Protective Role of Galanin during Chemically Induced Inflammation in Zebrafish Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. NAX 5055 Treatment
2.3. Chemically Induced Inflammation Assay
2.4. Acridine Orange Staining
2.5. Imaging
2.6. Quantification of Fluorescent Cells
2.7. RNA Isolation and Quantitative PCR
2.8. Statistical Analysis
3. Results
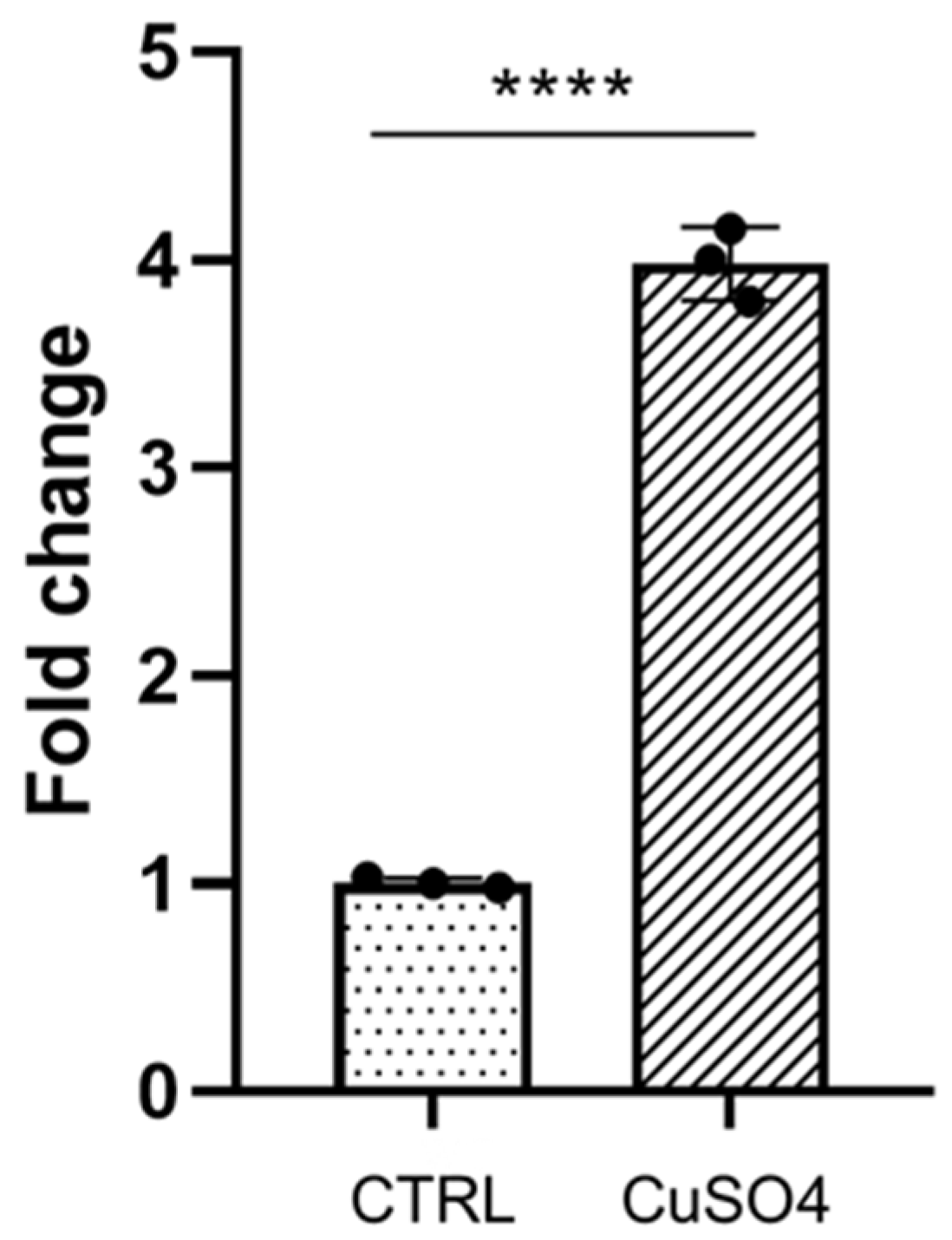
3.1. Galanin Expression Is Induced during Inflammation
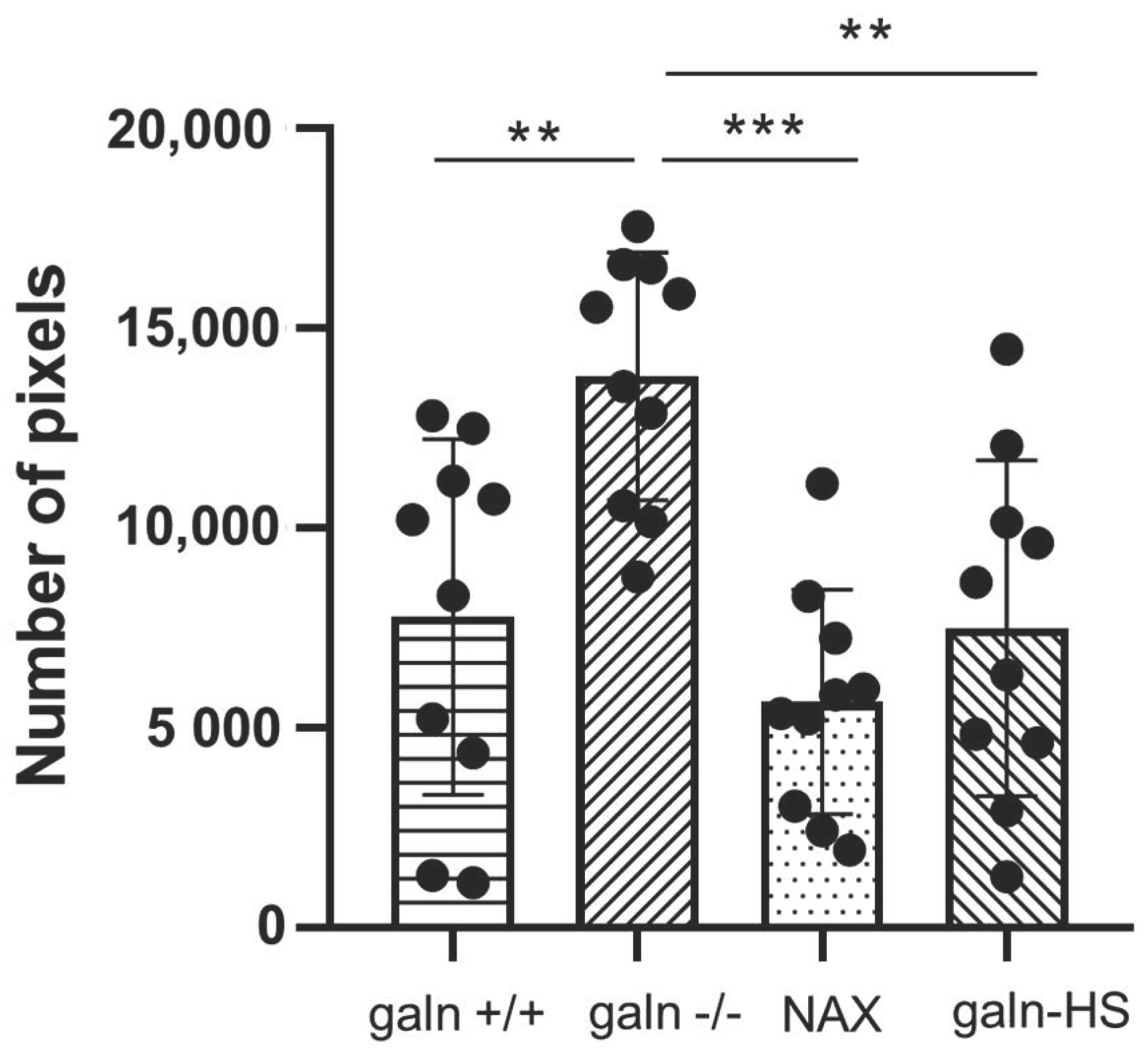
3.2. The Effect of Chemically Induced Inflammation and Lateral Line Damage Depends on Galanin Expression
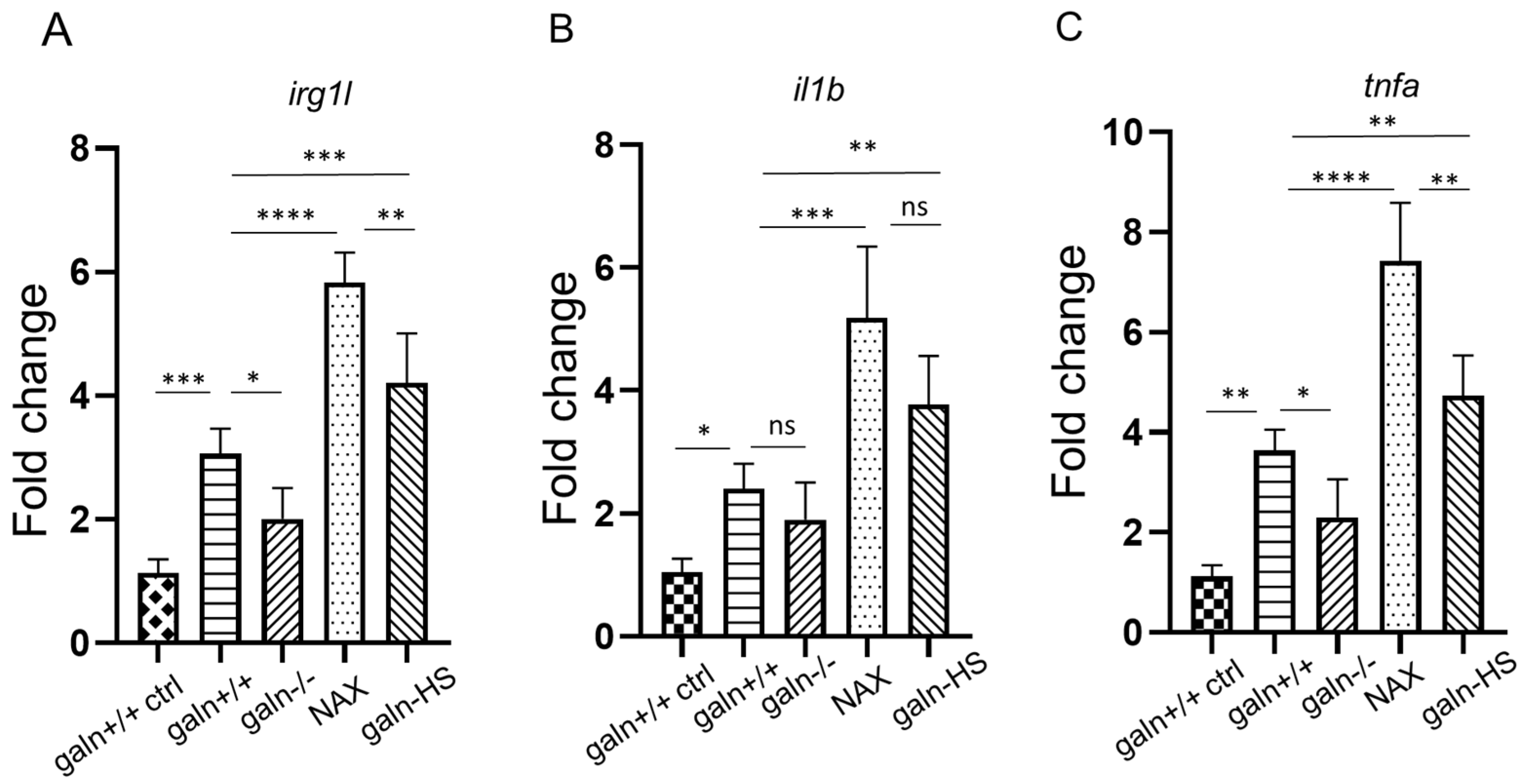
3.3. Galanin Modifies the Expression of irg1l, il1b and tnfa
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 2007, 7, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Rivest, S. Interactions between the immune and neuroendocrine systems. Prog. Brain Res. 2010, 181, 43–53. [Google Scholar] [PubMed]
- Sterner-Kock, A.; Braun, R.K.; van der Vliet, A.; Schrenzel, M.D.; McDonald, R.J.; Kabbur, M.B.; Vulliet, P.R.; Hyde, D.M. Substance P primes the formation of hydrogen peroxide and nitric oxide in human neutrophils. J. Leukoc. Biol. 1999, 65, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Kinhult, J.; Egesten, A.; Uddman, R.; Cardell, L.O. PACAP enhances the expression of CD11b, CD66b andCD63 in human neutrophils. Peptides 2002, 23, 1735–1739. [Google Scholar] [CrossRef]
- Monneret, G.; Arpin, M.; Venet, F.; Maghni, K.; Debard, A.L.; Pachot, A.; Lepape, A.; Bienvenu, J. Calcitonin gene related peptide and N-procalcitonin modulate CD11b upregulation in lipopolysaccharide activated monocytes and neutrophils. Intensive Care Med. 2003, 29, 923–928. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Stanojevic, S.; Micic, S.; Vujic, V.; Kovacevic-Jovanovic, V.; Mitic, K.; von Horsten, S.; Kosec, D. Neuropeptide Y (NPY) modulates oxidative burst and nitric oxide production in carrageenan-elicited granulocytes from rat air pouch. Peptides 2006, 27, 3208–3215. [Google Scholar] [CrossRef]
