Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva
Abstract
Simple Summary
Abstract
1. Introduction
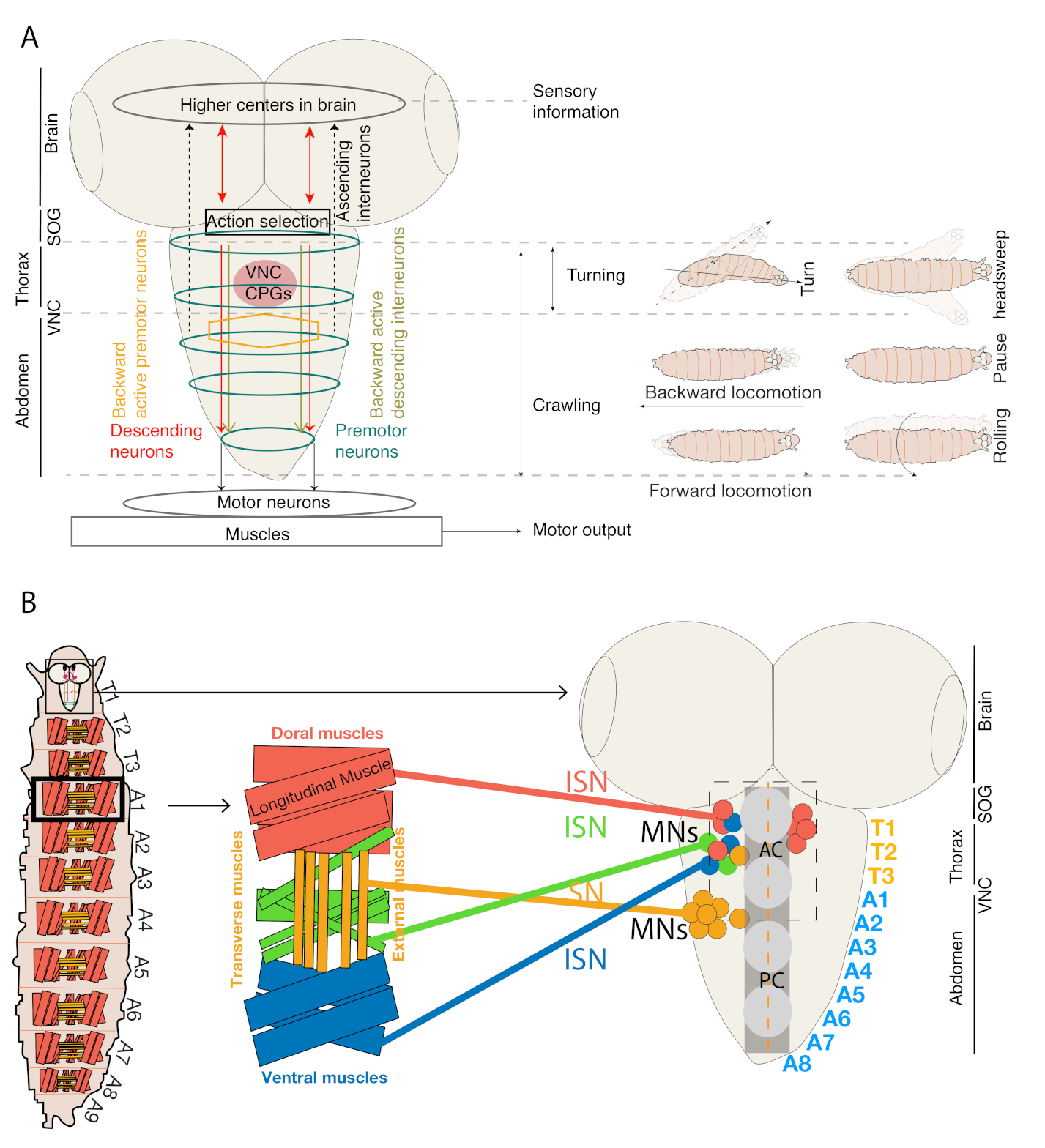
2. The Topological Organization, Myotopic Map, and Neuro-Muscular Architecture of Drosophila Larva
3. Neural Circuitry in Thorax and Abdominal Regions Controls Movements Related to Distinct Motor Patterns during Exploratory Behavior in Drosophila Larva
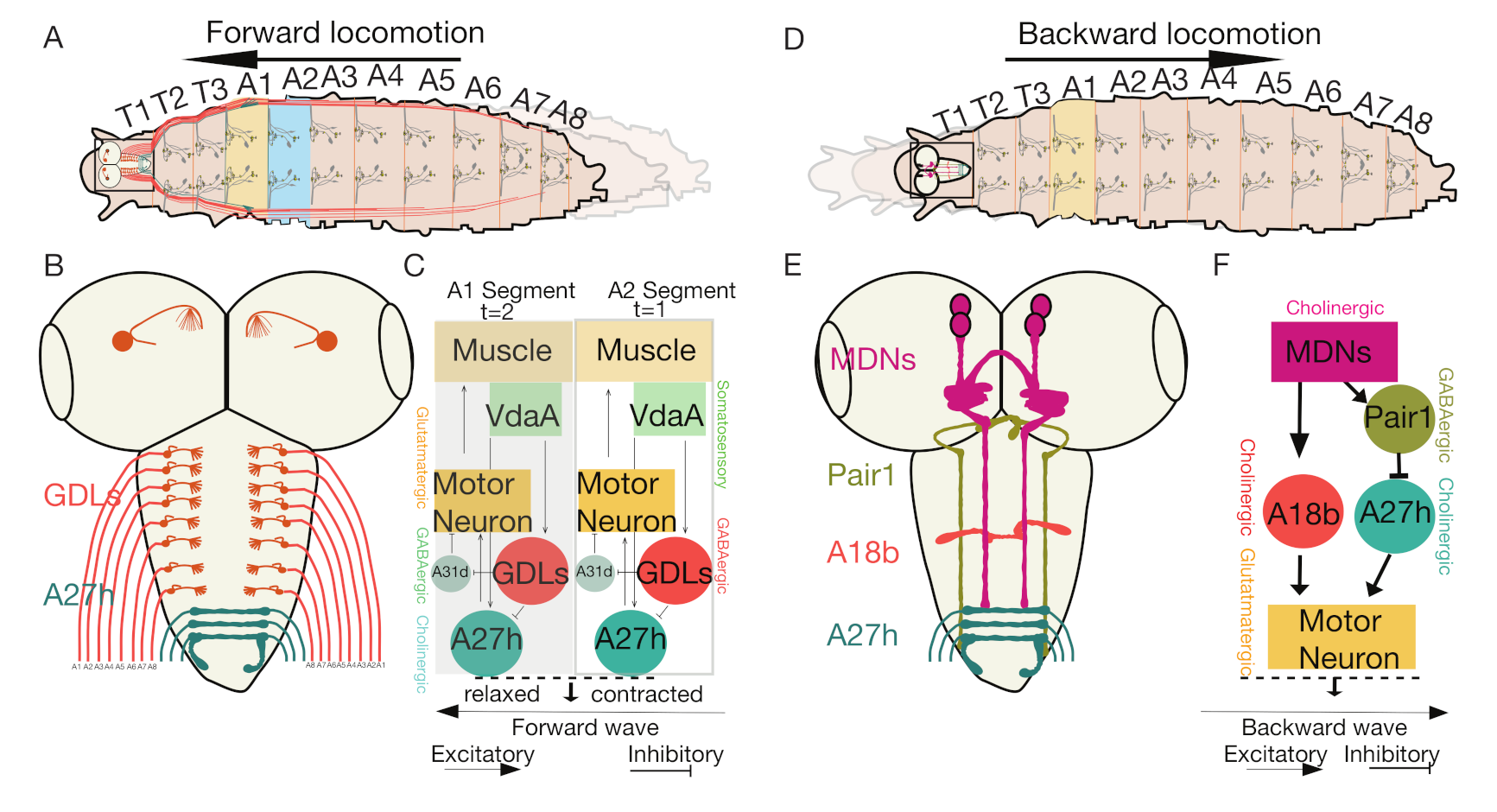
4. The Neuronal Circuits That Control Forward and Backward Locomotion in Drosophila Larvae
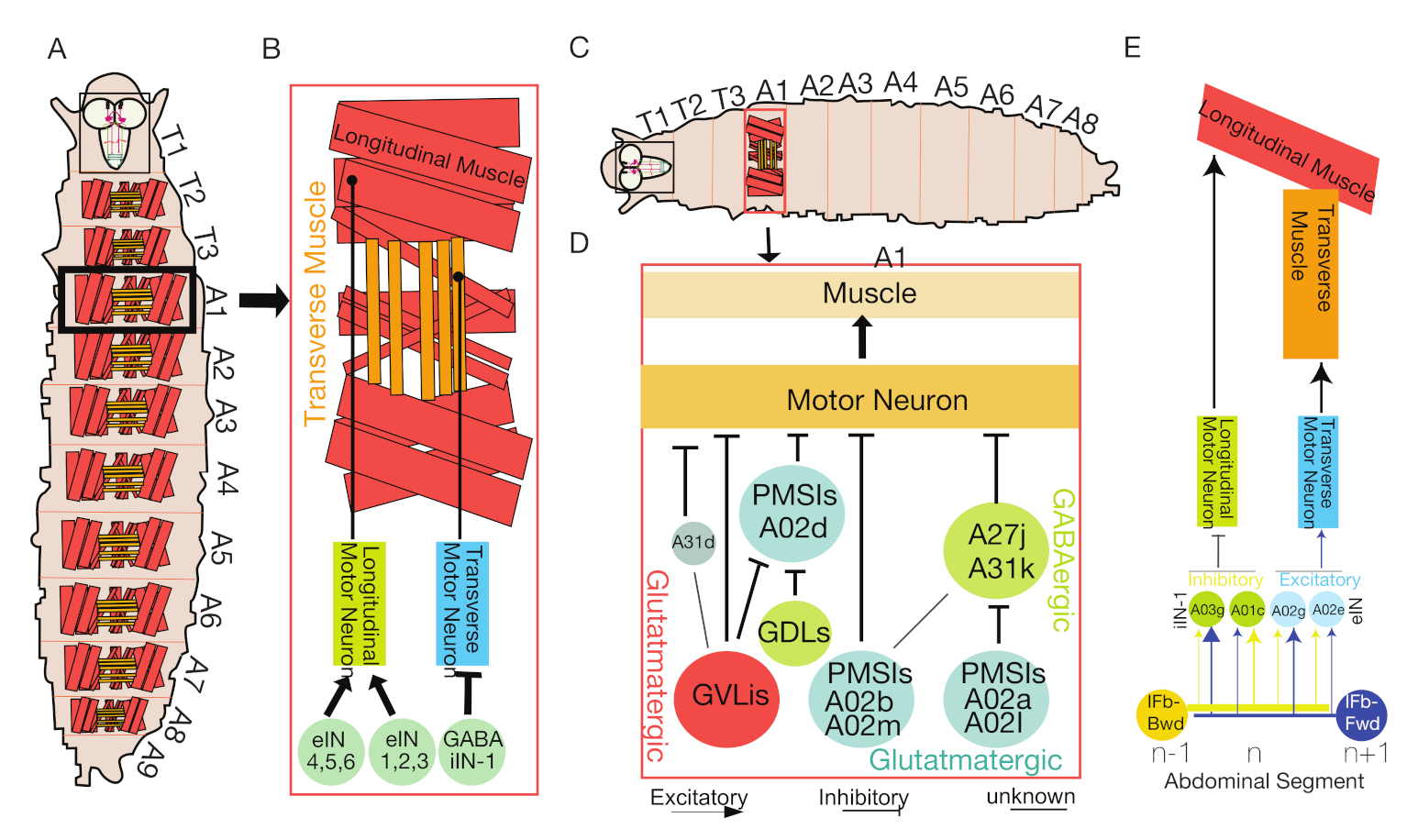
4.1. The Interneurons That Control Intrasegmental Muscle Coordination
4.2. Intersegmental Feedback Neurons Regulate Bidirectional Larval Movements
4.3. The Interneurons That Regulate Feedforward Wave Propagation
4.4. Moon Descending Neurons Activate Backward Locomotion
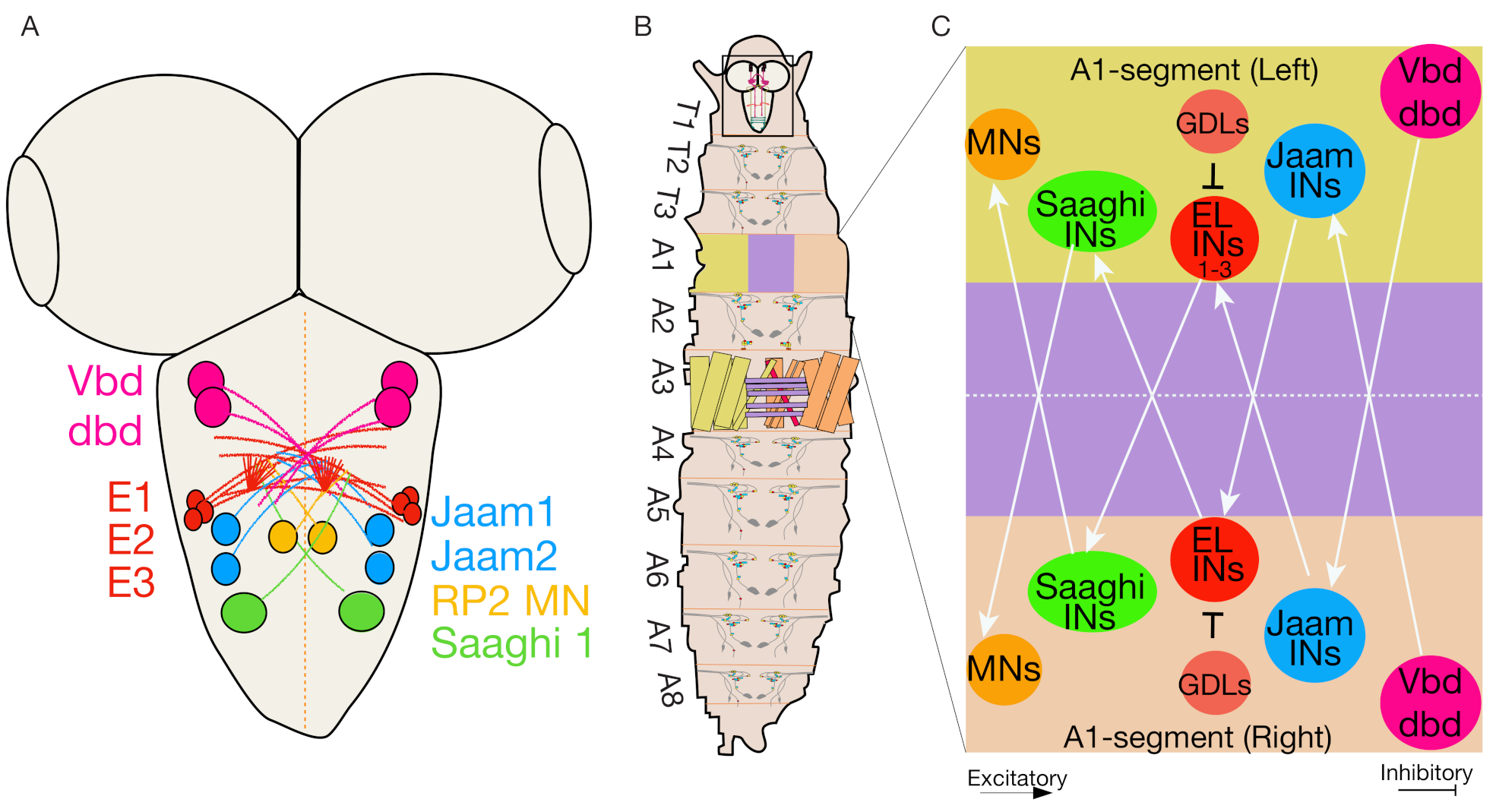
4.5. Even-Skipped Lateral Interneurons Regulate Bilateral Motor Coordination for Symmetric Motor Output
4.6. Thoracic Neuronal Network Regulates Turning and Head-Sweeps
4.7. Distinct PMSIs Regulate Specific Locomotion Types
5. Sensory Neurons Mediate Defensive Behaviors
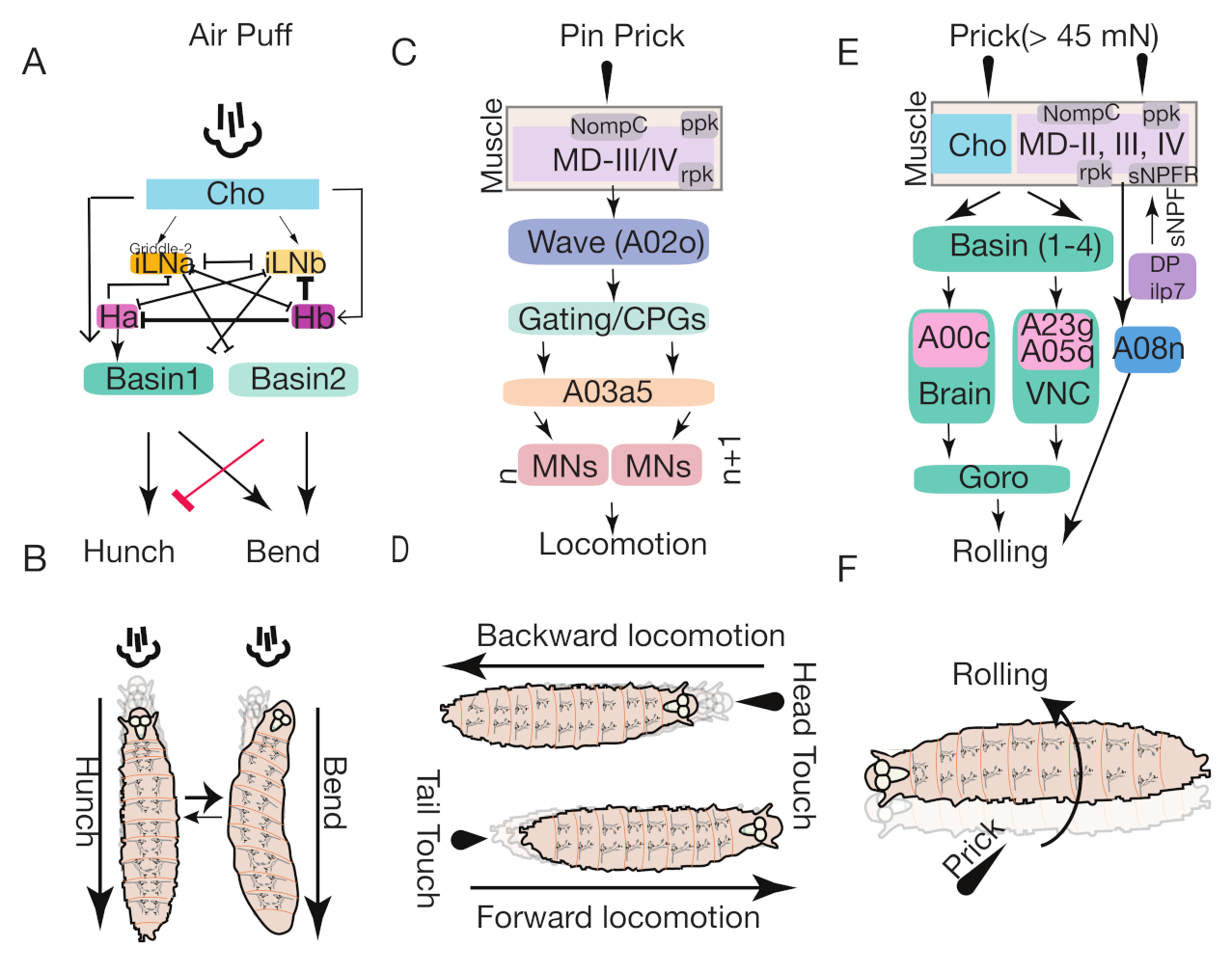
5.1. Mechanical Nociception
5.1.1. Chordotonal Organ Mediated Pathway
5.1.2. Wave Neurons Mediated Pathway
5.1.3. Basins-Goro Neurons Mediated Pathway
5.1.4. DP-ilp-sNPF Receptor Mediated Pathway
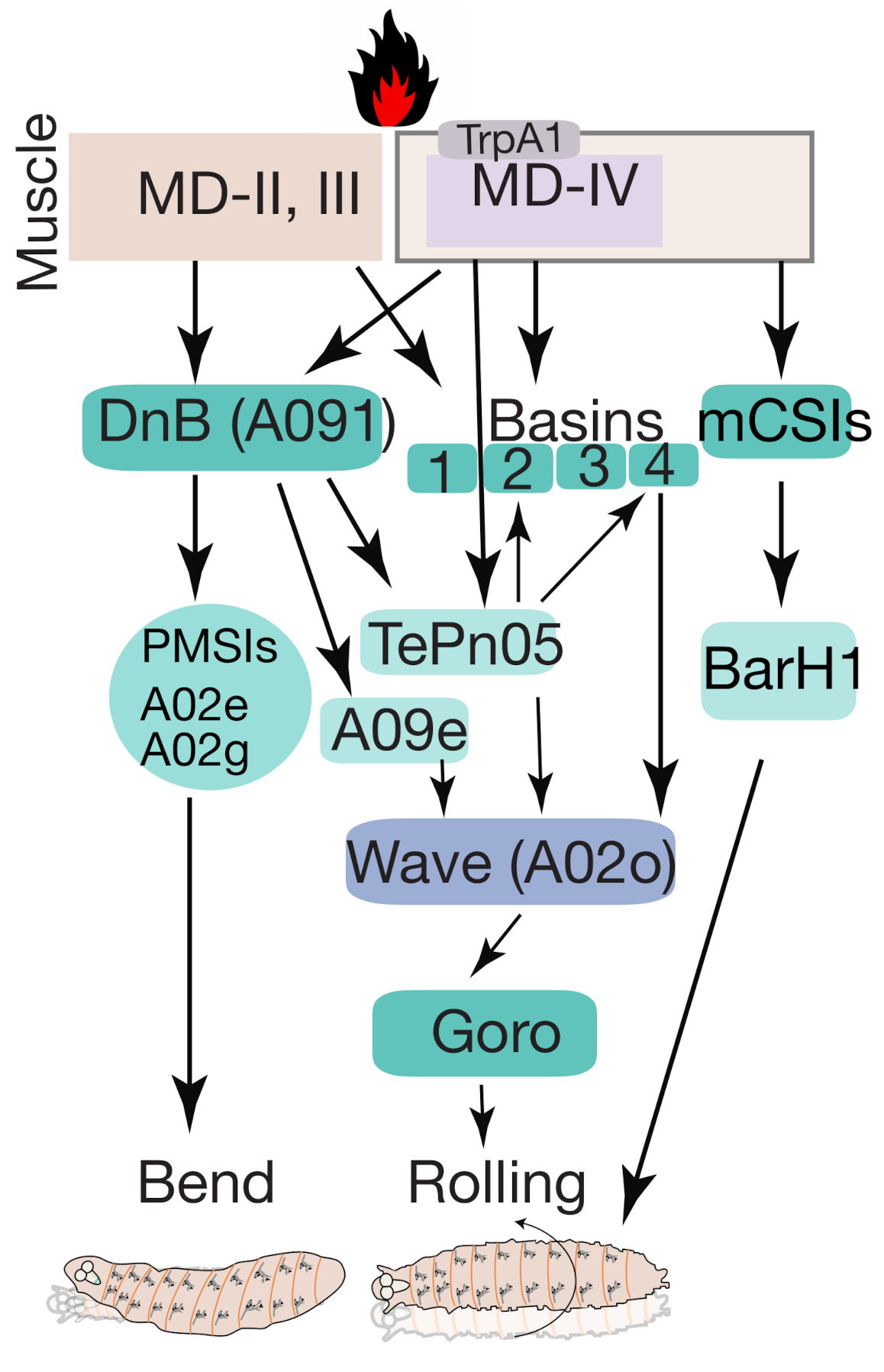
5.2. Thermo-Nociception
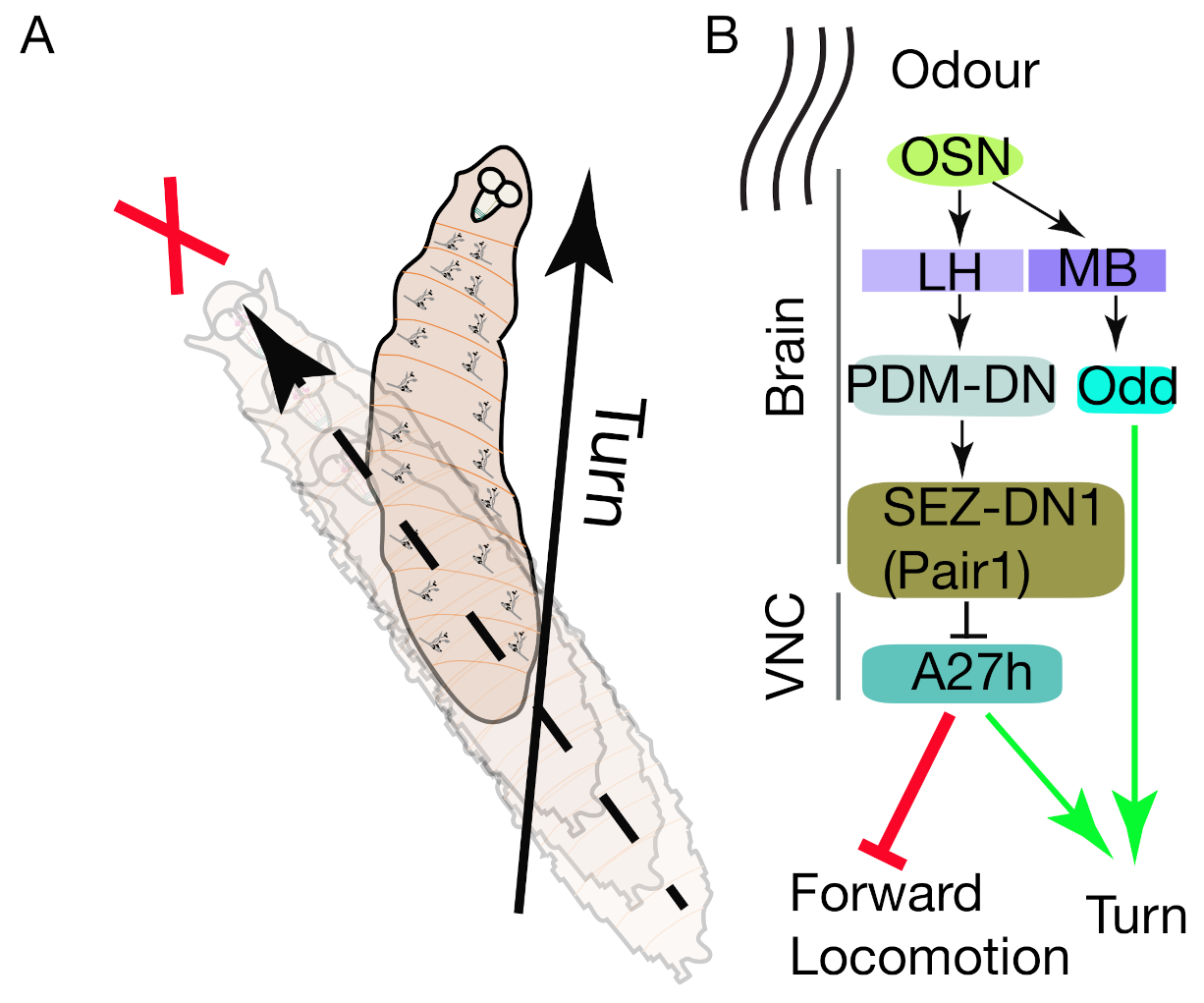
5.3. Chemotaxis
5.4. Chemo-Nociception
5.5. Proprioception or Kinaesthesia
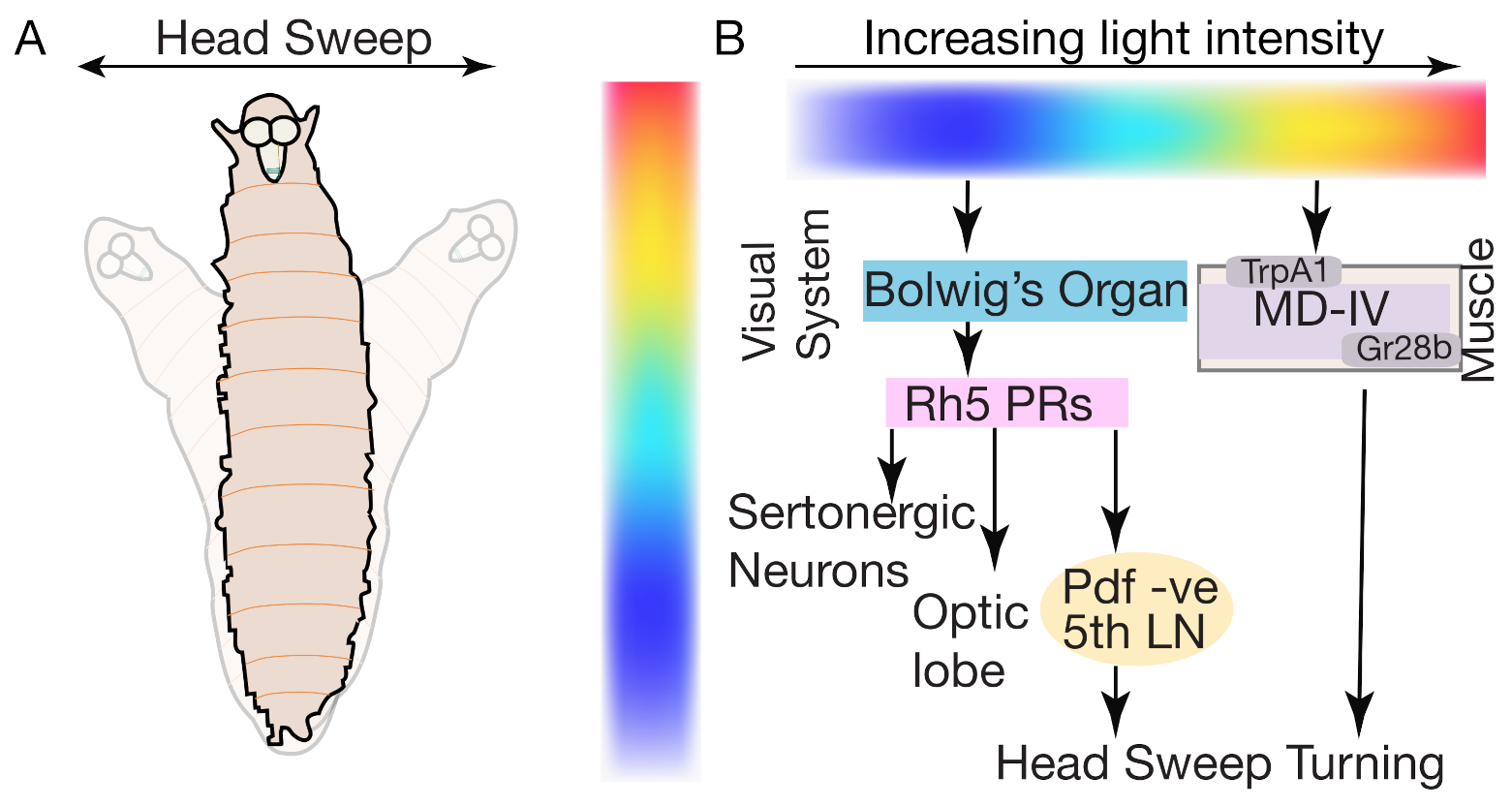
5.6. Phototaxis
6. Sensory Feedback Neurons Are Critical for Motor Circuit Maturation
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selverston, A.I. Neuronal Mechanisms for Rhythmic Motor Pattern Generation in a Simple System. In Neural Control of Locomotion; Advances in Behavioral Biology; Springer: Boston, MA, USA, 1976; pp. 377–399. [Google Scholar]
- Marder, E.; Calabrese, R.L. Principles of rhythmic motor pattern generation. Physiol. Rev. 1996, 76, 687–717. [Google Scholar] [CrossRef]
- Grillner, S. The motor infrastructure: From ion channels to neuronal networks. Nat. Rev. Neurosci. 2003, 4, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Park, J.; Taniguchi, A.; Kohsaka, H.; Nakae, K.; Nonaka, S.; Ishii, S.; Nose, A. System level analysis of motor-related neural activities in larval Drosophila. J. Neurogenet. 2019, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.W.; Carbone, M.A.; Yamamoto, A.; Morgan, T.J.; Mackay, T.F.C. Quantitative genomics of locomotor behavior in Drosophila melanogaster. Genome Biol. 2007, 8, R172. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Lin, C.-Y.; Chiang, A.-S. Internal representations of smell in the Drosophila brain. J. Biomed. Sci. 2007, 14, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The Coding of Temperature in the Drosophila Brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef]
- Frank, D.D.; Jouandet, G.C.; Kearney, P.J.; Macpherson, L.J.; Gallio, M. Temperature representation in the Drosophila brain. Nature 2015, 519, 358–361. [Google Scholar] [CrossRef]
- Enjin, A.; Zaharieva, E.E.; Frank, D.D.; Mansourian, S.; Suh, G.S.B.; Gallio, M.; Stensmyr, M.C. Humidity Sensing in Drosophila. Curr. Biol. 2016, 26, 1352–1358. [Google Scholar] [CrossRef]
- Leslie, B.; Vosshall, M.C. Sensory Systems. Curr. Opin. Neurobiol. 2009, 19, 343. [Google Scholar]
- Martinez, P.; Sprecher, S.G. Of Circuits and Brains: The Origin and Diversification of Neural Architectures. Front. Ecol. Evol. 2020, 8, 82. [Google Scholar] [CrossRef]
- Kohsaka, H.; Okusawa, S.; Itakura, Y.; Fushiki, A.; Nose, A. Development of larval motor circuits in Drosophila. Dev. Growth Differ. 2012, 54, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.Q.; Zarin, A.A.; Carreira-Rosario, A.; Doe, C.Q. Neural circuits driving larval locomotion in Drosophila. Neural Dev. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, R.L.; De Schutter, E. Motor-pattern-generating networks in invertebrates: Modeling our way toward understanding. Trends Neurosci. 1992, 15, 439–445. [Google Scholar] [CrossRef]
