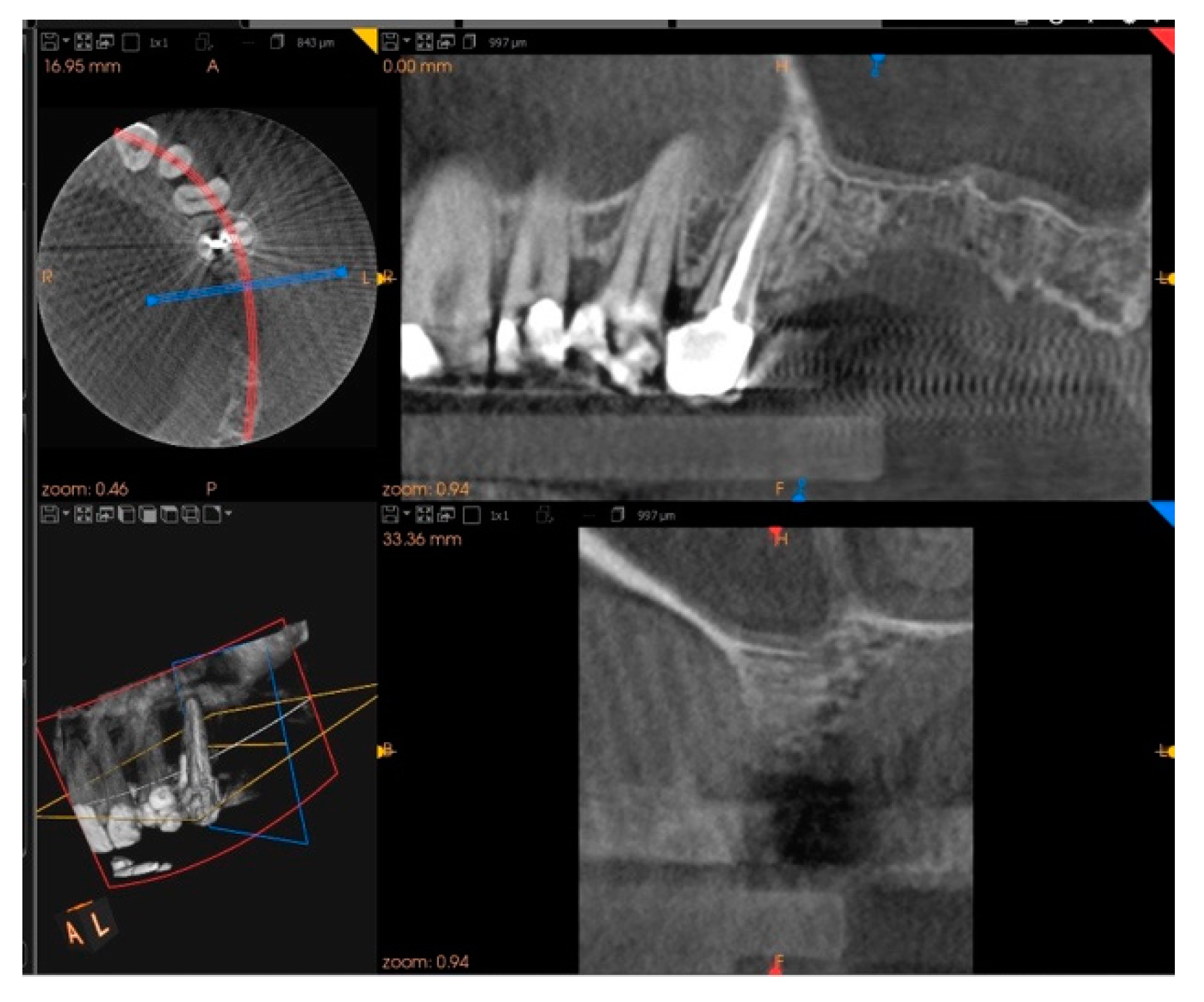
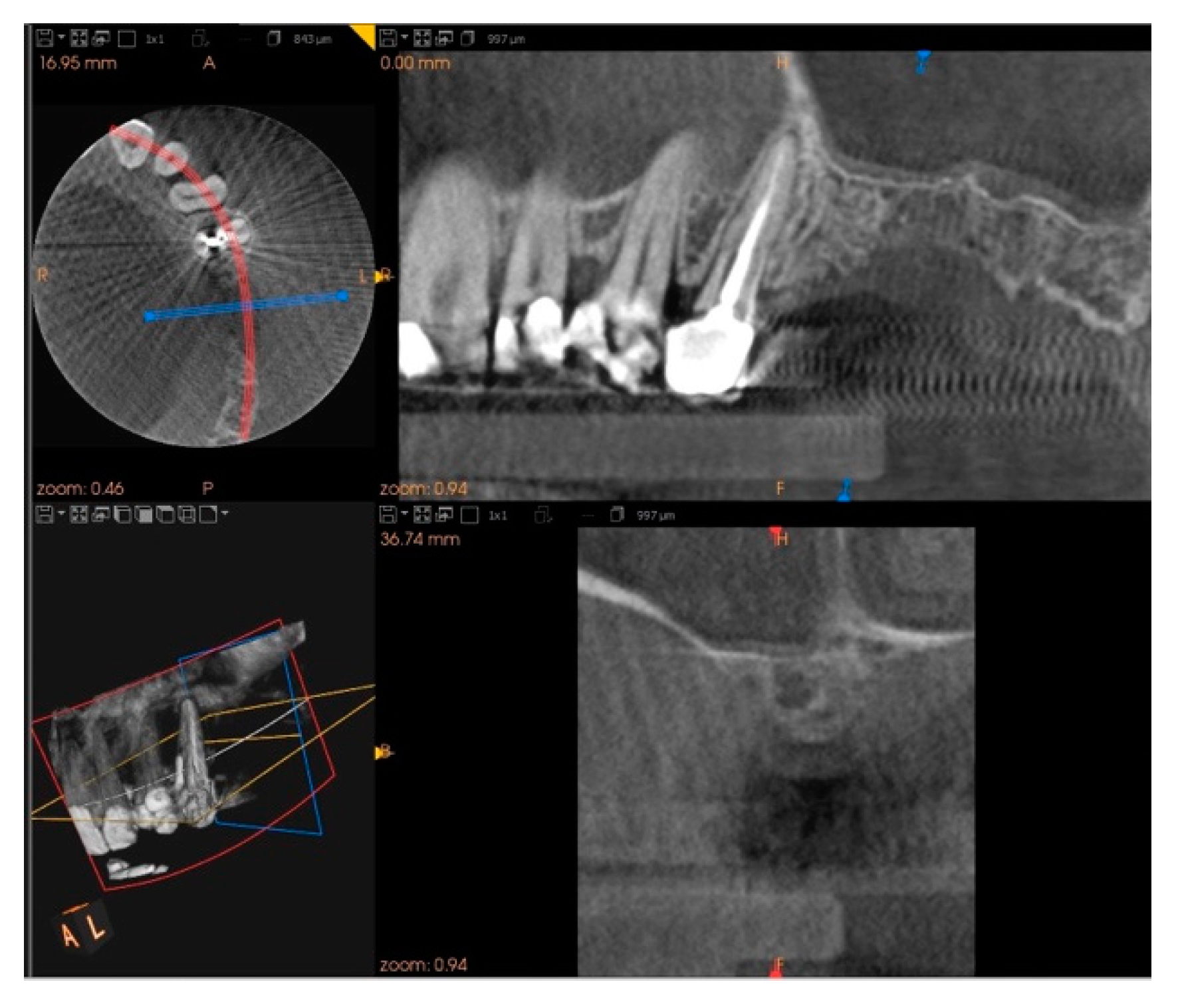
2. Case Report
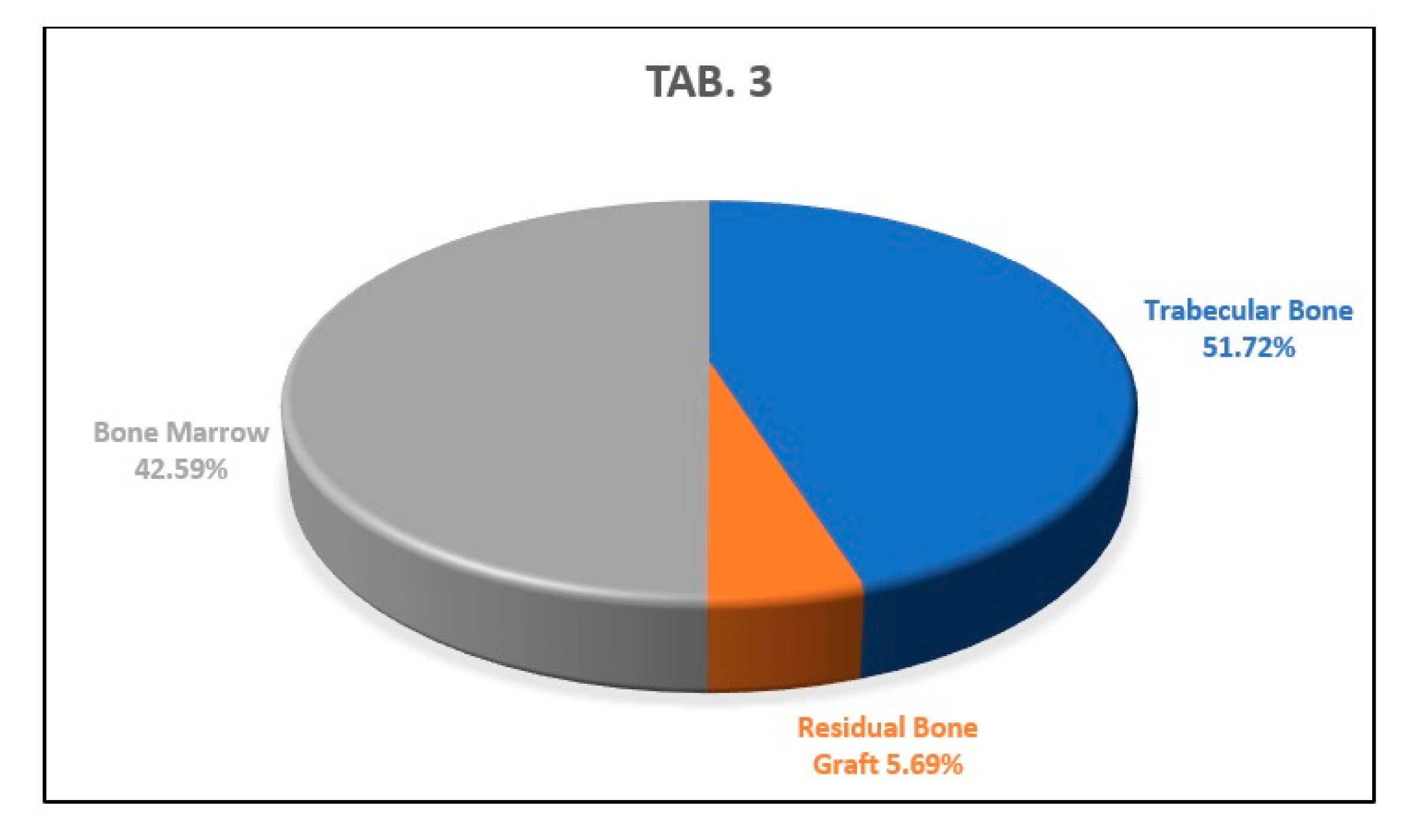
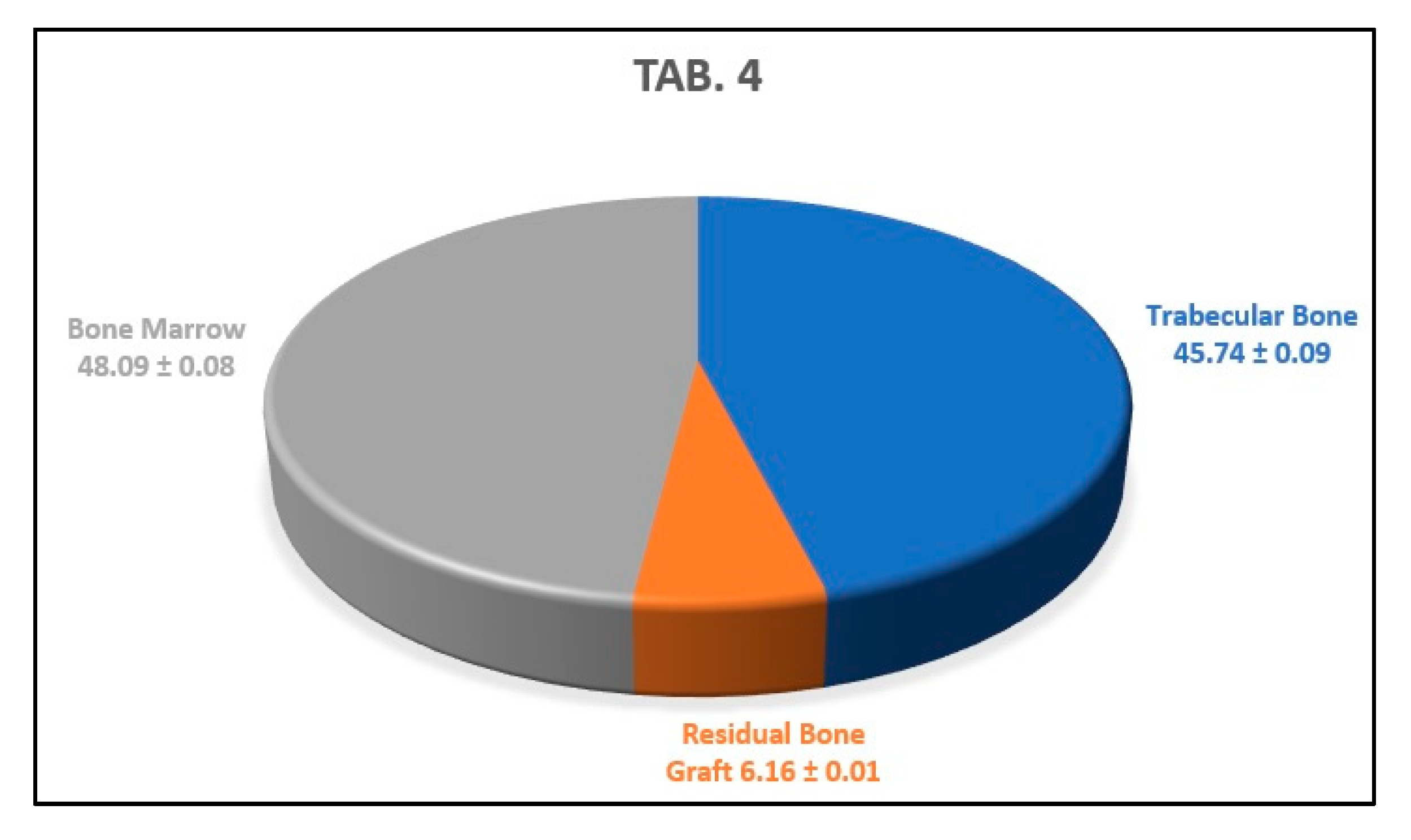
3. Results
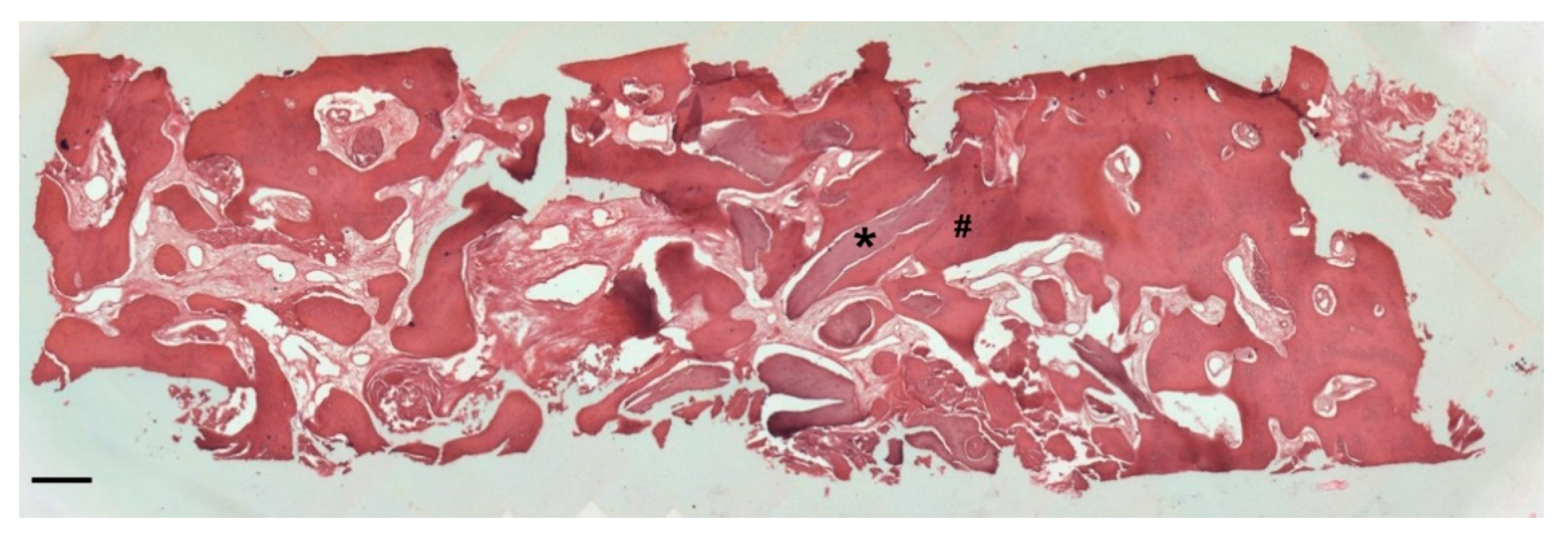
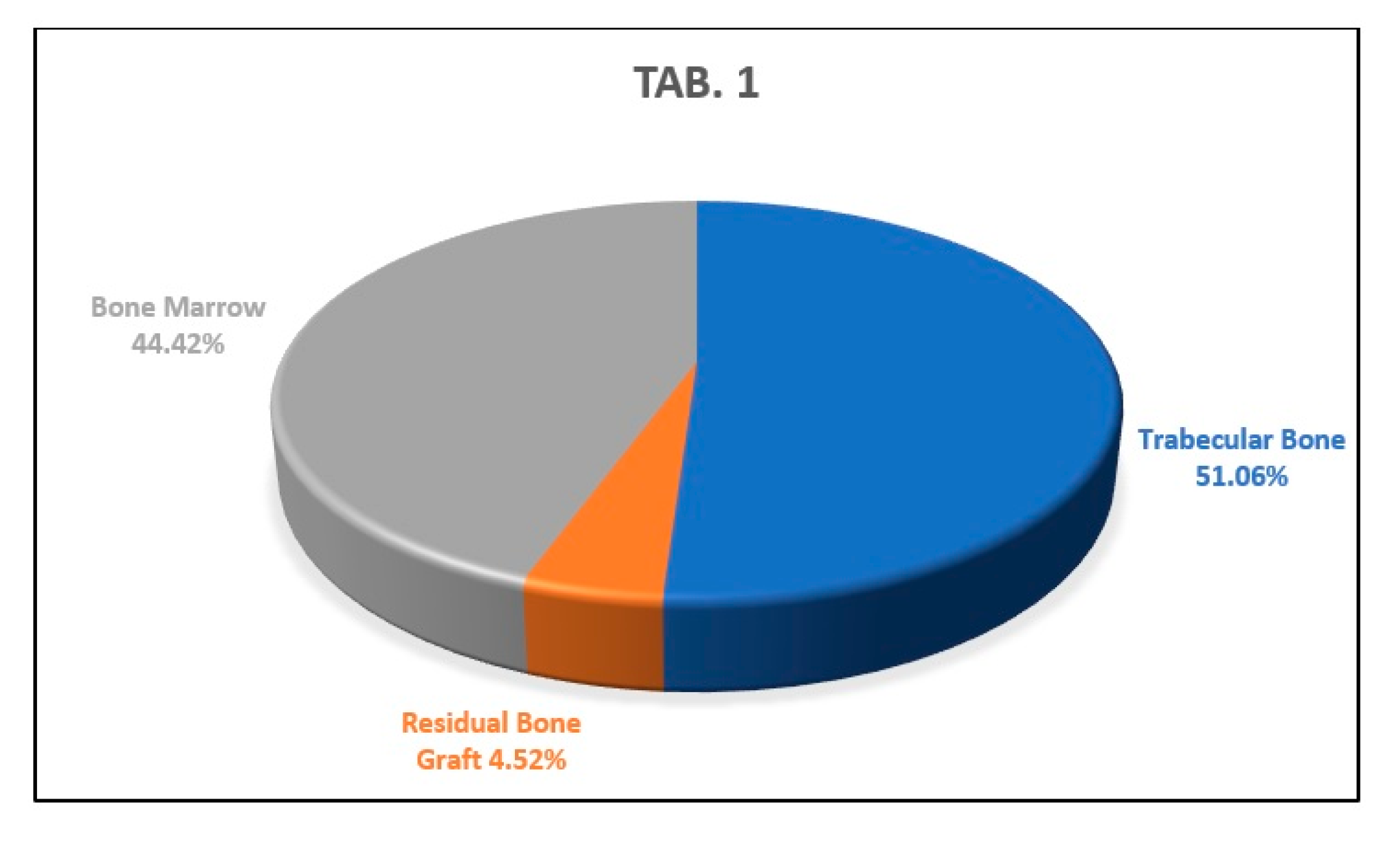
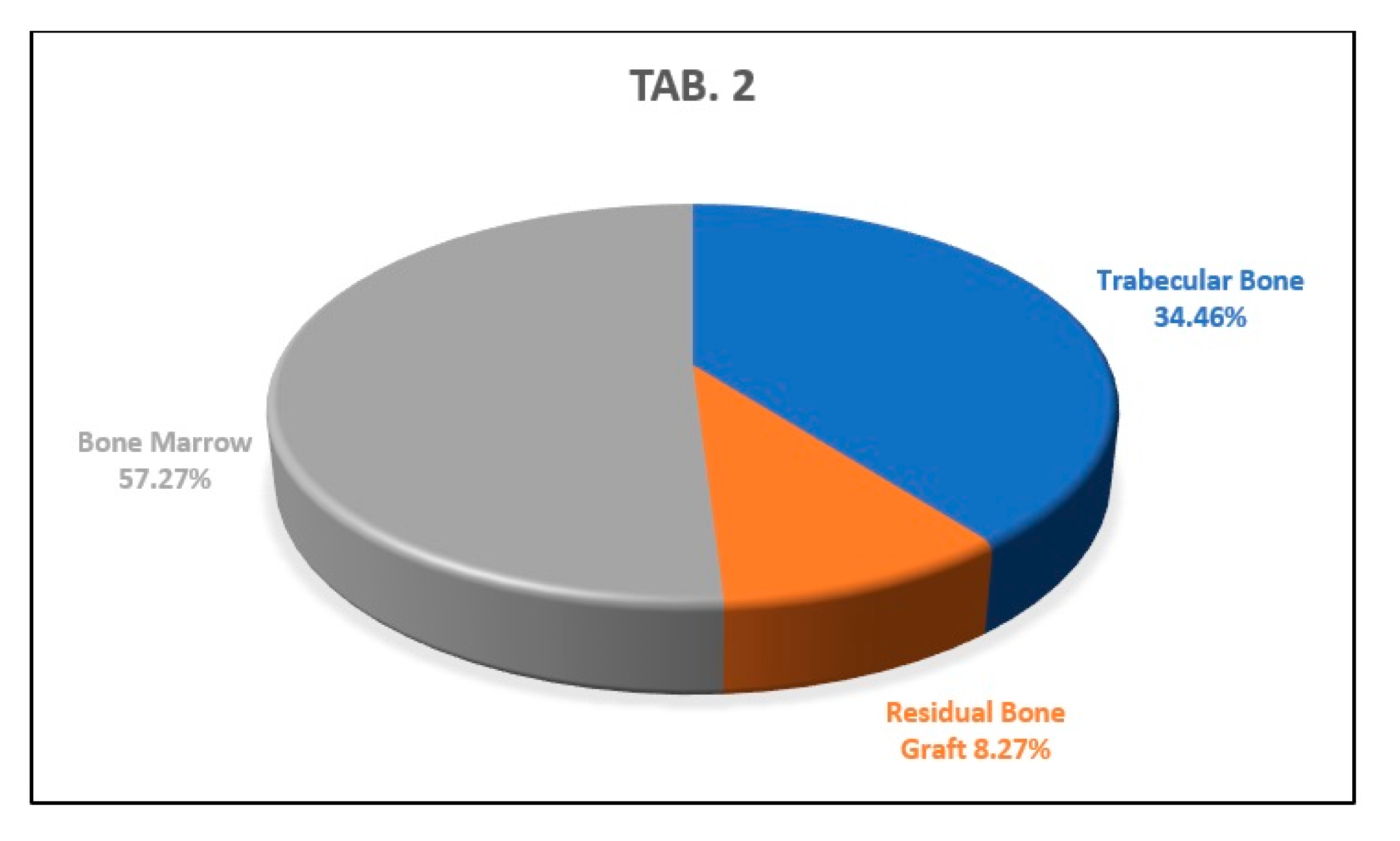
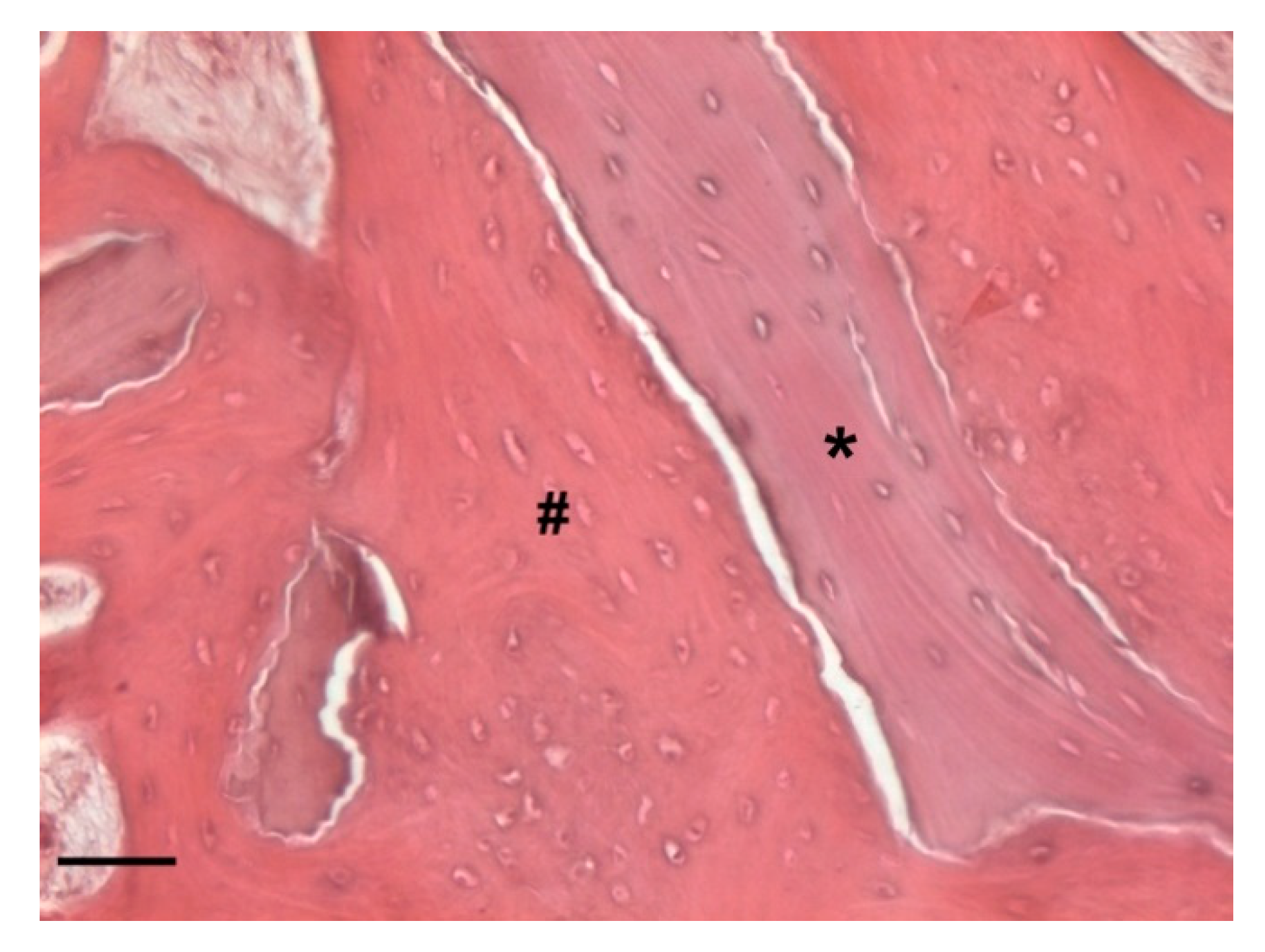
Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPs | Bisphosphonates |
| BRONJ | Bisphosphonate-related osteonecrosis of the jaw |
| ONJ | Osteonecrosis of the jaw |
| AAOMS | American Association of Oral and Maxillofacial Surgeons |
| CBCT | Cone-beam computer tomography |
| OPT | Orthopantomography |
References
- Murray, H.; Clark, M.; Locker, D.; Kay, E.J. Reasons for tooth extractions in dental practices in Ontario, Canada according to tooth type. Int. Dent. J. 1997, 47, 3–8. [Google Scholar] [CrossRef]
- Schropp, L. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. J. Prosthet. Dent. 2004, 91, 92. [Google Scholar]
- Thomason, J.M.; Kelly, S.A.; Bendkowski, A.; Ellis, J.S. Two implant retained overdentures—A review of the literature supporting the McGill and York consensus statements. J. Dent. 2012, 4, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Vittorini Orgeas, G.; Clementini, M.; De Risi, V.; de Sanctis, M. Surgical Techniques for Alveolar Socket Preservation: A Sys-tematic Review. Int. J. Oral. Maxillofac. Implant. 