- Tatemoto, K.; Rokaeus, A.; Jornvall, H.; McDonald, T.J.; Mutt, V. Galanin–a novel biologically active peptide from porcine intestine. FEBS Lett. 1983, 164, 124–128. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.l.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, signaling, and pharmacology of galanin peptides and receptors: Three decades of emerging diversity. Pharmacol. Rev. 2015, 67, 118–175. [Google Scholar] [CrossRef]
- Fang, P.; Yu, M.; Guo, L.; Bo, P.; Zhang, Z.; Shi, M. Galanin and its receptors: A novel strategy for appetite control and obesity therapy. Peptides 2012, 36, 331–339. [Google Scholar] [CrossRef]
- Lang, R.; Kofler, B. The galanin peptide family in inflammation. Neuropeptides 2010, 45, 1–8. [Google Scholar] [CrossRef]
- Kofler, B.; Liu, M.L.; Jacoby, A.S.; Shine, J.; Iismaa, T.P. Molecular cloning and characterisation of the mouse preprogalanin gene. Gene 1996, 182, 71–75. [Google Scholar] [PubMed]
- Rauch, I.; Lundström, L.; Hell, M.; Sperl, W.; Kofler, B. Galanin message-associated peptide suppresses growth and the budded-to-hyphal-form transition of Candida albicans. Antimicrob. Agents Chemother. 2007, 51, 4167–4170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kriz, J. Inflammation in Ischemic Brain Injury: Timing Is Important. Crit. Rev. Neurobiol. 2006, 18, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Matkowskyj, K.A.; Yung, K.; Hecht, G.; Benya, R.V. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory neurotransmitter systems and behavior: Towards zebrafish models of neurodegenerative diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef]
- Zarkadis, I.K.; Mastellos, D.; Lambris, J.D. Phylogenetic aspects of the complement system. Dev. Comp. Immunol. 2001, 25, 745–762. [Google Scholar] [CrossRef]
