Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging
Abstract
Simple Summary
Abstract
1. Introduction
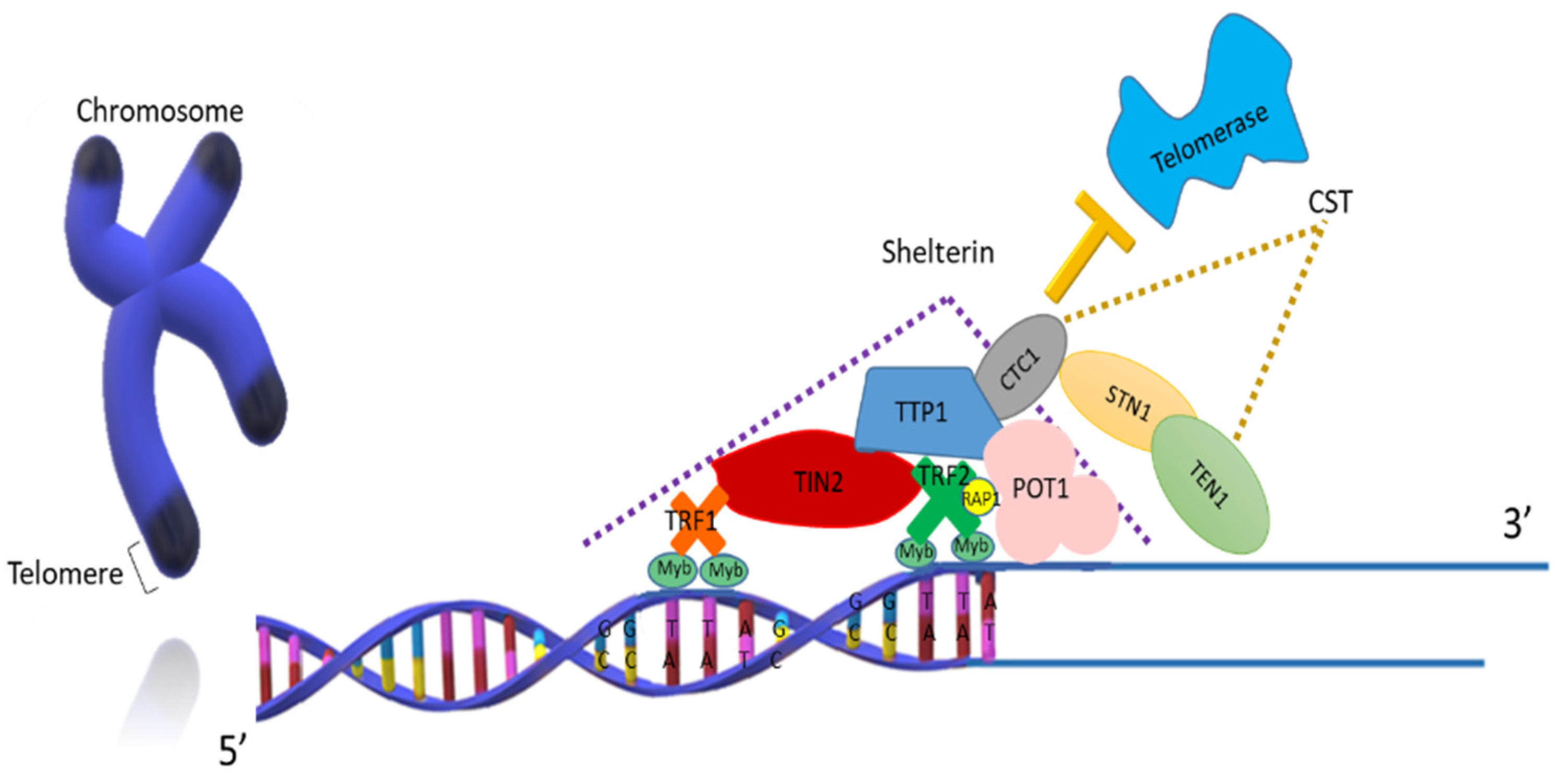
1.1. Structure and Function of Telomeres
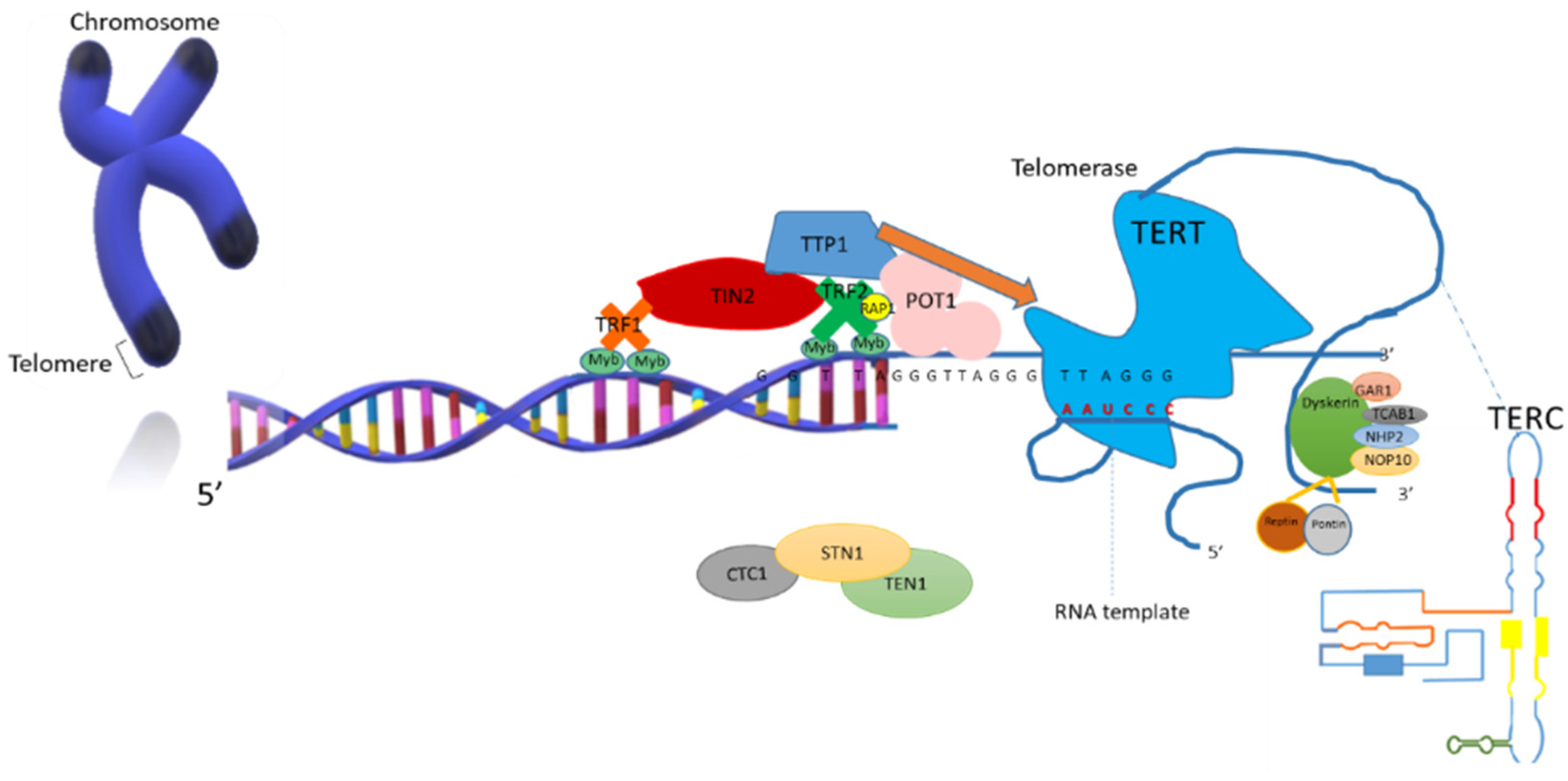
1.2. Telomerase Structure and Function
2. Telomeric Length and Cell Fate in Aging
3. Reactive Oxygen Species and Antioxidants
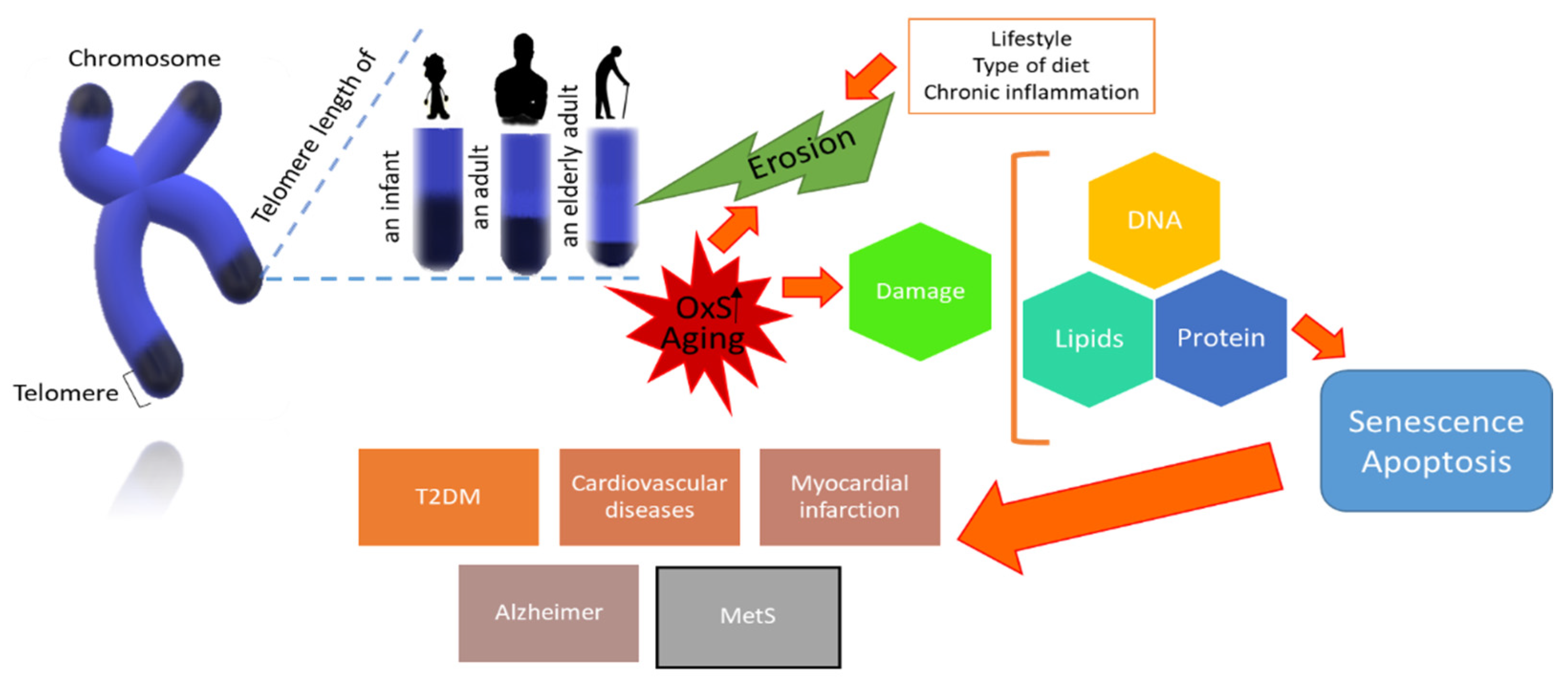
4. Telomere Shortening and Oxidative Stress
5. Telomere Length and Age-Related Diseases and Oxidative Stress
6. Damage Repair of the Telomeric DNA
7. Relationship of Oxidative Stress with the Metabolic Syndrome and Telomeric Length
8. Components of the MetS and Its Relationship with Oxidative Stress and Telomeric Length
9. Telomere Length and Inflammation in the MetS
10. Effect of Healthy Lifestyles on Oxidative Stress and the Telomere Length
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shay, J.W. Telomeres and aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, D.; Songyang, Z. The telosome/shelterin complex and its functions. Genome Biol. 2008, 9, 232. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.R.A. Telómeros y telomerasas. Rev. Cubana Investig. Biomed. 1999, 18, 121–129. [Google Scholar]
- Rubtsova, M.P.; Vasilkova, D.P.; Malyavko, A.N.; Naraikina, Y.V.; Zvereva, M.I.; Dontsova, O.A. Telomere lengthening and other functions of telomerase. Acta Nat. 2012, 4, 44–61. [Google Scholar] [CrossRef]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef]
- Chen, L.Y.; Redon, S.; Lingner, J. The human CST complex is a terminator of telomerase activity. Nature 2012, 488, 540–544. [Google Scholar] [CrossRef]
- Giraud-Panis, M.J.; Pisano, S.; Poulet, A.; Le-Du, M.H.; Gilson, E. Structural identity of telomeric complexes. FEBS Lett. 2010, 584, 3785–3799. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.R.A. El complejo proceso de la duplicación de los telómeros. Rev. Cubana Genet. Comunit. 2015, 9, 4–13. [Google Scholar]
- van Steensel, B.; De Lange, T. Control of telomere length by the human telomeric protein TRF1. Nature 1997, 385, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Smith, S.; Chong, L.; Elias, P.; De Lange, T. TRF1 is a dimer and bends telomeric DNA. EMBO J. 1997, 16, 1785–1794. [Google Scholar] [CrossRef]
- Ye, J.Z.; Donigian, J.R.; Van Overbeek, M.; Loayza, D.; Luo, Y.; Krutchinsky, A.N.; Chait, B.T.; De Lange, T. TIN2 Binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J. Biol. Chem. 2004, 279, 47264–47271. [Google Scholar] [CrossRef]
- Hanaoka, S.; Nagadoi, A.; Nishimura, Y. Comparison between TRF2 and TRF1 of their telomeric DNA-bound structures and DNA-binding activities. Protein Sci. 2005, 14, 119–130. [Google Scholar] [CrossRef]
- Stansel, R.M.; de Lange, T.; Griffith, J.D. T-loop assembly in vitro involves binding of TRF2 near the 3’ telomeric overhang. EMBO J. 2001, 20, 5532–5540. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Cech, T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001, 292, 1171–1175. [Google Scholar] [CrossRef]
- Baumann, P.; Price, C. Pot1 and telomere maintenance. FEBS Lett. 2010, 584, 3779–3784. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Zaug, A.J.; Kim, H.J.; Cech, T.R. Reconstitution of human shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance telomerase processivity. Nat. Commun. 2017, 8, 1075. [Google Scholar] [CrossRef]
- Gilson, E.; Gasser, S.M. Repressor activator protein 1 and its ligands: Organising chromatin domains. In Nucleic Acids and Molecular Biology; Eckstein, F., Lilley, D.M.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 9, pp. 308–309. [Google Scholar]
- Takai, K.K.; Kibe, T.; Donigian, J.R.; Frescas, D.; de Lange, T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol. Cell 2011, 44, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Podell, E.; Zaug, A.; Yang, Y.; Baciu, P.; Cech, T.R.; Le, M. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 2007, 445, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; O’Connor, M.; Chan, D.; Laegeler, A.; Qin, J.; Songyang, Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 2004, 6, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Smith, E.M.; Tesmer, V.M.; Nandakumar, J. Structural and functional consequences of a disease mutation in the telomere protein TPP1. Proc. Natl. Acad. Sci. USA 2016, 113, 13021–13026. [Google Scholar] [CrossRef]
- Sexton, A.N.; Regalado, S.G.; Lai, C.S.; Cost, G.J.; O’Neil, C.M.; Urnov, F.D.; Gregory, P.D.; Jaenisch, R.; Collins, K.; Hockemeyer, D. Genetic and molecular identification of three human TPP1 functions in telomerase action: Recruitment, activation, and homeostasis set point regulation. Genes Dev. 2014, 28, 1885–1899. [Google Scholar] [CrossRef]
- Nandakumar, J.; Bell, C.; Weidenfeld, I.; Zaug, A.; Leinwand, L.; Cech, T. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 2012, 492, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Dionne, I.; Wellinger, R.J.; Holm, C. The function of DNA polymerase alpha at telomeric G tails is important for telomere homeostasis. Mol. Cell. Biol. 2000, 20, 786–796. [Google Scholar]
- Huang, C.; Jia, P.; Chastain, M.; Shiva, O.; Chai, W. The human CTC1/STN1/TEN1 complex regulates telomere maintenance in ALT cancer cells. Exp. Cell Res. 2017, 355, 95–104. [Google Scholar] [CrossRef]
- Stewart, J.A.; Wang, F.; Chaiken, M.F.; Kasbek, C.; Chastain, P.D.; Wright, W.E.; Price, C.M. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 2012, 31, 3537–3549. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.; Puglisi, A.; Dmitriev, P.V.; Lopes, M.; Shore, D. Pol12, the B subunit of DNA polymerase alpha, functions in both telomere capping and length regulation. Genes Dev. 2004, 18, 992–1006. [Google Scholar] [CrossRef]
- Feng, X.; Hsu, S.; Bhattacharjee, A.; Wang, Y.; Diao, J.; Price, C.M. CTC1-STN1 terminates telomerase while STN1-TEN1 enables C-strand synthesis during telomere replication in colon cancer cells. Nat. Commun. 2018, 9, 2827. [Google Scholar] [CrossRef]
- Bryan, C.; Rice, C.; Harkisheimer, M.; Schultz, D.C.; Skordalakes, E. Structure of the human telomeric Stn1-Ten1 capping complex. PLoS ONE 2013, 8, e66756. [Google Scholar] [CrossRef] [PubMed]
- Kasbek, C.; Wang, F.; Price, C.M. Human TEN1 maintains telomere integrity and functions in genome-wide replication restart. J. Biol. Chem. 2013, 288, 30139–30150. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Wynford-Thomas, D.; Kipling, D. Cancer and the knockout mouse. Nature 1997, 389, 551–552. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Blackburn, E.H. Telomere states and cell fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef]
- Wu, A.; Ichihashi, M.; Ueda, M. Correlation of the expression of human telomerase subunits with telomerase activity in normal skin and skin tumors. Cancer 1999, 86, 2038–2044. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Cheng, J.; Collins, K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3’ end. Mol. Cell. Biol. 1999, 19, 567–576. [Google Scholar] [CrossRef]