- Selverston, A.I. Invertebrate central pattern generator circuits. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2329–2345. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.; Jeffrey, V.; Fujioka, M.; Jaynes, J.B.; Bate, M. Embryonic origins of a motor system: Motor dendrites form a myotopic map in Drosophila. PLoS Biol. 2003, 1, E41. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Stewart, J.C.; Ott, S.; Chlebikova, K.; Chua, J.Y.; Koh, T.-W.; Ho, J.; Claridge-Chang, A. Optogenetic inhibition of behavior with anion channelrhodopsins. Nat. Methods 2017, 14, 271–274. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef]
- Cardona, A.; Saalfeld, S.; Preibisch, S.; Schmid, B. An integrated micro-and macroarchitectural analysis of the Drosophila brain by computer-assisted serial section electron microscopy. PLoS Biol. 2010, 8, e1000502. [Google Scholar] [CrossRef]
- Cardona, A. Towards semi-automatic reconstruction of neural circuits. Neuroinformatics 2013, 11, 31–33. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Sanes, J.R. Ome sweet ome: What can the genome tell us about the connectome? Curr. Opin. Neurobiol. 2008, 18, 346–353. [Google Scholar] [CrossRef]
- Kohsaka, H.; Zwart, M.F.; Fushiki, A.; Fetter, R.D.; Truman, J.W.; Cardona, A.; Nose, A. Regulation of forward and backward locomotion through intersegmental feedback circuits in Drosophila larvae. Nat. Commun. 2019, 10, 2654. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Rosario, A.; Zarin, A.A.; Clark, M.Q.; Manning, L.; Fetter, R.D.; Cardona, A.; Doe, C.Q. MDN brain descending neurons coordinately activate backward and inhibit forward locomotion. Elife 2018, 7, e38554. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Cocanougher, B.T.; Niki, S.; Miyamoto, D.; Kohsaka, H.; Kazama, H.; Fetter, R.D.; Truman, J.W.; Zlatic, M.; Cardona, A.; et al. Divergent Connectivity of Homologous Command-like Neurons Mediates Segment-Specific Touch Responses in Drosophila. Neuron 2017, 96, 1373–1387.e6. [Google Scholar] [CrossRef] [PubMed]
- Fushiki, A.; Zwart, M.F.; Kohsaka, H.; Fetter, R.D.; Cardona, A.; Nose, A. A circuit mechanism for the propagation of waves of muscle contraction in Drosophila. Elife 2016, 5, e13253. [Google Scholar] [CrossRef] [PubMed]
- Zarin, A.A.; Mark, B.; Cardona, A.; Litwin-Kumar, A.; Doe, C.Q. A multilayer circuit architecture for the generation of distinct locomotor behaviors in Drosophila. Elife 2019, 8, e51781. [Google Scholar] [CrossRef] [PubMed]
- Zwart, M.F.; Pulver, S.R.; Truman, J.W.; Fushiki, A.; Fetter, R.D.; Cardona, A.; Landgraf, M. Selective Inhibition Mediates the Sequential Recruitment of Motor Pools. Neuron 2016, 91, 944. [Google Scholar] [CrossRef] [PubMed]
- Jovanic, T.; Winding, M.; Cardona, A.; Truman, J.W.; Gershow, M.; Zlatic, M. Neural Substrates of Drosophila Larval Anemotaxis. Curr. Biol. 2019, 29, 554–566.e4. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Schneider-Mizell, C.M.; Fetter, R.D.; Aleman, J.V.; Franconville, R.; Rivera-Alba, M.; Mensh, B.D.; Branson, K.M.; Simpson, J.H.; Truman, J.W.; et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature 2015, 520, 633–639. [Google Scholar] [CrossRef]
- Jovanic, T.; Schneider-Mizell, C.M.; Shao, M.; Masson, J.-B.; Denisov, G.; Fetter, R.D.; Mensh, B.D.; Truman, J.W.; Cardona, A.; Zlatic, M. Competitive Disinhibition Mediates Behavioral Choice and Sequences in Drosophila. Cell 2016, 167, 858–870.e19. [Google Scholar] [CrossRef]
- Burgos, A.; Honjo, K.; Ohyama, T.; Qian, C.S.; Shin, G.J.-E.; Gohl, D.M.; Silies, M.; Tracey, W.D.; Zlatic, M.; Cardona, A.; et al. Nociceptive interneurons control modular motor pathways to promote escape behavior in Drosophila. Elife 2018, 7, e26016. [Google Scholar] [CrossRef]