- Novoa, B.; Figueras, A. Zebrafish: Model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 2012, 946, 253–275. [Google Scholar]
- Steinke, D.; Salzburger, W.; Braasch, I.; Meyer, A. Many genes in fish have species-specific asymmetric rates of molecular evolution. BMC Genom. 2006, 8, 7–20. [Google Scholar] [CrossRef]
- Podlasz, P.; Jakimiuk, A.; Chmielewska-Krzesinska, M.; Kasica, N.; Nowik, N.; Kaleczyc, J. Galanin regulates blood glucose level in the zebrafish: A morphological and functional study. Histochem. Cell Biol. 2016, 145, 105–117. [Google Scholar] [CrossRef]
- Li, L.; Wei, S.; Huang, Q.; Feng, D.; Zhang, S.; Liu, Z. A novel galanin receptor 1a gene in zebrafish: Tissue distribution, developmental expression roles in nutrition regulation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 164, 159–167. [Google Scholar] [CrossRef]
- Hernández, P.P.; Moreno, V.; Olivari, F.A.; Allende, M.L. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio). Brain Res. 2008, 1244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti-Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- White, H.S.; Scholl, E.A.; Klein, B.D.; Flynn, S.P.; Pruess, T.H.; Green, B.R.; Zhang, L.; Bulaj, B. Developing novel antiepileptic drugs: Characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics 2009, 6, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Podlasz, P.; Jakimiuk, A.; Kasica-Jarosz, N.; Czaja, K.; Wasowicz, K. Neuroanatomical Localization of Galanin in Zebrafish Telencephalon and Anticonvulsant Effect of Galanin Overexpression. ACS Chem. Neurosci. 2018, 9, 3049–3059. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- d’Alençon, C.A.; Peña, O.A.; Wittmann, C.; Gallardo, V.E.; Jones, R.A.; Loosli, F.; Liebel, U.; Grabher, C.; Allende, M.L. A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol. 2010, 8, 151. [Google Scholar] [CrossRef]