- Yang, J.; Chang, E.; Cherry, A.M.; Bangs, C.D.; Oeii, Y.; Bodnari, A.; Bronsteini, A.; Chiui, C.P.; Herron, G.S. Human endothelial cell life extensión by telomerase expression. J. Biol. Chem. 1999, 271, 21141–21148. [Google Scholar] [CrossRef]
- Perez, M.; Dubner, D.; Michelin, S.; Gisone, P.; Carosella, E. Telómeros y reparación de daño genómico su implicancia en patología humana. Medicina (Buenos Aires) 2002, 62, 593–603. [Google Scholar]
- Rostamiani, K.; Klauck, S.M.; Heiss, N.; Poustka, A.; Khaleghi, M.; Rosales, R.; Metzenberg, A.B. Novel mutations of the DKC1 gene in individuals affected with dyskeratosis congénita. Blood Cells Mol. Dis. 2010, 44, 88. [Google Scholar] [CrossRef]
- Venteicher, A.S.; Abreu, E.B.; Meng, Z.; McCann, K.E.; Terns, R.M.; Veenstra, T.D.; Terns, M.P.; Artandi, S.E. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 2009, 323, 644–648. [Google Scholar] [CrossRef]
- Stern, J.L.; Zyner, K.G.; Pickett, H.A.; Cohen, S.B.; Bryan, T.M. Telomerase recruitment requires both TCAB1 and Cajal bodies independently. Mol. Cell. Biol. 2012, 32, 2384–2395. [Google Scholar] [CrossRef]
- Wang, Q.; Sawyer, I.; Sung, M.; Sturgill, D.; Shevstsov, S.P.; Pegoraro, G.; Hakim, O.; Baek, S.; Hager, G.L. Cajal bodies are linked to genome conformation. Nat. Commun. 2016, 7, 10966. [Google Scholar] [CrossRef]
- Pogacic, V.; Dragon, F.; Filipowicz, W. Human H/ACA small nucleolar RNPs and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol. Cell. Biol. 2000, 20, 9028–9040. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Baccon, J.; Charroux, B.; Dreyfuss, G. The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1. Curr. Biol. 2001, 11, 1079–1088. [Google Scholar] [CrossRef]
- Vulliamy, T.; Beswick, R.; Kirwan, M.; Marrone, A.; Digweed, M.; Walne, A.; Dokal, I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc. Natl. Acad. Sci. USA 2008, 105, 8073–8078. [Google Scholar] [CrossRef]
- Venteicher, A.S.; Meng, Z.; Mason, P.J.; Veenstra, T.D.; Artandi, S.E. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell 2008, 132, 945–957. [Google Scholar] [CrossRef]
- Sekaran, V.G.; Soares, J.; Jarstfer, M.B. Structures of telomerase subunits provide functional insights. Biochim. Biophys. Acta Proteins Proteom 2010, 1804, 1190–1201. [Google Scholar] [CrossRef]
- Baek, S.H. When ATPases pontin and reptin met telomerase. Dev. Cell 2008, 14, 459–461. [Google Scholar] [CrossRef][Green Version]
- Harley, C. Telomerase and cancer therapeutics. Nat. Rev. Cancer 2008, 8, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Bryan, T.M.; Englezou, A.; Dalla-Pozza, L.; Dunham, M.A.; Reddel, R.R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 1997, 3, 1271–1274. [Google Scholar] [CrossRef]
- Webb, C.J.; Wu, Y.; Zakian, V.A. DNA repair at telomeres: Keeping the ends intact. Cold Spring Harb. Perspect. Biol. 2013, 5, a012666. [Google Scholar] [CrossRef]
- Frenck, R.W.; Blackburn, E.H.; Shannon, K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci. USA 1998, 95, 5607–5610. [Google Scholar] [CrossRef]
- Bree, R.T.; Stenson-Cox, C.; Grealy, M.; Byrnes, L.; Gorman, A.M.; Samali, A. Cellular longevity: Role of apoptosis and replicative senescence. Biogerontology 2002, 3, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Kuhlow, D.; Florian, S.; von Figura, G.; Weimer, S.; Schulz, N.; Petzk, K.J.; Zarse, K.; Pfeiffer, A.F.; Rudolph, K.L.; Ristow, M. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging (Albany N. Y.) 2010, 2, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Daubenmier, J.; Lin, J.; Blackburn, E.; Hecht, F.M.; Kristeller, J.; Maninger, N.; Kuwata, M.; Bacchetti, P.; Havel, P.J.; Epel, E. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology 2012, 37, 917–928. [Google Scholar] [CrossRef]
- Tristano, A.; Chollet, M.A.; Willson, M.L.; Adjounian, H.; Correa, M.F.; Borges, A. Actividad de la telomerasa en leucocitos de sangre periférica de pacientes con hipertensión arterial esencial. Med. Clin. Barc. 2003, 120, 365–369. [Google Scholar] [CrossRef]
- Maeda, T.; Oyama, J.I.; Higuchi, Y.; Arima, T.; Mimori, K.; Makino, N. The correlation between the telomeric parameters and the clinical laboratory data in the patients with brain infarct and metabolic disorders. J. Nutr. Health Aging 2010, 14, 793–797. [Google Scholar] [CrossRef]
- Richter, T.; von Zglinicki, T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 2007, 42, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Farr, C.; Fantes, J.; Goodfellow, P.; Cooke, H. Functional reintroduction of human telomeres into mammalian cells. Proc. Natl. Acad. Sci. USA 1991, 88, 7006–7010. [Google Scholar] [CrossRef] [PubMed]
- Capper, R.; Britt-Compton, B.; Tankimanova, M.; Rowson, J.; Letsolo, B.; Man, S.; Haughton, M.; Baird, D.M. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007, 21, 2495–2508. [Google Scholar] [CrossRef]
- Di Leonardo, A.; Linke, S.P.; Clarkin, K.; Wahl, G.M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994, 8, 2540–2551. [Google Scholar] [CrossRef]
- Bhayadia, R.; Schmidt, B.M.W.; Melk, A.; Hömme, M. Senescence-induced oxidative stress causes endothelial dysfunction. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.B.; Song, S.; Johnson, F.B. Contributions of telomere biology to human age-related disease. In Handbook of the Biology of Aging; Kaeberlein, M.R., Martin, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 205–239. [Google Scholar]
- Jiang, H.; Ju, Z.; Rudolph, K.L. Telomere shortening and ageing. Z. Gerontol. Geriatr. 2007, 40, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kaminker, P.; Campisi, J. Telomeres, aging and cancer: In search of a happy ending. Oncogene 2002, 21, 503–511. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; Van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Telomeres and cell senescence—Size matters not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- iedernhofer, L.; Robbins, P. Senotherapeutics for healthy ageing. Nat. Rev. Drug Discov. 2018, 17, 377. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Viel, T.; Chinta, S.; Rane, A.; Chamoli, M.; Buck, H.; Andersen, J. Microdose lithium reduces cellular senescence in human astrocytes—A potential pharmacotherapy for COVID-19? Aging 2020, 12, 10035–10040. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.M.; De-Paula, V.J.R.; Ikenaga, E.H.; Costa, L.R.; Catanozi, S.; Schaeffer, E.L.; Gattaz, W.F.; Kerr, D.S.; Forlenza, O.V. Chronic lithium treatment increases telomere length in parietal cortex and hippocampus of triple-transgenic alzheimer’s disease mice. J. Alzheimers Dis. 2018, 63, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, L.; Wei, Y.; Xu, D.; Melas, P.A.; Mathé, A.A.; Schalling, M.; Lavebratt, C.; Backlund, L. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl. Psychiatry. 2013, 3, e261. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular senescence in type 2 diabetes: A therapeutic opportunity. Diabetes 2015, 64, 2289–2298. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Zhu, Y.; Langhi, L.G.; Tchkonia, T.; Krüger, P.; Fielder, E.; Victorello, S.; Ruswhandi, R.A.; Giorgadze, N.; Pirtskhalava, T.; et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019, 29, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Karlseder, J.; Broccoli, D.; Dai, Y.; Hardy, S.; de Lange, T. p53-and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 1999, 283, 1321–1325. [Google Scholar] [CrossRef]