2013, 28, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.; Eduardo, C.; Pizzigatti, M.E.; Comenalli, F.; Pelegrine, A. Clinical and histomorphometric evaluation of extrac-tion sockets treated with an autologous bone marrow graft. Clin. Oral. Implant. Res. 2010, 21, 535–542. [Google Scholar] [CrossRef]
- Froum, S.; Cho, S.; Rosenberg, E.; Rohrer, M.; Tarnow, D. Histological Comparison of Healing Extraction Sockets Implanted with Bioactive glass or demineralized Freeze-Dried Bone Allograft: A Pilot Study. J. Periodontol. 2002, 73, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Carlos, E. The Application of Deproteinized Bovine Bone Mineral for Ridge Preservation Prior to Implantation. Clinical and Histological Observations in a Case Report. J. Periodontol. 1998, 69, 1062–1067. [Google Scholar] [CrossRef]
- Guarnieri, R.; Pecora, G.; Fini, M.; Aldini, N.; Giardino, R.; Orsini, G.; Piattelli, A. Medical Grade Calcium Sulfate Hemihydrate in Healing of Human Extraction Sockets: Clinical and Histological Observations at 3 Months. J. Periodontol. 2004, 75, 902–908. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; MIller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge Preservation with Freeze-Dried Bone Allograft and a Collagen Membrane Compared to Extraction Alone for Implant Site Development: A Clinical and Histologic Study in Humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Falco, A.; Amoroso, C.; Berardini, M.; D’Archivio, L.A. Retrospective Study of Clinical and Radiologic Outcomes of 69 Consecutive Maxillary Sinus Augmentations Associated with Functional Endoscopic Sinus Surgery. Int. J. Oral. Maxillofac. Implants 2015, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, J.; Ravida, A.; Starch-Jensen, T.; Tattan, M.; Suárez-López Del Amo, F. The Influence of Different Grafting Materials on Alveolar Ridge Preservation: A Systematic Review. J. Oral. Maxillofac. 2019, 10, e6. [Google Scholar] [CrossRef] [PubMed]
- Faria-Almeida, R.; Astramskaite-Januseviciene, I.; Puisys, A.; Correia, F. Extraction Socket Preservation with or without Membranes, Soft Tissue Influence on Post Extraction Alveolar Ridge Preservation: A Systematic Review. J. Oral. Maxillofac. Res. 2019, 10, e5. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral. Implant. Res. 2011, 5, 22–38. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral. Maxillofac. Implants 2011, 26, 1057–1062. [Google Scholar] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral. Maxillofac. Implants 2011, 26, 866–872. [Google Scholar]
- Di Stefano, D. Treatment of a ridge atrophy and two peri-implant defects with equine bone and an equine pericardium membrane: Clinical and histological outcome. Stomatolog 2013, 19, 32–37. [Google Scholar]