- Tastekin, I.; Khandelwal, A.; Tadres, D.; Fessner, N.D.; Truman, J.W.; Zlatic, M.; Cardona, A.; Louis, M. Sensorimotor pathway controlling stopping behavior during chemotaxis in the Drosophila melanogaster larva. Elife 2018, 7, e38740. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.; Kunz, T.; Harris, K.-L.; Whitington, P.M.; Technau, G.M. Morphological characterization of the entire interneuron population reveals principles of neuromere organization in the ventral nerve cord of Drosophila. J. Neurosci. 2011, 31, 15870–15883. [Google Scholar] [CrossRef] [PubMed]
- Heckscher, E.S.; Long, F.; Layden, M.J.; Chuang, C.-H.; Manning, L.; Richart, J.; Pearson, J.C.; Crews, S.T.; Peng, H.; Myers, E.; et al. Atlas-builder software and the eNeuro atlas: Resources for developmental biology and neuroscience. Development 2014, 141, 2524–2532. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.E.; Soll, D.R.; Wu, C.F. Coordination and modulation of locomotion pattern generators in Drosophila larvae: Effects of altered biogenic amine levels by the tyramine β hydroxlyase mutation. J. Neurosci. 2006, 26, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Huser, A.; Rohwedder, A.; Apostolopoulou, A.A.; Widmann, A.; Pfitzenmaier, J.E.; Maiolo, E.M.; Selcho, M.; Pauls, D.; von Essen, A.; Gupta, T.; et al. The serotonergic central nervous system of the Drosophila larva: Anatomy and behavioral function. PLoS ONE 2012, 7, e47518. [Google Scholar] [CrossRef]
- Okusawa, S.; Kohsaka, H.; Nose, A. Serotonin and downstream leucokinin neurons modulate larval turning behavior in Drosophila. J. Neurosci. 2014, 34, 2544–2558. [Google Scholar] [CrossRef]
- Kohsaka, H.; Takasu, E.; Morimoto, T.; Nose, A. A group of segmental premotor interneurons regulates the speed of axial locomotion in Drosophila larvae. Curr. Biol. 2014, 24, 2632–2642. [Google Scholar] [CrossRef]
- Babski, H.; Jovanic, T.; Surel, C.; Yoshikawa, S.; Zwart, M.F.; Valmier, J.; Thomas, J.B.; Enriquez, J.; Carroll, P.; Garcès, A. A GABAergic Maf-expressing interneuron subset regulates the speed of locomotion in Drosophila. Nat. Commun. 2019, 10, 4796. [Google Scholar] [CrossRef]
- Berni, J.; Pulver, S.R.; Griffith, L.C.; Bate, M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr. Biol. 2012, 22, 1861–1870. [Google Scholar] [CrossRef]
- Lahiri, S.; Shen, K.; Klein, M.; Tang, A.; Kane, E.; Gershow, M.; Garrity, P.; Samuel, A.D.T. Two alternating motor programs drive navigation in Drosophila larva. PLoS ONE 2011, 6, e23180. [Google Scholar] [CrossRef]
- Green, C.H.; Burnet, B.; Connolly, K.J. Organization and patterns of inter- and intraspecific variation in the behaviour of Drosophila larvae. Anim. Behav. 1983, 31, 282–291. [Google Scholar] [CrossRef]
- Kernan, M.; Cowan, D.; Zuker, C. Genetic dissection of mechanosensory transduction: Mechanoreception-defective mutations of Drosophila. Neuron 1994, 12, 1195–1206. [Google Scholar] [CrossRef]
- Kim, D.; Alvarez, M.; Lechuga, L.M.; Louis, M. Species-specific modulation of food-search behavior by respiration and chemosensation in Drosophila larvae. Elife 2017, 6, e.27057. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D., Jr.; Wilson, R.I.; Laurent, G.; Benzer, S. painless, a Drosophila gene essential for nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Gomez-Marin, A.; Partoune, N.; Stephens, G.J.; Louis, M. Automated tracking of animal posture and movement during exploration and sensory orientation behaviors. PLoS ONE 2012, 7, e41642. [Google Scholar] [CrossRef]
- Gerber, B.; Stocker, R.F. The Drosophila larva as a model for studying chemosensation and chemosensory learning: A review. Chem. Senses 2007, 32, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.; Thor, S. Development of Drosophila motoneurons: Specification and morphology. Semin. Cell Dev. Biol. 2006, 17, 3–11. [Google Scholar] [CrossRef]