- Abrams, J.M.; White, K.; Fessler, L.I.; Steller, H. Programmed cell death during Drosophila embryogenesis. Development 1993, 117, 29–43. [Google Scholar]
- Stoop, E.J.; Schipper, T.; Rosendahl Huber, S.K.; Nezhinsky, A.E.; Verbeek, F.J.; Gurcha, S.S.; Besra, G.S.; Vandenbroucke-Grauls, C.M.; Bitter, W.; van der Sar, A.M. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis. Models Mech. 2011, 4, 526–536. [Google Scholar] [CrossRef]
- Isakov, N.; Altman, A. Regulation of immune system cell functions by protein kinase C. Front. Immunol. 2013, 4, 384. [Google Scholar] [CrossRef]
- Wehbi, V.L.; Tasken, K. Molecular Mechanisms for cAMP-Mediated Immunoregulation in T cells-Role of Anchored Protein Kinase A Signaling Units. Front. Immunol. 2016, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Zhang, X.; Zhang, Q.; Dagerlind, A.; Nilsson, S.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Central and peripheral expression of galanin in response to inflammation. Neuroscience 1995, 68, 563–576. [Google Scholar] [CrossRef]
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Locker, F.; Schlager, S.; Kofler, B. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci. Rep. 2019, 9, 7237. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C.; Pope, R.; Butzkueven, H.; Holder, H.; Vanderplank, P.; Lowrey, P.; Day, M.J.; Gundlach, A.L.; Kilpatrick, T.J.; Scolding, N.; et al. A role for galanin in human and experimental inflammatory demyelination. Proc. Natl. Acad. Sci. USA 2009, 106, 15466–15471. [Google Scholar] [CrossRef] [PubMed]
- Botz, B.; Kemény, Á.; Brunner, S.M.; Locker, F.; Csepregi, J.; Mócsai, A.; Pintér, E.; McDougall, J.J.; Kofler, B.; Helyes, Z. Lack of Galanin 3 Receptor Aggravates Murine Autoimmune Arthritis. J. Mol. Neurosci. 