- Multani, A.S.; Ozen, M.; Narayad, S.; Kumar, V.; Chandra, J.; McConkey, D.J.; Newman, R.A.; Pathak, S. Caspase-dependent apoptosis induced by telomere cleavage and TRF2 loss. Neoplasia 2000, 2, 339–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keefe, D.L.; Franco, S.; Liu, L.; Trimarchi, J.; Cao, B.; Weitzen, S.; Agarwal, S.; Blasco, M.A. Telomere length predicts embryo fragmentation after in vitro fertilization in women—Toward a telomere theory of reproductive aging in women. Am. J. Obstet. Gynecol. 2005, 192, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.L.; Liu, L.; Marquard, K. Telomeres and aging-related meiotic dysfunction in women. Cell. Mol. Life Sci. 2007, 64, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Rampazzo, E.; Rocca, M.S.; Keppel, S.; Frigo, A.C.; De Rossi, A.; Foresta, C. In young men sperm telomere length is related to sperm number and parental age. Hum. Reprod. 2017, 28, 3370–3376. [Google Scholar] [CrossRef]
- Baccetti, B.; Collodel, G.; Piomboni, P. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9). J. Submicrosc. Cytol. Pathol. 1996, 28, 587–596. [Google Scholar]
- Amir, S.; Vakonaki, E.; Tsiminikaki, K.; Tzatzarakis, M.N.; Michopoulou, V.; Flamourakis, M.; Kalliantasi, K.; Karzi, V.; Fragkiadaki, P.; Renieri, E.A.; et al. Sperm telomere length: Diagnostic and prognostic biomarker in male infertility (Review). World Acad. Sci. J. 2019, 1, 259–263. [Google Scholar] [CrossRef]
- Koju, N.; Taleb, A.; Zhou, J.; Lv, G.; Yang, J.; Cao, X.; Lei, H.; Ding, Q. Pharmacological strategies to lower crosstalk between nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondria. Biomed. Pharmacother. 2019, 111, 1478–1498. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Arista-Ugalde, T.L.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. The biological significance of oxidative stress effects of fruits as natural edible antioxidants. Curr. Pharm. Des. 2018, 24, 4807–4824. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Thomas, D.D. Oxidative stress. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin, Germany, 2013; pp. 1813–1818. [Google Scholar]
- Kehrer, J. The Haber-Weiss reaction and mechanism of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Lindahl, T.; Barnes, D.E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–134. [Google Scholar] [CrossRef]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol. Cell 2019, 75, 117–130. [Google Scholar] [CrossRef]
- Urbaniak, S.K.; Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. 8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a potential biomarker for gestational diabetes mellitus (GDM) development. Molecules 2020, 25, 202. [Google Scholar] [CrossRef] [PubMed]
- Mut-Salud, N.; Álvarez, P.J.; Garrido, J.M.; Carrasco, E.; Aránega, A.; Rodríguez-Serrano, F. Antioxidant intake and antitumor therapy: Toward nutritional recommendations for optimal results. Oxid. Med. Cell. Longev. 2016, 2016, 6719534. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or negative actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Osterod, M.; Hollenbach, S.; Hengstler, J.G.; Barnes, D.E.; Lindahl, T.; Epe, B. Age-related and tissue-specific accumulation of oxidative DNA base damage in 7, 8-dihydro-8-oxoguanine-DNA glycosylase (Ogg1) deficient mice. Carcinogenesis 2001, 22, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging. Mutat. Res. 1992, 275, 257–266. [Google Scholar] [CrossRef]
- Saretzki, G.; von Zglinicki, T. Replicative aging, telomeres, and oxidative stress. Ann. N. Y. Acad. Sci. 2002, 959, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. J. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- León, R.M.L.; Cedeño, M.R.; Rivero, M.R.J.; García, P.D.L.; Bordón, G.L. La teoría del estrés oxidativo como causa directa del envejecimiento celular. Medisur 2018, 16, 699–710. [Google Scholar]
- Chen, Q.M.; Liu, J.; Merrett, J.B. Apoptosis or senescence-like growth arrest: Influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem. J. 2000, 347, 543–551. [Google Scholar] [CrossRef]
- Holt, S.E.; Glinsky, V.V.; Ivanova, A.B.; Glinsky, G.V. Resistance to apoptosis in human cells conferred by telomerase function and telomere stability. Mol. Carcinog. 1999, 25, 241–248. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Bald, E. Investigation on the origin of sperm DNA fragmentation: Role of apoptosis, immaturity and oxidative stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Henle, E.S.; Han, Z.; Tang, N.; Rai, P.; Luo, Y.; Linn, S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J. Biol. Chem. 1999, 274, 962–971. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Martin-Ruiz, C.M.; Saretzki, G. Telomeres, cell senescence and human ageing. Signal. Transduct. 2005, 5, 103–114. [Google Scholar] [CrossRef]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- Mikhelson, V.M.; Gamaley, I.A. Telomere shortening is a sole mechanism of aging in mammals. Curr. Aging Sci. 2012, 5, 203–208. [Google Scholar] [CrossRef]
- Cattan, V.; Mercier, N.; Gardner, J.P.; Regnault, V.; Labat, C.; Mäki-Jouppila, J.; Nzietchueng, R.; Benetos, A.; Kimura, M.; Aviv, A.; et al. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radic. Biol. Med. 2008, 44, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Seo, H.W.; Jung, G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology 2018, 67, 1378–1391. [Google Scholar] [CrossRef]
- Opresko, P.; Fan, J.; Danzy, S.; Wilson, D.M.; Bohr, V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005, 33, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fischer, A.; Reagan, J.D.; Yan, L.J.; Ames, B.N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. USA 1995, 92, 4337–4341. [Google Scholar] [CrossRef]
- Ahmed, W.; Lingner, J. PRDX1 Counteracts catastrophic telomeric cleavage events that are triggered by DNA repair activities post oxidative damage. Cell Rep. 2020, 33, 108347. [Google Scholar] [CrossRef] [PubMed]
- Kuzminov, A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc. Natl. Acad. Sci. USA 2001, 98, 8241–8246. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; Martínez-Maldonado, M.L.; Vivaldo-Martínez, M. What is the onset age of human aging and old age? Int. J. Gerontol. 2016, 10, 56. [Google Scholar] [CrossRef]
- WHO. Ageing and Life-Course. Facts about Ageing. Available online: https://www.who.int/ageing/about/facts/en/ (accessed on 7 December 2020).