- Di Stefano, D.A.; Artese, L.; Iezzi, G.; Piattelli, A.; Pagnutti, S.; Piccirilli, M.; Perrotti, V. Alveolar ridge regeneration with equine spongy bone: A clinical, histological, and immunohistochemical case series. Clin. Implant. Dent. Relat. Res. 2009, 11, 90–100. [Google Scholar] [CrossRef]
- Nancollas, G.H.; Tang, R.; Phipps, R.J.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.G.G.; Ebetino, F.H. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dobson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and Frequency of Bisphosphonate-Associated Osteonecrosis of the Jaws in Australia. J. Oral. Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of Osteonecrosis of the Jaw in Patients with Oral Bisphosphonate Exposure. YJOMS 2010, 68, 243–253. [Google Scholar] [CrossRef]
- Fung, P.P.L.; Bedogni, G.; Bedogni, A.; Petrie, A.; Porter, S.; Campisi, G.; Bagan, J.; Fusco, V.; Saia, G.; Acham, S.; et al. Time to onset of bisphosphonate-related osteonecrosis of the jaws: A multicentre retrospective cohort study. Oral. Diseases 2017, 23, 477–483. [Google Scholar] [CrossRef]
- Diniz-Freitas, M.; Limeres, J. Prevention of medication-related osteonecrosis of the jaws secondary to tooth extractions. A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2016, 21, e250–e259. [Google Scholar] [CrossRef] [PubMed]