2016, 59, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Locker, F.; Vidali, S.; Holub, B.S.; Stockinger, J.; Brunner, S.M.; Ebner, S.; Koller, A.; Trost, A.; Reitsamer, H.A.; Schwarzenbacher, D.; et al. Lack of Galanin Receptor 3 Alleviates Psoriasis by Altering Vascularization, Immune Cell Infiltration, and Cytokine Expression. J. Investig. Dermatol. 2018, 138, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ramspacher, A.; Neudert, M.; Koller, A.; Schlager, S.; Kofler, B.; Brunner, S.M. Influence of the regulatory peptide galanin on cytokine expression in human monocytes. Ann. N. Y. Acad. Sci. 2019, 1455, 185–195. [Google Scholar] [CrossRef]
| Gene | Forward 5′-3′ | Reverse 5′-3′ | Accession |
|---|---|---|---|
| irg1l | GGTTAGAAGCAAGTCCTC | TGTGTTCATCCTCCTCAG | NM_001077607 |
| il1b | GAACAGAATGAAGCACATCAAACC | ACGGCACTGAATCCACCAC | NM_212844 |
| tnfa | AGACCTTAGACTGGAGAGATGAC | CAAAGACACCTGGCTGTAGAC | NM_212829 |
| galn | AAGGATACTCCCAGTGCAAGG | CTTTCCTGCCAGTCCGTGTT | NM_001346239 |
| ppial | ACACTGAAACACGGAGGCAAAG | CATCCACAACCTTCCCGAACAC | AY391451 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowik, N.; Przyborowska, A.; Sienkiewicz, W.; Podlasz, P. Protective Role of Galanin during Chemically Induced Inflammation in Zebrafish Larvae. Biology 2021, 10, 99. https://doi.org/10.3390/biology10020099
Nowik N, Przyborowska A, Sienkiewicz W, Podlasz P. Protective Role of Galanin during Chemically Induced Inflammation in Zebrafish Larvae. Biology. 2021; 10(2):99. https://doi.org/10.3390/biology10020099
Chicago/Turabian StyleNowik, Natalia, Anna Przyborowska, Waldemar Sienkiewicz, and Piotr Podlasz. 2021. "Protective Role of Galanin during Chemically Induced Inflammation in Zebrafish Larvae" Biology 10, no. 2: 99. https://doi.org/10.3390/biology10020099
APA StyleNowik, N., Przyborowska, A., Sienkiewicz, W., & Podlasz, P. (2021). Protective Role of Galanin during Chemically Induced Inflammation in Zebrafish Larvae. Biology, 10(2), 99. https://doi.org/10.3390/biology10020099