- Llanes, B.C. Envejecimiento demográfico y necesidad de desarrollar las competencias profesionales en enfermería geriátrica. Rev. Haban. Cien. Med. 2015, 14, 89–96. [Google Scholar]
- Ridout, K.K.; Ridout, S.J.; Goonan, K.; Tyrka, A.R.; Price, L.H. Telomeres and Early Life Stress: Neuroendocrinology and Neurobiology Handbook of Stress Series; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 185–193. [Google Scholar]
- Brouilette, S.; Singh, R.K.; Thompson, J.R.; Goodall, A.H.; Samani, N.J. White cell telomere length and risk of premature myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.; Dickinson, H.O.; Keys, B.; Rowan, E.; Kenny, R.A.; von Zglinicki, T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006, 60, 174–180. [Google Scholar] [CrossRef]
- Valdes, A.M.; Richars, J.B.; Gardner, J.P.; Swaminathan, R.; Kimura, M.; Xiaobin, L.; Aviv, A.; Spector, T.D. Telomere length in leukocytes correlates with bone mineral density and is shirter in women with osteoporosis. Osteoporos. Int. 2007, 18, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev., M.A.; Vishnyakova, K.S.; Yegorov, Y.E. Telomere-dependent senescent phenotype of lens epithelial cells as a biological marker of aging and cataractogenesis: The role of oxidative stress intensity and specific mechanism of phospholipid hydroperoxide toxicity in lens and aqueous. Fundam. Clin. Pharmacol. 2011, 25, 139–162. [Google Scholar] [CrossRef]
- Weischer, M.; Bojesen, S.E.; Cawthon, R.M.; Freiberg, J.J.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 822–829. [Google Scholar] [CrossRef]
- Vera, E.; Blasco, M.A. Beyond average: Potential for measurement of short telomeres. Aging (Albany N. Y.) 2012, 4, 379–392. [Google Scholar] [CrossRef]
- Kirchner, H.; Shaheen, F.; Kalscheuer, H.; Schmid, S.M.; Oster, H.; Lehnert, H. The Telomeric complex and metabolic disease. Genes 2017, 8, 176. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Sleiman, F.; Nasreddine, L.; Nasrallah, M.; Nakhoul, N.; Isma’eel, H.; Tamim, H. Short telomere length is associated with aging, central obesity, poor sleep and hypertension in lebanese individuals. Aging Dis. 2018, 9, 77–89. [Google Scholar] [CrossRef]
- Soerensen, M.; Thinggaard, M.; Nygaard, M.; Dato, S.; Tan, Q.; Hjelmborg, J.; Andersen-Ranberg, K.; Stevnsner, T.; Bohr, V.A.; Kimura, M.; et al. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: A cross-sectional and longitudinal analysis. Aging Cell 2012, 11, 223–227. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association between telomere length and risk of cancer and non-neoplastic diseases: A mendelian randomization study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valle, V.; Méndez-Sánchez, N. Estrés oxidativo, antioxidantes y enfermedad. Med. Sur 2013, 20, 161–168. [Google Scholar]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere length: A review of methods for measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef]
- Richter, T.; Proctor, C. The role of intracellular peroxide levels on the development and maintenance of telomere-dependent senescence. Exp. Gerontol. 2007, 42, 1043–1052. [Google Scholar] [CrossRef]
- Aviv, A.; Valdés, A.; Spector, T. Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. Int. J. Epidemiol. 2006, 35, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Weng, N. Interplay between telomere length and telomerase in human leukocyte differentiation and aging. J. Leukoc. Biol. 2001, 70, 861–867. [Google Scholar]
- Strandberg, T.E.; Saijonmaa, O.; Tilvis, R.S.; Pitkälä, K.H.; Strandberg, A.Y.; Miettinen, T.A.; Fyhrquist, F. Association of telomere length in older men with mortality and midlife body mass index and smoking. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, Y.; Xu, G.; Snetselaar, L.G.; Ludewig, G.; Wallace, R.B.; Bao, W. Association between body iron status and leukocyte telomere length, a biomarker of biological aging, in a nationally representative sample of us adults. J. Acad. Nutr. Diet. 2019, 119, 617–625. [Google Scholar] [CrossRef]
- Marzetti, E.; Lorenzi, M.; Antocicco, M.; Bonassi, S.; Celi, M.; Mastropaolo, S.; Settanni, S.; Valdiglesias, V.; Landi, F.; Bernabei, R.; et al. Shorter telomeres in peripheral blood mononuclear cells from older persons with sarcopenia: Results from an exploratory study. Front. Aging Neurosci. 2014, 6, 233. [Google Scholar] [CrossRef]
- Visala, R.D.; Boyle, G.M.; Parsons, P.G.; Watson, K.; Jones, G.L. Influence of ageing, heat shock treatment and in vivo total antioxidant status on gene-expression profile and protein synthesis in human peripheral lymphocytes. Mech. Ageing Dev. 2003, 124, 55–69. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; Ruiz-Ramos, M.; Sánchez-Rodríguez, M.; Retana-Ugalde, R.; Muñoz-Sánchez, J.L. Aging-related oxidative stress in healthy humans. Tohoku J. Exp. Med. 2007, 213, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Huerta, J.M.; González, S.; Fernández, S.; Patterson, A.M.; Lasheras, C. Lipid peroxidation, antioxidant status and survival in institutionalised elderly: A five-year longitudinal study. Free Radic. Res. 2006, 40, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Saretzki, G.; von Zglinicki, T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 1998, 239, 152–160. [Google Scholar] [CrossRef]
- Kruk, P.A.; Rampino, N.J.; Bohr, V.A. DNA damage and repair in telomeres: Relation to aging. Proc. Indian Natl. Sci. 1995, 92, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beasejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Shafirovich, V.; Geacintov, N.E. Removal of oxidatively generated DNA damage by overlapping repair pathways. Free Radic. Biol. Med. 2017, 107, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar]
- Miller, A.S.; Balakrishnan, L.; Buncher, N.A.; Opresko, P.L.; Bambara, R.A. Telomere proteins POT1, TRF1 and TRF2 augment long-patch base excision repair in vitro. Cell Cycle 2012, 11, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Dianov, G.; Bischoff, C.; Piotrowski, J.; Bohr, V.A. Repair pathways for processing of 8-oxoguanine in DNA by mammalian cell extracts. J. Biol. Chem. 1998, 273, 33811–33816. [Google Scholar] [CrossRef] [PubMed]
- Rhee, D.B.; Ghosh, A.; Lu, J.; Bohr, V.A.; Liu, Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair 2011, 10, 34–44. [Google Scholar] [CrossRef]
- Karihtala, P.; Kauppila, S.; Puistola, U.; Jukkola-Vuorinen, A. Absence of the DNA repair enzyme human 8-oxoguanine glycosylase is associated with an aggressive breast cancer phenotype. Br. J. Cancer 2012, 106, 344–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubo, N.; Morita, M.; Nakashima, Y.; Kitao, H.; Egashira, A.; Saeki, H.; Oki, E.; Kakeji, Y.; Oda, Y.; Maehara, Y. Oxidative DNA damage in human esophageal cancer: Clinicopathological analysis of 8-hydroxydeoxyguanosine and its repair enzyme. Dis. Esophagus 2014, 27, 285–293. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Repair of telomeric DNA prior to replicative senescence. Mech. Ageing Dev. 2000, 118, 23–34. [Google Scholar] [CrossRef]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Sola-Carvajal, A.; Cancila, V.; Revêchon, G.; Ong, P.F.; Jones-Weinert, C.W.; Arzt, E.W.; Lattanzi, G.; Dreesen, O.; Tripodo, C.; et al. Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of Hutchinson–Gilford Progeria Syndrome. Nat. Commun. 2019, 10, 1–11. [Google Scholar]
- Cusanelli, E.; Chartrand, P. Telomeric nonconding RNA: Telomeric repeat-containg RNA in telomere biology. Wiley Interdiscip. Rev. RNA 2014, 5, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.; Feuerhahn, S.; Delafontaine, J.; Riethman, H.; Rougemont, J.; Lingner, J. Functional characterization of the TERRA transcriptome at damaged telomeres. Nat. Commun. 2014, 5, 5379. [Google Scholar] [CrossRef] [PubMed]
- Santosh, B.; Varshney, A.; Yadava, P.K. Non-coding RNAs: Biological functions and applications. Cell Biochem. Funct. 2015, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Bettin, N.; Oss Pegorar, C.; Cusanelli, E. The emerging roles of TERRA in telomere maintenance and genome stability. Cells 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Aguado, J.; Sepe, S.; Iannello, F.; Nguyen, Q.; Pitchiaya, S.; Carninci, P.; di Fagagna, F.D.A. DNA damage response inhibition at dysfunctional telomeres by modulation of telomeric DNA damage response RNAs. Nat. Commun. 2017, 8, 13980. [Google Scholar] [CrossRef] [PubMed]
- Genua, M.I.; Miró, B.; Hernández, R.; Martínez, M.; Miró, M.; Pardo, C. Geriatría. In Farmacia Hospitalaria; Bonal, J., Domínguez-Gil., Gamundi, M.C., Napal, V., Valverde, E., Eds.; Doyma: Madrid, Spain, 2002; pp. 959–992. [Google Scholar]
- Domínguez, L.J.; Barbagallo, M. The biology of the metabolic syndrome and aging. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Llabre, M.M.; Dong, C.; Elkind, M.S.; Stern, Y.; Rundek, T.; Sacco, R.L.; Wright, C.B. Modeling metabolic syndrome and its association with cognition: The Northern Manhattan study. J. Int. Neuropsychol. Soc. 2014, 20, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Aguilar-Salinas, C.A.; Jiménez-Corona, A.; Shamah-Levy, T.; Rauda, J.; Ávila-Burgos, L.; Villalpando, S.; Lazcano-Ponce, E. Metabolic syndrome in Mexican adults. Results from the National Health and Nutrition Survey 2006. Salud. Publica Mex. 2010, 52, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Roiland, R.; Chen, D.G.; Qiu, C. Linking cognition and frailty in middle and old age: Metabolic syndrome matters. Int. J. Geriatr. Psychiatry 2015, 30, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Avelar, T.M.T.; Storch, A.S.; Castro, L.A.; Azevedo, G.V.M.M.; Ferraz, L.; Lopes, P.F. Oxidative stress in the pathophysiology of metabolic syndrome: Which mechanisms are involved? J. Bras. Patol. Med. Lab. 2015, 51, 231–239. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Hajian-Tilaki, K.; Omidvar, S.; Nasiri Amiri, F. Association of lipid peroxidation and antioxidant status with metabolic syndrome in Iranian healthy elderly women. Biomed. Rep. 2017, 7, 331–336. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Peña-Orihuela, P.; Perez-Martinez, P.; Fuentes, F.; Marin, C.; Tunez, I.; Tinahones, F.J.; Perez-Jimenez, F.; Roche, H.M.; et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp. Mol. Med. 2013, 45, e28. [Google Scholar] [CrossRef] [PubMed]