- Lnenicka, G.A.; Atwood, H.L.; Marin, L. Morphological transformation of synaptic terminals of a phasic motoneuron by long-term tonic stimulation. J. Neurosci. 1986, 6, 2252–2258. [Google Scholar] [CrossRef]
- Kurdyak, P.; Atwood, H.L.; Stewart, B.A.; Wu, C.F. Differential physiology and morphology of motor axons to ventral longitudinal muscles in larval Drosophila. J. Comp. Neurol. 1994, 350, 463–472. [Google Scholar] [CrossRef]
- Lnenicka, G.A.; Keshishian, H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J. Neurobiol. 2000, 43, 186–197. [Google Scholar] [CrossRef]
- Newman, Z.L.; Hoagland, A.; Aghi, K.; Worden, K.; Levy, S.L.; Son, J.H.; Lee, L.P.; Isacoff, E.Y. Input-Specific Plasticity and Homeostasis at the Drosophila Larval Neuromuscular Junction. Neuron 2017, 93, 1388–1404.e10. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Chiba, A. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev. Biol. 2001, 229, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.D.; Wen, Y.; Jan, Y.-N. Patterning and organization of motor neuron dendrites in the Drosophila larva. Dev. Biol. 2009, 336, 213–221. [Google Scholar] [CrossRef]
- Schaefer, J.E.; Worrell, J.W.; Levine, R.B. Role of intrinsic properties in Drosophila motoneuron recruitment during fictive crawling. J. Neurophysiol. 2010, 104, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.; Bossing, T.; Technau, G.M.; Bate, M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J. Neurosci. 1997, 17, 9642–9655. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, M.; Augart, C.; Budnik, V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J. Neurosci. 1993, 13, 3692–3704. [Google Scholar] [CrossRef] [PubMed]
- Mauss, A.; Tripodi, M.; Evers, J.F.; Landgraf, M. Midline signalling systems direct the formation of a neural map by dendritic targeting in the Drosophila motor system. PLoS Biol. 2009, 7, e1000200. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kohsaka, H.; Nose, A. Gap junction–mediated signaling from motor neurons regulates motor generation in the central circuits of larval drosophila. J. Neurosci. 2017, 37, 2045–2060. [Google Scholar] [CrossRef]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef]
- Seelig, J.D.; Jayaraman, V. Neural dynamics for landmark orientation and angular path integration. Nature 2015, 521, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, V.; Laurent, G. Evaluating a genetically encoded optical sensor of neural activity using electrophysiology in intact adult fruit flies. Front. Neural Circuits 2007, 1, 3. [Google Scholar] [CrossRef]
- Han, X.; Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2007, 2, e299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, W.; Wang, Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur. J. Neurosci. 2007, 26, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Pulver, S.R.; Pashkovski, S.L.; Hornstein, N.J.; Garrity, P.A.; Griffith, L.C. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 2009, 101, 3075–3088. [Google Scholar] [CrossRef]
- Inagaki, H.K.; Jung, Y.; Hoopfer, E.D.; Wong, A.M.; Mishra, N.; Lin, J.Y.; Tsien, R.Y.; Anderson, D.J. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 2014, 11, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Heckscher, E.S.; Lockery, S.R.; Doe, C.Q. Characterization of Drosophila larval crawling at the level of organism, segment, and somatic body wall musculature. J. Neurosci. 2012, 32, 12460–12471. [Google Scholar] [CrossRef]
- Hughes, C.L.; Thomas, J.B. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 2007, 35, 383–396. [Google Scholar] [CrossRef]
- Crisp, S.; Evers, J.F.; Fiala, A.; Bate, M. The development of motor coordination in Drosophila embryos. Development 2008, 135, 3707–3717. [Google Scholar] [CrossRef]
- Dixit, R.; Vijayraghavan, K.; Bate, M. Hox genes and the regulation of movement in Drosophila. Dev. Neurobiol. 2008, 68, 309–316. [Google Scholar] [CrossRef]