- Barasch, A.; Cunha-Cruz, J.; Curro, F.A.; Hujoel, P.; Sung, A.H.; Vena, D.; Voinea-Driffin, A.E. Risk factors for osteonecrosis of the jaws: A case-control study from the CONDOR dental PBRN. J. Dent. Res. 2011, 90, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.O.; Toth, B.B.; Altundag, K.; Guarnieri, V.; Adamus, A.; Nookag, A.K.; Sayegh, G.; Johnson, M.M.; Gagel, R.F.; Hortobagyi, G.N. Osteonecrosis of the jaw in patients receiving intravenous bisphosphonate therapy. J. Clin. Oncol. 2006, 24, 8528. [Google Scholar] [CrossRef]
- Pazianas, M.; Miller, P.; Blumentals, W.A.; Bernal, M.; Kothawala, P. A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: Prevalence, risk factors, and clinical characteristics. Clin. Ther. 2007, 29, 1548–1558. [Google Scholar] [CrossRef]
- Basile, M.; Marchegiani, F.; Novak, S.; Kalajzic, I.; Di Pietro, R. Human amniotic fluid stem cells attract osteoprogenitor cells in bone healing. J. Cell. Physiol. 2020, 235, 4643–4654. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Prathigudupu, R.S.; Jairaj, A.; Khader, M.A.; Rajeev, K.; Khader, A.A. Role of Synthetic Hydroxyapatite-In Socket Preservation: A Systematic Review and Meta-analysis. J. Contemp. Dent. Pract. 2019, 20, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Clementini, M.; Tiravia, L.; De Risi, V.; Vittorini Orgeas, G.; Mannocci, A.; de Sanctis, M. Dimensional changes after immediate implant placement with or without simultaneous regenerative procedures: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Pun, L.; Lau, K.Y.; Li, K.Y.; Wong, M.C. A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin. Oral. Implants. Res. 2012, 23, 39–66. [Google Scholar] [CrossRef]