- Carrier, A. Metabolic syndrome and oxidative stress: A complex relationship. Antioxid. Redox Signal. 2017, 26, 429–431. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of millimolar steady-state hydrogen peroxide exposure on inflammatory and redox gene expression in immune cells from humans with metabolic syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef] [PubMed]
- Abril-Ulloa, V.; Flores-Mateo, G.; Solà-Alberich, R.; Manuel-y-Keenoy, B.; Arija, V. Ferritin levels and risk of metabolic syndrome: Meta-analysis of observational studies. BMC Public Health 2014, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, A.; Szeto, I.M.; Wu, W.; Ren, Z.; Li, T.; Feng, H.; Wang, P.; Wang, Y.; Zhang, Y. Association of serum ferritin with metabolic syndrome in eight cities in China. Food Sci. Nutr. 2020, 8, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R.F. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men-the third national health and nutrition examination survey. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 639–645. [Google Scholar] [CrossRef]
- Halle, M.; Konig, D.; Berg, A.; Keul, J.; Baumstark, M.W. Relationship of serum ferritin concentrations with metabolic cardiovascular risk factors in men without evidence for coronary artery disease. Atherosclerosis 1997, 128, 235–240. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, S.K.; Choi, W.J.; Kwak, K.M.; Kang, D.; Lee, S.H.; Lee, J.H. Association between serum ferritin and hypertension according to the working type in Korean men: The fifth Korean national health and nutrition examination survey 2010–2012. Ann. Occup. Environ. Med. 2018, 30, 40. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef]
- Fujita, K.; Nishizawa, H.; Funahashi, T.; Shimomura, I.; Shimabukuro, M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ. J. 2006, 70, 1437–1442. [Google Scholar] [CrossRef]
- Sankhla, M.; Sharma, T.K.; Mathur, K.; Rathor, J.S.; Butolia, V.; Gadhok, A.K.; Kaushik, G.G. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin. Lab. 2012, 58, 385–392. [Google Scholar] [PubMed]
- Mattson, M.P. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp. Gerontol. 2009, 44, 625–633. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (Chayote) on telomerase levels and antioxidant capacity in older adults with metabolic syndrome. Antioxidants 2020, 9, 634. [Google Scholar] [CrossRef]
- Holvoet, P.; De Keyzer, D.; Jacobs, D.R. Oxidized LDL and the metabolic syndrome. Future Lipidol. 2008, 3, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Uribarri, J.; Zhu, L.; Chen, X.; Swamy, S.; Zhao, Z.; Grosjean, F.; Simonaro, C.; Kuchel, G.A.; Schnaider-Beeri, M.; et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc. Natl. Acad. Sci. USA 2014, 111, 4940–4945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mishra, S.; Ajmera, P.; Mathur, S. Oxidative stress in metabolic syndrome. Indian J. Clin Biochem 2005, 20, 145–149. [Google Scholar] [CrossRef][Green Version]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The metabolic syndrome and antioxidant concentrations: Findings from the third national health and nutrition examination survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Spanidis, Y.; Mpesios, A.; Stagos, D.; Goutzourelas, N.; Bar-Or, D.; Karapetsa, M.; Zakynthinos, E.; Spandidos, D.A.; Tsatsakis, A.M.; Leon, G.; et al. Assessment of the redox status in patients with metabolic syndrome and type 2 diabetes reveals great variations. Exp. Ther. Med. 2016, 11, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef] [PubMed]
- Skalicky, J.; Muzakova, V.; Kandar, R.; Meloun, M.; Rousar, T.; Palicka, V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin. Chem. Lab. Med. 2008, 46, 499–505. [Google Scholar] [CrossRef]
- Mahjoub, S.; Masrour-Roudsari, J. Role of oxidative stress in pathogenesis of metabolic syndrome. Caspian J. Intern. Med. 2012, 3, 386–396. [Google Scholar] [PubMed]
- Tian, R.; Zhang, L.N.; Zhang, T.T.; Pang, H.Y.; Chen, L.F.; Shen, Z.J.; Liu, Z.; Fang, Q.; Zhang, S.Y. Association between oxidative stress and peripheral leukocyte telomere length in patients with premature coronary artery disease. Med. Sci. Monit. 2017, 23, 4382–4390. [Google Scholar] [CrossRef] [PubMed]
- McAninch, D.; Bianco-Miotto, T.; Gatford, K.L.; Leemaqz, S.Y.; Andraweera, P.H.; Garrett, A.; Plummer, M.D.; Dekker, G.A.; Roberts, C.T.; Smithers, L.G.; et al. The metabolic syndrome in pregnancy and its association with child telomere length. Diabetologia 2020, 63, 2140–2149. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of nutrition on telomere health: Systematic review of observational cohort studies and randomized clinical trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef] [PubMed]
- Rentoukas, E.; Tsarouhas, K.; Kaplanis, I.; Korou, E.; Nikolaou, M.; Marathonitis, G.; Kokkinou, S.; Haliassos, A.; Mamalaki, A.; Kouretas, D.; et al. Connection between telomerase activity in pbmc and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PLoS ONE 2012, 7, e35739. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Seluanov, A.; Pereira-Smith, O.M. Evidence that high telomerase activity may induce a senescent-like growth arrest in human fibroblasts. J. Biol. Chem. 2003, 278, 7692–7698. [Google Scholar] [CrossRef]
- Braun, S.; Bitton-Worms, K.; LeRoith, D. The link between the metabolic syndrome and cancer. Int. J. Biol. Sci. 2011, 7, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, T.; Lukanova, A.; Jonsson, H.; Tretli, S.; Ulmer, H.; Manjer, J.; Stocks, T.; Selmer, R.; Nagel, G.; Almquist, M.; et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Autelitano, M.; Bisanti, L. Metabolic syndrome and cancer risk. Eur. J. Cancer 2008, 4, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Lorente, M.A.; Cano-Martin, A.C.; Blasco, M.A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 2019, 10, 4723. [Google Scholar] [CrossRef] [PubMed]
- Salpea, K.D.; Maubaret, C.G.; Kathagen, A.; Ken-Dror, G.; Gilroy, D.W.; Humphries, S.E. The effect of pro-inflammatory conditioning and/or high glucose on telomere shortening of aging fibroblasts. PLoS ONE 2013, 8, e73756. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, S.S.I.; McLennan, S.V.; Keech, A.C.; Twigg, S.M. Shortening of telomere length by metabolic factors in diabetes: Protective effects of fenofibrate. J. Cell Commun. Signal. 2019, 13, 523–530. [Google Scholar] [CrossRef]
- Smith, D.L.; Mattison, J.A.; Desmond, R.A.; Gardner, J.P.; Kimura, M.; Roth, G.S.; Ingram, D.K.; Allison, D.V.; Aviv, A. Telomere dynamics in Rhesus Monkeys: No apparent effect of caloric restriction. J. Gerontol. 2011, 66A, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Kao, T.W.; Chang, Y.W.; Wu-Jung, C.; Peng, T.C.; Wu, L.W.; Yang, H.F.; Chen, W.L. Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. PLoS ONE 2017, 12, e0180687. [Google Scholar] [CrossRef] [PubMed]
- Révész, D.; Milaneschi, Y.; Verhoeven, J.E.; Penninx, B.W. Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 4607–4615. [Google Scholar] [CrossRef]
- Higuchi, Y.; Maeda, T.; Guan, J.Z.; Oyama, J.; Sugano, M.; Makino, N. Diagonal earlobe crease are associated with shorter telomere in male Japanese patients with metabolic syndrome. Circ. J. 2009, 73, 274–279. [Google Scholar] [CrossRef]
- Iglesias-Molli, A.E.; Panero, J.; Dos Santos, P.C.; González, C.D.; Vilariño, J.; Sereday, M.; Cerrone, G.E.; Slavutsky, I.; Frechtel, G.D. Metabolically healthy obese women have longer telomere length than obese women with metabolic syndrome. PLoS ONE 2017, 12, e0174945. [Google Scholar] [CrossRef]
- Khalangot, M.D.; Krasnienkov, D.S.; Chizhova, V.P.; Korkushko, O.V.; Shatilo, V.B.; Kukharsky, V.M.; Kravchenko, V.I.; Kovtun, V.A.; Guryanov, V.G.; Vaiserman, A.M. Additional impact of glucose tolerance on telomere length in persons with and without metabolic syndrome in the elderly Ukraine population. Front. Endocrinol. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Révész, D.; Milaneschi, Y.; Verhoeven, J.E.; Lin, J.; Penninx, B.W. Longitudinal associations between metabolic syndrome components and telomere shortening. J. Clin. Endocrinol. Metab. 2015, 100, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Huzen, J.; Wong, L.S.; van Veldhuisen, D.J.; Samani, N.J.; Zwinderman, A.H.; Codd, V.; Cawthon, R.M.; Benus, G.F.; van der Horst, I.C.; Navis, G.; et al. Telomere length loss due to smoking and metabolic traits. J. Intern. Med. 2014, 275, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Morioka, K.; Maruyama, N.; Kitagawa, N.; Tanaka, T.; Hori, Y.; et al. Oxidative stress is associated with adiposity and insulin resistance in men. J. Clin. Endocrinol. Metab. 2003, 88, 4673–4676. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.I.; Tuluca, A.; Ives, S.J.; Reynolds, T.H. High-fat diet induced obesity and age influence the telomere shelterin complex and telomerase gene expression in mouse ad-ipose tissue. Physiol. Rep. 2020, 8, e14461. [Google Scholar] [CrossRef] [PubMed]
- Al-Aubaidy, H.A.; Jelinek, H.F. Oxidative stress and triglycerides as predictors of subclinical atherosclerosis in prediabetes. Redox Rep. 2014, 19, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Li, W.; Le, G.W. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef]
- Le, N.A. Postprandial triglycerides, oxidative stress, and inflammation. In Triglycerides and Cholesterol; Waisundara, V.Y., Jovandaric, M.Z., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–13. [Google Scholar]
- Lupachyk, S.; Watcho, P.; Hasanova, N.; Julius, U.; Obrosova, I.G. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: Role for oxidative–nitrosative stress. Free Radic. Biol. Med. 2012, 52, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; de Faria, E.C.; Chantepie, S.; Chapman, M.J. A normotriglyceridemic, low HDL-cholesterol phenotype is characterised by elevated oxidative stress and HDL particles with attenuated antioxidative activity. Atherosclerosis 2005, 182, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Aviv, A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis 2009, 205, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Touyz, R.M. Oxidative stress and hypertension: Current concepts. Curr. Hypertens. Rep. 2010, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- de Champlain, J.; Wu, R.; Girouard, H.; Karas, M.; Midaoui, A.E.; Laplante, M.; Wu, L. Oxidative stress in hypertension. Clin Exp. Hypertens. 2004, 26, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.C.; Reckelhoff, J.F. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999, 34, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Du Plooy, C.; Mels, M.C.; Huisman, H.; Kruger, R. The association of endothelin-1 with markers of oxidative stress in a biethnic South African cohort: The SABPA study. Hypertens. Res. 2017, 40, 189–195. [Google Scholar] [CrossRef]