- Pulver, S.R.; Griffith, L.C. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat. Neurosci. 2010, 13, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Crisp, S.J.; Evers, J.F.; Bate, M. Endogenous patterns of activity are required for the maturation of a motor network. J. Neurosci. 2011, 31, 10445–10450. [Google Scholar] [CrossRef] [PubMed]
- Marder, E.; Bucher, D. Central pattern generators and the control of rhythmic movements. Curr. Biol. 2001, 11, R986–R996. [Google Scholar] [CrossRef]
- MacKay-Lyons, M. Central pattern generation of locomotion: A review of the evidence. Phys. Ther. 2002, 82, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Pulver, S.R.; Bayley, T.G.; Taylor, A.L.; Berni, J.; Bate, M.; Hedwig, B. Imaging fictive locomotor patterns in larval Drosophila. J. Neurophysiol. 2015, 114, 2564–2577. [Google Scholar] [CrossRef] [PubMed]
- Berni, J. Genetic dissection of a regionally differentiated network for exploratory behavior in Drosophila larvae. Curr. Biol. 2015, 25, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Gjorgjieva, J.; Berni, J.; Evers, J.F.; Eglen, S.J. Neural circuits for peristaltic wave propagation in crawling Drosophila larvae: Analysis and modeling. Front. Comput. Neurosci. 2013, 7, 24. [Google Scholar] [CrossRef]
- Dougherty, K.J.; Zagoraiou, L.; Satoh, D.; Rozani, I.; Doobar, S.; Arber, S.; Jessell, T.M.; Kiehn, O. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 2013, 80, 920–933. [Google Scholar] [CrossRef]
- Slater, G.; Levy, P.; Chan, K.L.A.; Larsen, C. A central neural pathway controlling odor tracking in Drosophila. J. Neurosci. 2015, 35, 1831–1848. [Google Scholar] [CrossRef]
- Tastekin, I.; Riedl, J.; Schilling-Kurz, V.; Gomez-Marin, A.; Truman, J.W.; Louis, M. Role of the subesophageal zone in sensorimotor control of orientation in Drosophila larva. Curr. Biol. 2015, 25, 1448–1460. [Google Scholar] [CrossRef]
- Heckscher, E.S.; Zarin, A.A.; Faumont, S.; Clark, M.Q.; Manning, L.; Fushiki, A.; Schneider-Mizell, C.M.; Fetter, R.D.; Truman, J.W.; Zwart, M.F.; et al. Even-Skipped(+) Interneurons Are Core Components of a Sensorimotor Circuit that Maintains Left-Right Symmetric Muscle Contraction Amplitude. Neuron 2015, 88, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, E.H.; Vanhoven, M.K.; Bendesky, A.; Wang, G.; Fetter, R.D.; Shen, K.; Bargmann, C.I. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 2008, 57, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Itakura, Y.; Kohsaka, H.; Ohyama, T.; Zlatic, M.; Pulver, S.R.; Nose, A. Identification of Inhibitory Premotor Interneurons Activated at a Late Phase in a Motor Cycle during Drosophila Larval Locomotion. PLoS ONE 2015, 10, e0136660. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.E.; Song, W.; Looger, L.L.; Jan, L.Y.; Jan, Y.N. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 2010, 67, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, D.A.; Levine, R.B. Synaptic interactions between a muscle-associated proprioceptor and body wall muscle motor neurons in larval and Adult manduca sexta. J. Neurophysiol. 1996, 76, 1597–1610. [Google Scholar] [CrossRef]
- Bidaye, S.S.; Machacek, C.; Wu, Y.; Dickson, B.J. Neuronal control of Drosophila walking direction. Science 2014, 344, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Suster, M.L.; Kania, A.; Liao, M.; Asakawa, K.; Charron, F.; Kawakami, K.; Drapeau, P. A novel conserved evx1 enhancer links spinal interneuron morphology and cis-regulation from fish to mammals. Dev. Biol. 2009, 325, 422–433. [Google Scholar] [CrossRef][Green Version]
- Moran-Rivard, L.; Kagawa, T.; Saueressig, H.; Gross, M.K.; Burrill, J.; Goulding, M. Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron 2001, 29, 385–399. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Long, H.; Thomas, J.B. A subset of interneurons required for Drosophila larval locomotion. Mol. Cell. Neurosci. 2016, 70, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wreden, C.C.; Meng, J.L.; Feng, W.; Chi, W.; Marshall, Z.D.; Heckscher, E.S. Temporal Cohorts of Lineage-Related Neurons Perform Analogous Functions in Distinct Sensorimotor Circuits. Curr. Biol. 2017, 27, 1521–1528.e4. [Google Scholar] [CrossRef]