- Baümer, D.; Zuhr, O.; Rebele, S.; Hürzeler, M. Socket Shield Technique for immediate implant placement - clinical, radiographic and volumetric data after 5 years. Clin. Oral. Implants. Res. 2017, 28, 1450–1458. [Google Scholar] [CrossRef]
- Hürzeler, M.B.; Zuhr, O.; Schupbach, P.; Rebele, S.F.; Emmanouilidis, N.; Fickl, S. The socket-shield technique: A proof-of-principle report. J. Clin. Periodontol. 2010, 37, 855–862. [Google Scholar] [CrossRef]
- Salama, M.; Ishikawa, T.; Salama, H.; Funato, A.; Garber, D. Advantages of the root submergence technique for pontic site development in esthetic implant therapy. Int. J. Periodontics. Restorative. Dent. 2007, 27, 521–527. [Google Scholar]
- Capparé, P.; Teté, G.; Romanos, G.E.; Nagni, M.; Sannino, G.; Gherlone, E.F. The ‘All-on-four’ protocol in HIV-positive patients: A prospective, longitudinal 7-year clinical study. Int. J. Oral. Implantol. 2019, 12, 501–510. [Google Scholar]
- Willenbacher, M.; Al-Nawas, B.; Berres, M.; Kämmerer, P.W.; Schiegnitz, E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2016, 18, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, V.; Camargo, P.M.; Klokkevold, P.R.; Weinlaender, M.; Kenney, E.B.; Dimitrijevic, B.; Nedic, M. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J. Periodontol. 1998, 69, 1044–1049. [Google Scholar] [CrossRef]