- Monickaraj, F.; Gokulakrishnan, K.; Prabu, P.; Sathishkumar, C.; Anjana, R.M.; Rajkumar, J.S.; Mohan, V.; Balasubramanyam, M. Convergence of adipocyte hypertrophy, telomere shortening and hypoadiponectinemia in obese subjects and in patients with type 2 diabetes. Clin. Biochem. 2012, 45, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Demissie, S.; Levy, D.; Benjamin, E.J.; Cupples, L.A.; Gardner, J.P.; Herbert, A.; Kimura, M.; Larson, M.G.; Meigs, J.B.; Keaney, J.F.; et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006, 5, 325–330. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Balasubramanyam, M.; Mohan, V. Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabet. Med. 2005, 22, 1151–1156. [Google Scholar] [CrossRef]
- Sampson, M.J.; Winterbone, M.K.; Hughes, J.C.; Dozio, N.; Hughes, D.A. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006, 29, 283–289. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Balasubramanyam, M.; Ravikumar, R.; Deepa, R.; Mohan, V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis 2007, 195, 83–89. [Google Scholar] [CrossRef]
- Salpea, K.D.; Talmud, P.J.; Cooper, J.A.; Maubaret, C.G.; Stephens, J.W.; Abelak, K.; Humphries, S.E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 2010, 209, 42–50. [Google Scholar] [CrossRef]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse association between adiposity and telomere length: The fels longitudinal study. Am. J. Hum. Biol. 2011, 23, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Cawthon, R.M.; Blackburn, E.H.; Harris, T.B.; Li, R.; Sanders, J.L.; Newman, A.B.; Nalls, M.; Cummings, S.R.; Hsueh, W.C. Shorter telomeres are associated with obesity and weight gain in the elderly. Int. J. Obes. (Lond.) 2012, 36, 1176–1179. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, Y.T.; Cai, Q.; Qu, S.; Cai, H.; Li, H.L.; Wu, J.; Ji, B.T.; Yang, G.; Chow, W.H.; et al. Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity (Silver Spring) 2013, 21, 2582–2588. [Google Scholar] [CrossRef]
- Chen, S.; Yeh, F.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Lee, E.T.; Howard, B.V.; Zhao, J. Short leukocyte telomere length is associated with obesity in American Indians: The strong heart family study. Aging (Albany N. Y.) 2014, 6, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.P.; Li, S.; Srinivasan, S.R.; Chen, W.; Kimura, M.; Lu, X.; Berenson, G.S.; Aviv, A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation 2005, 111, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Telomere length of subcutaneous adipose tissue cells is shorter in obese and formerly obese subjects. Int. J. Obes. 2010, 34, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Harte, A.L.; da Silva, N.F.; Miller, M.A.; Cappuccio, F.P.; Kelly, A.; O’Hare, J.P.; Barnett, A.H.; Al-Daghri, N.M.; Al-Attas, O.; Alokail, M.; et al. Telomere length attrition, a marker of biological senescence, is inversely correlated with triglycerides and cholesterol in South Asian males with type 2 diabetes mellitus. Exp. Diabetes Res. 2012, 2012, 895185. [Google Scholar] [CrossRef]
- Laimer, M.; Melmer, A.; Lamina, C.; Raschenberger, J.; Adamovski, P.; Engl, J.; Ress, C.; Tschoner, A.; Gelsinger, C.; Mair, L.; et al. Telomere length increase after weight loss induced by bariatric surgery: Results from a 10 year prospective study. Int. J. Obes. 2016, 40, 773–778. [Google Scholar] [CrossRef]
- Rehkopf, D.H.; Needham, B.L.; Lin, J.; Blackburn, E.H.; Zota, A.R.; Wojcicki, J.M.; Epel, E.S. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: A cross-sectional study of US adults. PLoS Med. 2016, 13, e1002188. [Google Scholar] [CrossRef]
- Neuner, B.; Lenfers, A.; Kelsch, R.; Jäger, K.; Brüggmann, N.; van der Harst, P.; Walter, M. Telomere length is not related to established cardiovascular risk factors but does correlate with red and white blood cell counts in a german blood donor population. PLoS ONE 2015, 7, e0139308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zhou, K.W.; Yang, G.Z.; Chen, C. Association between lipoproteins and telomere length in US adults: Data from the NHANES 1999–2002. Lipids Health Dis. 2019, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; Sahebkar, A.; Banach, M. Telomere length is associated with cardiometabolic factors in US adults. Angiology 2017, 69, 164–169. [Google Scholar] [CrossRef]
- Benetos, A.; Gardner, J.P.; Zureik, M.; Labat, C.; Xiaobin, L.; Adamopoulos, C.; Temmar, M.; Bean, K.E.; Thomas, F.; Aviv, A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 2004, 43, 182–185. [Google Scholar] [CrossRef]
- Tellechea, M.L.; Pirola, C.J. The impact of hypertension on leukocyte telomere length: A systematic review and meta-analysis of human studies. J. Hum. Hypertens. 2017, 31, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, X.; Jiang, H.; Zhang, Y.; Liu, H.; Qin, C.; Eisner, G.M.; Jose, P.; Rudolph, L.; Ju, Z. Short telomeres and prognosis of hypertension in a Chinese population. Hypertension 2009, 53, 639–645. [Google Scholar] [CrossRef]
- Jeanclos, E.; Schork, N.J.; Kyvik, K.O.; Kimura, M.; Skurnick, J.H.; Aviv, A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000, 36, 195–200. [Google Scholar] [CrossRef]
- Olivieri, F.; Lorenzi, M.; Antonicelli, R.; Testa, R.; Sirolla, C.; Cardelli, M.; Mariotti, S.; Marchegianif, F.; Marra, M.; Spazzafumoe, L.; et al. Leukocyte telomere shortening in elderly Type 2 DM patients with previous myocardial infarction. Atherosclerosis 2009, 206, 558–593. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Med. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- León-Pedroza, J.I.; González-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Inflamación sistémica de grado bajo y su relación con el desarrollo de enfermedades metabólicas: De la evidencia molecular a la aplicación clínica. Cir. Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Squassina, A.; Pisanu, C.; Vanni, R. Mood disorders, accelerated aging, and inflammation: Is the link hidden in telomeres? Cells 2019, 8, 52. [Google Scholar] [CrossRef]
- Ramírez, A.M.M.; Sánchez, R.C.; Pérez, D.A.; Millán, B.E. Evaluación del efecto de la ingesta de una alta carga de ácidos grasos saturados sobre los niveles séricos de la proteína C reactiva, alfa1-antitripsina, fibrinógeno y alfa1-glicoproteína ácida en mujeres obesas. Nutr. Hosp. 2010, 25, 72–79. [Google Scholar]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.C.; Satterfield, S.; et al. Health aging and body composition study. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Rode, L.; Nordestgaard, B.G.; Weischer, M.; Bojesen, S.E. Increased body mass index, elevated C-reactive protein, and short telomere length. J. Clin. Endocrinol. Metab. 2014, 99, E1671–E1675. [Google Scholar] [CrossRef]
- Ebron, K.; Andersen, C.J.; Aguilar, D.; Blesso, C.N.; Barona, J.; Dugan, C.E.; Jones, J.L.; Al-Sarraj, T.; Fernandez, M.L. A Larger body mass index is associated with increased atherogenic dyslipidemia, insulin resistance, and low-grade inflammation in individuals with metabolic syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 458–464. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Yin, L.; Hubbard, A.K.; Giardina, C. NF-kB regulates transcription of the mouse telomerase catalytic subunit. J. Biol. Chem. 2000, 275, 36671–36675. [Google Scholar] [CrossRef]
- Wang, J.C.; Bennett, M.R. Nuclear Factor-κΒ–mediated regulation of telomerase. The myc link. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2327–2328. [Google Scholar] [CrossRef] [PubMed]
- Mattiussi, M.; Tilman, G.; Lenglez, S.; Decottignies, A. Human telomerase represses ROS-dependent cellular responses to tumor necrosis factor-α without affecting NF-κB activation. Cell Signal. 2012, 24, 708–717. [Google Scholar] [CrossRef]
- Geng, H.; Wittwer, T.; Dittrich-Breiholz, O.; Kracht, M.; Schmitz, M.L. Phosphorylation of NF-kappaB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 2009, 10, 381–386. [Google Scholar] [CrossRef]
- Teo, H.; Ghosh, S.; Luesch, H.; Ghosh, A.; Wong, E.T.; Malik, N.; Orth, A.; de Jesus, P.; Perry, A.S.; Oliver, J.D.; et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene expression. Nat. Cell Biol. 2010, 12, 758–767. [Google Scholar] [CrossRef]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Sánchez, N.J.C.; López, Z.D.F.; Pinzón, D.O.A.; Sepúlveda, A.J.C. Adipocinas y síndrome metabólico: Múltiples facetas de un proceso fisiopatológico complejo. Rev. Colom. Cardiol. 2010, 17, 167–176. [Google Scholar] [CrossRef][Green Version]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Molina, M.T.; Antuna-Puente, B. Adiponectin: Anti-inflammatory and cardioprotective effects. Biochimie 2012, 94, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Al-Attas, O.S.; Al-Daghri, N.M.; Alokail, M.S.; Alfadda, A.; Bamakhramah, A.; Sabico, S.; Pritlove, D.; Harte, A.; Tripathi, G.; McTernan, P.G.; et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: The influence of circulating adiponectin. Eur. J. Endocrinol. 2010, 163, 601–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Syematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (chayote) on oxidative stress and pro-inflammatory markers in older adults with metabolic syndrome: An exploratory study. Antioxidants 2019, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Singaravelu, G.; Harley, C.B.; Flom, P.; Suram, A.; Raffaele, J.M. A natural product telomerase activator lengthens telomeres in humans: A randomized, double blind, and placebo controlled study. Rejuvenation Res. 2016, 19, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Thomas, M.S.; Lemos, B.S.; DiMarco, D.M.; Missimer, A.; Melough, M.; Chun, O.K.; Murillo, A.G.; Alyousef, H.M.; Medina-Vera, I. TA-65, A Telomerase activator improves cardiovascular markers in patients with metabolic syndrome. Curr. Pharm. Des. 2018, 24, 1905–1911. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Sng, E. Mode-specific physical activity and leukocyte telomere length among US adults: Implications of running on cellular aging. Prev. Med. Rep. 2016, 85, 17–19. [Google Scholar] [CrossRef]