- Rodriguez Moncalvo, V.G.; Campos, A.R. Role of serotonergic neurons in the Drosophila larval response to light. BMC Neurosci. 2009, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Moore, A.W. Convergent local identity and topographic projection of sensory neurons. J. Neurosci. 2011, 31, 17017–17027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, R.Y.; Zhong, L.; Xu, Y.; Johnson, T.; Zhang, F.; Deisseroth, K.; Tracey, W.D. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Carvalho, M.J.; Berh, D.; Braun, A.; Chen, Y.-C.; Eichler, K.; Eschbach, C.; Fritsch, P.M.J.; Gerber, B.; Hoyer, N.; Jiang, X.; et al. The Ol1mpiad: Concordance of behavioural faculties of stage 1 and stage 3 Drosophila larvae. J. Exp. Biol. 2017, 220, 2452–2475. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bellido, R.; Himmel, N.J.; Gutstein, H.B.; Cox, D.N.; Galko, M.J. An assay for chemical nociception in Drosophila larvae. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190282. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Galko, M.J. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev. Dyn. 2012, 241, 16–26. [Google Scholar] [CrossRef]
- Song, W.; Onishi, M.; Jan, L.Y.; Jan, Y.N. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2007, 104, 5199–5204. [Google Scholar] [CrossRef]
- Zhong, L.; Hwang, R.Y.; Tracey, W.D. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 2010, 20, 429–434. [Google Scholar] [CrossRef]
- Kim, S.E.; Coste, B.; Chadha, A.; Cook, B.; Patapoutian, A. The role of Drosophila Piezo in mechanical nociception. Nature 2012, 483, 209–212. [Google Scholar] [CrossRef]
- Terada, S.-I.; Matsubara, D.; Onodera, K.; Matsuzaki, M.; Uemura, T.; Usui, T. Neuronal processing of noxious thermal stimuli mediated by dendritic Ca(2+) influx in Drosophila somatosensory neurons. Elife 2016, 5, e12959. [Google Scholar] [CrossRef]
- Robertson, J.L.; Tsubouchi, A.; Tracey, W.D. Larval defense against attack from parasitoid wasps requires nociceptive neurons. PLoS ONE 2013, 8, e78704. [Google Scholar] [CrossRef] [PubMed]
- Ainsley, J.A.; Pettus, J.M.; Bosenko, D.; Gerstein, C.E.; Zinkevich, N.; Anderson, M.G.; Adams, C.M.; Welsh, M.J.; Johnson, W.A. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr. Biol. 2003, 13, 1557–1563. [Google Scholar] [CrossRef]
- Hoyer, N.; Petersen, M.; Tenedini, F.; Soba, P. Assaying Mechanonociceptive Behavior in Drosophila Larvae. Bio-Protocol 2018, 8, 82736. [Google Scholar] [CrossRef]
- Yoshino, J.; Morikawa, R.K.; Hasegawa, E.; Emoto, K. Neural Circuitry that Evokes Escape Behavior upon Activation of Nociceptive Sensory Neurons in Drosophila Larvae. Curr. Biol. 2017, 27, 2499–2504.e3. [Google Scholar] [CrossRef] [PubMed]
- Dason, J.S.; Cheung, A.; Anreiter, I.; Montemurri, V.A.; Allen, A.M.; Sokolowski, M.B. Drosophila melanogaster foraging regulates a nociceptive-like escape behavior through a developmentally plastic sensory circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 23286–23291. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Petersen, M.; Hoyer, N.; Spitzweck, B.; Tenedini, F.; Wang, D.; Gruschka, A.; Burchardt, L.S.; Szpotowicz, E.; Schweizer, M.; et al. Sensory integration and neuromodulatory feedback facilitate Drosophila mechanonociceptive behavior. Nat. Neurosci. 2017, 20, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Titlow, J.S.; Rice, J.; Majeed, Z.R.; Holsopple, E.; Biecker, S.; Cooper, R.L. Anatomical and genotype-specific mechanosensory responses in Drosophila melanogaster larvae. Neurosci. Res. 2014, 83, 54–63. [Google Scholar] [CrossRef]
- Tsubouchi, A.; Caldwell, J.C.; Tracey, W.D. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr. Biol. 2012, 22, 2124–2134. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Wang, Q.; Wang, Z. The role of PPK26 in Drosophila larval mechanical nociception. Cell Rep. 2014, 9, 1183–1190. [Google Scholar] [CrossRef]