- Otto, S.; Schreyer, C.; Hafner, S.; Mast, G.; Ehrenfeld, M.; Stürzenbaum, S.; Pautke, C. Bisphosphonate-related osteonecrosis of the jaws e Characteristics, risk factors, clinical features, localization and impact on oncological treatment. J. Cranio-Maxillofacial. Surg. 2012, 40, 303–309. [Google Scholar] [CrossRef]
- Gulati, M.; Govila, V.; Anand, V.; Anand, B. Implant Maintenance: A Clinical Update. Int. Sch. Res. Not. 2014, 908534. [Google Scholar] [CrossRef]
- Kauffmann, F.; Höhne, C.; Assaf, A.T.; Vollkommer, T.; Semmusch, J.; Reitmeier, A.; Stein, J.M.; Heiland, M.; Smeets, R.; & Rutkowski, R. The Influence of Local Pamidronate Application on Alveolar Dimensional Preservation after Tooth Extraction-An Animal Experimental Study. Int. J. Mol. Sci. 2020, 21, 3616. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Carrascal, N.; Delgado-Ruiz, R.A.; Gargallo-Albiol, J.; Maté-Sánchez, J.E.; Hernandez Alfaro, F.; Calvo-Guirado, J.L. Xenografts supplemented with Pamindronate placed in postextraction sockets to avoid crestal bone resorption. Experimental study in Fox hound dogs. Clin. Oral. Implant. Res. 2016, 27, 149–155. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Xia, Z.; Dunford, J.E.; Oppermann, U.; Kwaasi, A.; Hulley, P.A.; Kavanagh, K.; Triffitt, J.T.; Lundy, M.W.; Phipps, R.J.; et al. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann. N. Y. Acad. Sci. 2007, 1117, 209–257. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, A.; Bataccia, F.; Vittorini Orgeas, L.; Perfetti, F.; Basile, M.; Di Pietro, R. A Clinical and Histological Study about the Socket Preservation in a Patient under Oral Bisphosphonates Treatment: A Case Report. Biology 2021, 10, 262. https://doi.org/10.3390/biology10040262
Falco A, Bataccia F, Vittorini Orgeas L, Perfetti F, Basile M, Di Pietro R. A Clinical and Histological Study about the Socket Preservation in a Patient under Oral Bisphosphonates Treatment: A Case Report. Biology. 2021; 10(4):262. https://doi.org/10.3390/biology10040262
Chicago/Turabian StyleFalco, Antonello, Francesco Bataccia, Lorenzo Vittorini Orgeas, Federico Perfetti, Mariangela Basile, and Roberta Di Pietro. 2021. "A Clinical and Histological Study about the Socket Preservation in a Patient under Oral Bisphosphonates Treatment: A Case Report" Biology 10, no. 4: 262. https://doi.org/10.3390/biology10040262
APA StyleFalco, A., Bataccia, F., Vittorini Orgeas, L., Perfetti, F., Basile, M., & Di Pietro, R. (2021). A Clinical and Histological Study about the Socket Preservation in a Patient under Oral Bisphosphonates Treatment: A Case Report. Biology, 10(4), 262. https://doi.org/10.3390/biology10040262