- Denham, J.; O’Brien, B.J.; Prestes, P.R.; Brown, N.J.; Charchar, F.J. Increased expression of telomere-regulating genes in endurance athletes with long leukocyte telomeres. J. Appl. Physiol. 2016, 120, 148. [Google Scholar] [CrossRef]
- Gong, Y.; Tian, G.; Xue, H.; Zhang, X.; Zhao, Y.; Cheng, G. Higher adherence to the ‘vegetable-rich’ dietary pattern is related to longer telomere length in women. Clin. Nutr. 2018, 37, 1232–1237. [Google Scholar] [CrossRef]
- Rosado-Pérez, J.; Santiago-Osorio, E.; Ortiz, R.; Mendoza-Núñez, V.M. Tai Chi diminishes oxidative stress in Mexican older adults. J. Nutr. Health Aging 2012, 16, 642–646. [Google Scholar] [CrossRef]
- Goon, J.A.; Aini, A.N.; Musalmah, M.; Anum, M.Y.; Nazaimoon, W.W.; Ngah, W.W. Effect of Tai Chi exercise on DNA damage, antioxidant enzymes, and oxidative stress in middle-age adults. J. Phys. Act. Health 2009, 6, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Saßenroth, D.; Meyer, A.; Salewsky, B.; Kroh, M.; Norman, K.; Steinhagen-Thiessen, E.; Demuth, I. Sports and exercise at different ages and leukocyte telomere length in later life-data from the Berlin Aging Study II (BASE-II). PLoS ONE 2015, 10, e0142131. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Vakonaki, E.; Salataj, E.; Kouvidi, E.; Nikitovic, D.; Kovatsi, L.; et al. Association of nutraceutical supplements with longer telomere length. Int J. Mol. Med. 2019, 44, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Simoes, T.M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Doménech, M.; Ponferrada-Ariza, E.; et al. Walnut consumption for two years and leukocyte telomere attrition in mediterranean elders: Results of a randomized controlled trial. Nutrients 2018, 10, 1907. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P.; Cheskin, L.J.; Banach, M. Serum lipophilic antioxidants levels are associated with leucocyte telomere length among US adults. Lipids Health Dis. 2018, 17, 1–6. [Google Scholar] [CrossRef]
- Ligi, P.; Cattaneo, M.; D’Angelo, A.; Sampietro, F.; Fermo, I.; Razzari, C.; Fontana, G.; Eugene, N.; Jacques, P.F.; Selhub, J. Telomere length in peripheral blood mononuclear cells is associated with folate status in men. J. Nutr. 2009, 139, 1273–1278. [Google Scholar]
- Xu, Q.; Parks, C.G.; DeRoo, L.A.; Cawthon, R.M.; Sandler, D.P.; Chen, H. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 2009, 89, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Zarezadeh, M.; Kalajahi, F.H. The relationship between vitamin D and telomere/telomerase: A comprehensive review. J. Fraility Aging 2021, 10, 2–9. [Google Scholar]
- Sen, A.; Marsche, G.; Freudenberger, P.; Schallert, M.; Toeglhofer, A.M.; Nagl, C.; Schmidt, R.; Launer, L.J.; Schmidt, H. Association between higher plasma lutein, zeaxanthin, and vitamin C concentrations and longer telomere length: Results of the Austrian stroke prevention study. J. Am. Geriatr. Soc. 2014, 62, 222–229. [Google Scholar] [CrossRef]
- Maleki, M.; Khelghati, N.; Alemi, F.; Bazdar, M.; Asemi, Z.; Majidinia, M.; Sadeghpoor, A.; Mahmoodpoor, A.; Jadidi-Niaragh, F.; Targhazeh, N.; et al. Stabilization of telomere by the antioxidant property of polyphenols: Anti-aging potential. J. Life Sci. 2020, 259, 118341. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Dietary fiber and telomere length in 5674 U.S. adults: An NHANES study of biological aging. Nutrients 2018, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in nurses’ health study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, L.; Cui, X.; Feng, L.; Zhao, X.; He, S.; Ping, F.; Li, W.; Li, Y. Influence of diet on leukocyte telomere length, markers of inflammation and oxidative stress in individuals with varied glucose tolerance: A Chinese population study. Nutr. J. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Crous-Bou, M.; Giovannucci, E.; De Vivo, I. Coffee consumption is positively associated with longer leukocyte telomere length in the nurses’ health study. J. Nutr. 2016, 146, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; Banach, M. Mineral and vitamin consumption and telomere length among adults in the United States. Pol. Arch. Intern. Med. 2017, 127, 87–90. [Google Scholar]
- Lee, J.Y.; Shin, C.; Baik, I. Longitudinal associations between micronutrient consumption and leukocyte telomere length. J. Hum. Nutr. Diet. 2017, 30, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav. Immun. 2013, 28, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, C.M.; Guarnieri, L.; et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Gutlapalli, S.D.; Kondapaneni, V.; Toulassi, I.A.; Poudel, S.; Zeb, M.; Choudhari, J.; Cancarevic, I. The effects of resveratrol on telomeres and post myocardial infarction remodeling. Cureus 2020, 12, e11482. [Google Scholar]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Hypoglycemic effect of resveratrol: A systematic review and meta-analysis. Antioxidants 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Bath, S.; Elbarbry, F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Wu, J.C.; Ho, C.T.; Badmaev, V. Effects of water extract of Curcuma longa (L.) roots on immunity and telomerase function. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Carroll, J.E.; Esquivel, S.; Goldberg, A.; Seeman, T.E.; Effros, R.B.; Dock, J.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Insomnia and telomere length in older adults. Sleep 2016, 39, 559–564. [Google Scholar] [CrossRef]
- Kim, K.S.; Kwak, J.W.; Lim, S.J.; Park, Y.K.; Yang, H.S.; Kim, H.J. Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea. Aging Dis. 2016, 7, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, A.; Miralles, C.; Barbe, F.; Vila, M.; Pons, S.; Agusti, A.G. Abnormal lipid peroxidation in patients with sleep apnoea. Eur. Respir. J. 2000, 16, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Cribbet, M.R.; Carlisle, M.; Cawthon, R.M.; Uchino, B.N.; Williams, P.G.; Smith, T.W.; Gunn, H.E.; Light, K.C. Cellular aging and restorative processes: Subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep 2014, 37, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Tempaku, P.F.; Mazzotti, D.R.; Tufik, S. Telomere length as a marker of sleep loss and sleep disturbances: A potential link between sleep and cellular senescence. Sleep Med. 2015, 16, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Marcon, F.; Siniscalchi, E.; Andreoli, C.; Allione, A.; Fiorito, G.; Medda, E.; Crebelli, R. Telomerase activity, telomere length and hTERT DNA methylation in peripheral blood mononuclear cells from monozygotic twins with discordant smoking habits. Environ. Mol. Mutagen. 2017, 58, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Astuti, Y.; Wardhana, A.; Watkins, J.; Wulaningsih, W. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 2017, 158, 480–489. [Google Scholar] [CrossRef]
- Ozguner, F.; Koyu, A.; Cesur, G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol. Ind. Health 2005, 21, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Laraia, B.A.; Needham, B.L.; Rehkopf, D.H.; Adler, N.E.; Lin, J.; Blackburn, E.H.; Epel, E.S. Soda and cell aging: Associations between sugar-sweetened beverage consumption and leukocyte telomere length in healthy adults from the National health and nutrition examination surveys. Am. J. Public Health 2014, 104, 2425–2431. [Google Scholar] [CrossRef]
- Wojcicki, J.M.; Medrano, R.; Lin, J.; Epel, E. Increased cellular aging by 3 years of age in latino, preschool children who consume more sugar-sweetened beverages: A pilot study. Child. Obes. 2018, 14, 3149–3157. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Diez-Roux, A.; Jenny, N.S.; Fitzpatrick, A.L.; Jacobs, D.R. Dietary patterns, food groups, and telomere length in the multi-ethnic study of atherosclerosis (MESA). Am. J. Clin. Nutr. 2008, 88, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Whooley, M.A.; Vittinghoff, E.; Roberts, J.D.; Heckbert, S.R.; Fitzpatrick, A.L.; Lin, J.; Leung, C.; Mukamal, K.J.; Marcus, G.M. Alcohol consumption and leukocyte telomere length. Sci. Rep. 2019, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
| Population with MetS | Determinations | Objective | Findings | Ref. |
|---|---|---|---|---|
|
7370 patients (56–73 years old). |
Evaluation of the components of the MetS. The average TL in leukocytes was determined by qPCR. | To investigate gender differences and their association between TL and MetS. | An increase in the number of MetS components is associated with shorter TL in the female population. | [211] |
|
2842 patients (18–65 years old). Follow-up for 6 years |
Evaluation of the components of the MetS. Basal TL in leukocytes was determined by qPCR. | To associate TL with the metabolic profile and with the MetS components. | Short TL is associated with higher MetS component scores, which persist even after 6 years. | [212] |
|
34 male patients (55–68 years old). |
Evaluation of the components of the MetS. The TL in leukocytes was determined by TRF. The bilateral ELC was determined by observation of a deep cut in both ears through the earlobe. | To determine if the ELC is related to telomeric shortening. | Bilateral ELC is a dermatological indicator associated with excessive telomere loss in patients with MetS. | [213] |
|
400 women (18–86 years old). |
Evaluation of the components of the MetS. The TL in leukocytes was determined by qPCR. | To determine the TL and its association with the metabolic condition in obese women. | TL is related to MetS and with a greater number of metabolic abnormalities. | [214] |
|
115 subjects (43–87 years old) |
The TL in leukocytes was determined by qPCR. Test 2hPG. | To establish the relationship between TL with the different components of MetS, glucose tolerance, and age. | MetS is associated with shorter telomeres. For its part, the 2hPG level showed a relationship with TL regardless of the presence of MetS. | [215] |
|
1808 patients (18–65 years old) Follow-up for 6 years |