- Gorczyca, D.A.; Younger, S.; Meltzer, S.; Kim, S.E.; Cheng, L.; Song, W.; Lee, H.Y.; Jan, L.Y.; Jan, Y.N. Identification of Ppk26, a DEG/ENaC Channel Functioning with Ppk1 in a Mutually Dependent Manner to Guide Locomotion Behavior in Drosophila. Cell Rep. 2014, 9, 1446–1458. [Google Scholar] [CrossRef]
- Mauthner, S.E.; Hwang, R.Y.; Lewis, A.H.; Xiao, Q.; Tsubouchi, A.; Wang, Y.; Honjo, K.; Skene, J.H.P.; Grandl, J.; Tracey, W.D., Jr. Balboa binds to pickpocket in vivo and is required for mechanical nociception in Drosophila larvae. Curr. Biol. 2014, 24, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.A.M.; Dweck, H.K.M.; Stökl, J.; Hofferberth, J.E.; Trona, F.; Weniger, K.; Rybak, J.; Seki, Y.; Stensmyr, M.C.; Sachse, S.; et al. Drosophila Avoids Parasitoids by Sensing Their Semiochemicals via a Dedicated Olfactory Circuit. PLoS Biol. 2015, 13, e1002318. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M. What and how do maggots smell? Biol. Rev. Camb. Philos. Soc. 1999, 74, 425–459. [Google Scholar] [CrossRef]
- Gershow, M.; Berck, M.; Mathew, D.; Luo, L.; Kane, E.A. Controlling airborne cues to study small animal navigation. Nature 2012, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Fosque, B.F.; Sun, Y.; Dana, H.; Yang, C.-T.; Ohyama, T.; Tadross, M.R.; Patel, R.; Zlatic, M.; Kim, D.S.; Ahrens, M.B.; et al. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science 2015, 347, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. Coordinated Movement: Watching Proprioception Unfold. Curr. Biol. 2019, 29, R202–R205. [Google Scholar] [CrossRef]
- Caldwell, J.C.; Miller, M.M.; Wing, S.; Soll, D.R.; Eberl, D.F. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc. Natl. Acad. Sci. USA 2003, 100, 16053–16058. [Google Scholar] [CrossRef]
- He, L.; Gulyanon, S.; Skanata, M.M.; Karagyozov, D.; Heckscher, E.S.; Krieg, M.; Tsechpenakis, G.; Gershow, M.; Tracey, W.D., Jr. Direction Selectivity in Drosophila Proprioceptors Requires the Mechanosensory Channel Tmc. Curr. Biol. 2019, 29, 945–956.e3. [Google Scholar] [CrossRef]
- Vaadia, R.D.; Li, W.; Voleti, V.; Singhania, A.; Hillman, E.M.C.; Grueber, W.B. Characterization of Proprioceptive System Dynamics in Behaving Drosophila Larvae Using High-Speed Volumetric Microscopy. Curr. Biol. 2019, 29, 935–944.e4. [Google Scholar] [CrossRef]
- Grueber, W.B.; Jan, L.Y.; Jan, Y.N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 2002, 129, 2867–2878. [Google Scholar]
- Guo, Y.; Wang, Y.; Zhang, W.; Meltzer, S.; Zanini, D.; Yu, Y.; Li, J.; Cheng, T.; Guo, Z.; Wang, Q.; et al. Transmembrane channel-like (tmc) gene regulates Drosophila larval locomotion. Proc. Natl. Acad. Sci. USA 2016, 113, 7243–7248. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Liu, T.; Zhang, W. Piezo-like Gene Regulates Locomotion in Drosophila Larvae. Cell Rep. 2019, 26, 1369–1377.e4. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.P.; Harris, L.R.; Campos, A.R.; Sokolowski, M.B. Sensorimotor transformation from light reception to phototactic behavior inDrosophila larvae (Diptera: Drosophilidae). J. Insect Behav. 1994, 7, 553. [Google Scholar] [CrossRef]
- Kane, E.A.; Gershow, M.; Afonso, B.; Larderet, I.; Klein, M.; Carter, A.R.; de Bivort, B.L.; Sprecher, S.G.; Samuel, A.D.T. Sensorimotor structure of Drosophila larva phototaxis. Proc. Natl. Acad. Sci. USA 2013, 110, E3868–E3877. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yuan, Q.; Vogt, N.; Looger, L.L.; Jan, L.Y.; Jan, Y.N. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 2010, 468, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Keene, A.C.; Mazzoni, E.O.; Zhen, J.; Younger, M.A.; Yamaguchi, S.; Blau, J.; Desplan, C.; Sprecher, S.G. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J. Neurosci. 2011, 31, 6527–6534. [Google Scholar] [CrossRef]