The TL in leukocytes was determined by qPCR. Anthropometric and biochemical parameters. | To determine whether the components of the MetS predict TL through time and if the alterations are parallel to telomeric attrition. | An increase in waist circumference and glucose, as well as low HDL-C concentrations, are associated with shorter TL. | [216] |
|
8074 patients (28–75 years old) Follow-up for 10 years | The TL in leukocytes was determined by qPCR. Anthropometric and biochemical parameters. | Evaluate the dynamics of TL and identify the factors associated with temporal changes in TL. | Conditions associated with MetS are factors that accelerate telomere attrition. | [217] |
| Components of MetS | Population | Determinations | Objective | Findings | Ref. |
|---|---|---|---|---|---|
| Obesity and OxS | 59 subjects: (26–57 years old) CTR (n = 20); Obese (n = 22); Non-obese T2DM (n = 10) and Obese-T2DM (n = 7). |
TL was determined by qPCR. Subcutaneous and visceral adipose tissue from subjects undergoing abdominal surgery. The size of the adipocytes was determined by histological staining. Lipid peroxidation by fluorometry. | To determine the association between adipocyte size and adipose tissue TL. | There is hypertrophy in adipocytes of obese, T2DM, and obese-T2DM subjects related to shortened TL. TBARS levels were higher in obese-T2DM and T2DM. | [232] |
| Hypertension, insulin resistance, and OxS | 327 men: (40–89 years old). |
The TL in leukocytes was determined by TRF. Determination of HOMA-IR. | To determine the association of TL with insulin resistance, OxS, and hypertension. | Hypertension, increased insulin resistance, OxS, and age are associated with shorter TL, being more evident in hypertensive patients, largely due to insulin resistance. | [233] |
|
Hyperglycemia and OxS | 120 subjects: (38–71 years old) CTR, with IGT, T2DM y T2DM− atherosclerosis. |
The TL in leukocytes was determined by TRF. Levels of TBARS, PCO, and CRP were measured by standard methodologies. IMT was assessed by ultrasonography. | To evaluate if the TL shortening occurs in the IGT stage and if it is greater in subjects with T2DM and atherosclerosis. | TL is lower in patients with T2DM and atherosclerosis. IGT and TL were negatively correlated with TBARS, PCO, and IMT. T2DM and TBARS are significant determinants of shortening. | [234] |
| Hyperglycemia and OxS | 21 subjects: (50–65 years old) with T2DM. |
TL was determined in monocytes by FISH. Oxidative damage by flow cytometry. | To establish if telomere shortening characterises T2DM. | TL in the diabetic group was lower and was associated with elevated levels of 8-oxoguanine. | [235] |
| Hyperglycemia and OxS | 80 subjects: (49–56 years old) T2DM (n = 40) CTR (n = 40) |
Lymphocyte TL was determined by TRF. Determine MDA plasma levels using TBARS. | To determine whether telomeric shortening occurs in T2DM patients. | TBARS levels showed a negative correlation with shortened telomeres in subjects with T2DM. | [236] |
| Hyperglycemia and OxS | 621 patients: T2DM (n = 173) (24–92 years old) CTR (n = 448) (18–61 years old). |
The TL in leukocytes was determined by qPCR. TAOS was determined by a photometric microassay. The patients were also genotyped for the UCP2 functional variants −866G> A and A55V. | To determine the association between TL and T2DM, OxS, and gene variation in UCP2. |
The shorter TL was associated with T2DM attributed to high OxS. Carriers of the UCP2 -866A allele have a shorter TL compared to common homozygotes. | [237] |
| Components of MetS | Population | Determinations | Objective | Findings | Ref. |
|---|---|---|---|---|---|
| Obesity | 309 participants (8–80 years old). | The average TL in leukocytes was determined by qPCR. Body fat was determined by DXA. The volume of adipose tissue was determined by MRI. Anthropometric indicators. | To evaluate the relationship between TL and adiposity. | Greater total and abdominal adiposity is associated with shorter TL, suggesting that obesity may accelerate the aging process. | [238] |
| Obesity | 2721 subjects: (70–79 years old). Follow-up for 7 years. | TL in leukocytes was determined by qPCR. Adipose levels: BMI, % of body fat (DXA), and ACT scan to determine visceral and subcutaneous fat. | To determine if TL can be a risk factor for increased accumulation of adipose tissue. | The shorter TL can be a risk factor for adiposity. | [239] |
| Obesity | 2912 women: (40–70 years old). |
TL in leukocytes was determined by qPCR. Anthropometric indicators. | To determine the association between TL and anthropometric indices. | Telomere shortening is associated with obesity, the circumference of the waist and hips. The normal weight maintains the TL. | [240] |
| Obesity | 3256 subjects: (14–93 years old). |
The TL in leukocytes was determined by qPCR. Obesity indexes (BMI, waist circumference, % body fat, waist-hip ratio, and waist-height). High sensitivity CRP test. | To determine the association between TL and obesity rates. | TL is inversely associated with all obesity parameters and with CRP. | [241] |
| Obesity and insulin resistance | 49 subjects: (21–43 years old). Follow-up for 10 years. |
The TL in leukocytes was determined by TRF. Determination of HOMA-IR. | To determine if insulin resistance accelerates telomere attrition. | An increase in body weight and HOMA-IR is associated with a decrease in TL and with aging. | [242] |
| Obesity, TG, and hypertension | 72 subjects: (45–60 years old). |
The TL in leukocytes was determined by TRF. Subcutaneous adipose tissue samples were obtained from patients undergoing surgical procedures. Anthropometric and biochemical parameters. | To establish the relationship between TL in adipose tissue cells, with age and obesity. | TL was negatively associated with BMI, TG, and SBP in obese patients, which could contribute to their comorbidities. | [243] |
| TG and hyperglycemia | 218 patients: (45–60 years old) T2DM (n = 142); CTR (n = 76). |
TL was measured by qPCR. Biochemical and anthropometric data were collected. | To assess whether metabolic status contributes to premature aging. | TL was reduced in men with T2DM and inversely correlated with TG and total cholesterol. | [244] |
| TG | 142 patients: (40–79 years old). Follow-up for 10 years. |
TL was measured by qPCR. Biochemical and anthropometric data were collected. | To investigate the effects of bariatric surgery-induced weight loss on TL. | TL was inversely associated with baseline plasma TG and cholesterol concentrations. | [245] |
| TG, HDL-C, glucose, and blood pressure | 7252 subjects: (20–84 years old) |
The TL in leukocytes was determined by qPCR. Anthropometric and biochemical measurements. | To examine the associations between TL and 17 cardiovascular biomarkers. | TL was inversely associated with BMI, waist circumference, % fat, TG, blood pressure, and CRP and positively with HDL-C | [246] |
| TG and HDL-C | 360 patients: (18–70 years old) |
The TL in leukocytes was determined by qPCR. Anthropometric and biochemical measurements. | To determine the association between TL and coronary risk factors. | There is no association between TL and coronary risk factors, including cholesterol, TG, and HDL-C | [247] |
| HDL-C | 6468 patients: (19–85 years old) |
The TL in leukocytes was determined by qPCR. Anthropometric and biochemical measurements. | To investigate whether lipoproteins are associated with TL. | TL is not associated with LDL-C and TG but is positively associated with HDL-C when telomere length is shorter. | [248] |
| HDL-C | 8892 subjects: (41–42 years old) | TL was measured by qPCR. Anthropometric and biochemical measurements were made. | To determine the relationship of TL with cardiometabolic risk profile. |
A positive association was found between HDL-C and TL. | [249] |
| Hypertension | 163 men: (60–64 years old). |
Lymphocyte TL was determined by TRF. Extracranial carotid plaques were evaluated by ultrasonography. | To examine the relationship between TL and atherosclerotic plaques with the presence of hypertension | A shorter TL is associated with a greater predisposition to carotid artery atherosclerosis | [250] |
| Hypertension | 3097 subjects: (23–76 years old) Hypertensive (n = 1415) |
Meta-analysis The TL in leukocytes was determined by qPCR and TRF. | To determine if TL is related to hypertension | Telomeres are shorter in hypertensive than in normotensive individuals. | [251] |
| Hypertension | 767 subjects: (30–80 years old). CTR (n = 379) Hypertensive (n = 388) | The relative length of the telomeres of the leukocytes was determined by qPCR | To investigate the association between TL and the risk and prognosis of hypertension | TL is significantly lower in patients with hypertension than in normotensive subjects. | [252] |
| Hypertension | 98 twins: (18–44 years old) |
The TL in leukocytes was determined by TRF. Anthropometric measurements | To investigate the relationship between TL and pulse pressure. | TL showed an inverse relationship with pulse pressure. | [253] |
| Hyperglycemia | 272 subjects: (61–76 years old) CTR (n = 104) T2DM (n = 103) T2DM + MI (n = 65) |
The TL in leukocytes was determined by qPCR. Glycaemic control markers: HbA1c, glucose, and waist–hip ratio. | To determine TL and its association with glycaemic control. | Patients with T2DM + MI have shorter TL than subjects with T2DM and CTR. Glycaemic control markers showed an inverse correlation with TL. | [254] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. https://doi.org/10.3390/biology10040253
Gavia-García G, Rosado-Pérez J, Arista-Ugalde TL, Aguiñiga-Sánchez I, Santiago-Osorio E, Mendoza-Núñez VM. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology. 2021; 10(4):253. https://doi.org/10.3390/biology10040253
Chicago/Turabian StyleGavia-García, Graciela, Juana Rosado-Pérez, Taide Laurita Arista-Ugalde, Itzen Aguiñiga-Sánchez, Edelmiro Santiago-Osorio, and Víctor Manuel Mendoza-Núñez. 2021. "Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging" Biology 10, no. 4: 253. https://doi.org/10.3390/biology10040253
APA StyleGavia-García, G., Rosado-Pérez, J., Arista-Ugalde, T. L., Aguiñiga-Sánchez, I., Santiago-Osorio, E., & Mendoza-Núñez, V. M. (2021). Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology, 10(4), 253. https://doi.org/10.3390/biology10040253