- Hassan, J.; Iyengar, B.; Scantlebury, N.; Rodriguez Moncalvo, V.; Campos, A.R. Photic input pathways that mediate the Drosophila larval response to light and circadian rhythmicity are developmentally related but functionally distinct. J. Comp. Neurol. 2005, 481, 266–275. [Google Scholar] [CrossRef]
- Humberg, T.-H.; Bruegger, P.; Afonso, B.; Zlatic, M.; Truman, J.W.; Gershow, M.; Samuel, A.; Sprecher, S.G. Dedicated photoreceptor pathways in Drosophila larvae mediate navigation by processing either spatial or temporal cues. Nat. Commun. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Humberg, T.-H.; Sprecher, S.G. Age- and Wavelength-Dependency of Drosophila Larval Phototaxis and Behavioral Responses to Natural Lighting Conditions. Front. Behav. Neurosci. 2017, 11, 66. [Google Scholar] [CrossRef]
- Fushiki, A.; Kohsaka, H.; Nose, A. Role of sensory experience in functional development of Drosophila motor circuits. PLoS ONE 2013, 8, e62199. [Google Scholar] [CrossRef]
- Harris-Warrick, R.M.; Marder, E. Modulation of neural networks for behavior. Annu. Rev. Neurosci. 1991, 14, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I. Beyond the connectome: How neuromodulators shape neural circuits. Bioessays 2012, 34, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zanini, D.; Giraldo, D.; Warren, B.; Katana, R.; Andrés, M.; Reddy, S.; Pauls, S.; Schwedhelm-Domeyer, N.; Geurten, B.R.H.; Göpfert, M.C. Proprioceptive Opsin Functions in Drosophila Larval Locomotion. Neuron 2018, 98, 67–74.e4. [Google Scholar] [CrossRef]
- Raad, H.; Ferveur, J.-F.; Ledger, N.; Capovilla, M.; Robichon, A. Functional Gustatory Role of Chemoreceptors in Drosophila Wings. Cell Rep. 2016, 15, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Peabody, N.C.; Vinson, C.R.; White, B.H. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 2006, 52, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.V.; Mohammad, F.; Chua, J.Y.; Lam, J.S.W.; Loberas, M.; Sahani, S.; Barros, C.S.; Claridge-Chang, A. A zinc-finger fusion protein refines Gal4-defined neural circuits. Mol. Brain 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Zaharieva, E.E.; Kearney, P.J.; Alpert, M.H.; Lin, T.-Y.; Turan, Z.; Lee, C.-H.; Gallio, M. Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 2015, 6, 10024. [Google Scholar] [CrossRef] [PubMed]
- Talay, M.; Richman, E.B.; Snell, N.J.; Hartmann, G.G.; Fisher, J.D.; Sorkaç, A.; Santoyo, J.F.; Chou-Freed, C.; Nair, N.; Johnson, M.; et al. Transsynaptic Mapping of Second-Order Taste Neurons in Flies by trans-Tango. Neuron 2017, 96, 783–795.e4. [Google Scholar] [CrossRef]
- Simpson, J.H.; Looger, L.L. Functional Imaging and Optogenetics in Drosophila. Genetics 2018, 208, 1291–1309. [Google Scholar] [CrossRef]
- Tao, X.; Lin, H.-H.; Lam, T.; Rodriguez, R.; Wang, J.W.; Kubby, J. Transcutical imaging with cellular and subcellular resolution. Biomed. Opt. Express 2017, 8, 1277–1289. [Google Scholar] [CrossRef]
- Brunet Avalos, C.; Maier, G.L.; Bruggmann, R.; Sprecher, S.G. Single cell transcriptome atlas of the Drosophila larval brain. Elife 2019, 8, e50354. [Google Scholar] [CrossRef] [PubMed]
- Cocanougher, B.T.; Wittenbach, J.D.; Long, X.; Kohn, A.B. Comparative single-cell transcriptomics of complete insect nervous systems. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kasai, Y.; Cagan, R. Drosophila as a tool for personalized medicine: A primer. Pers. Med. 2010, 7, 621–632. [Google Scholar] [CrossRef] [PubMed]
| Neurons | Neuron Identity | Drivers (#BDSC Stock) | Segmental Coordination | Function | References |
|---|---|---|---|---|---|
| GDL (A27j2) | GABAergic interneurons | GDL-Gal4 (9-20-Gal4, iav-GAL80), | Inter-segmental coordination | Forward and Backward locomotion | [25] |
| PMN (A27h) | Cholinergic premotor interneurons, excitatory | R36G02-Gal4 (#49939) | Inter-segmental coordination | Forward locomotion | [25] |
| PMN (A18b) | Cholinergic premotor interneurons, excitatory | R94E10-Gal4 (#40689) R94E10-LexA | Inter-segmental coordination | Backward locomotion, Escape behavior | [23] |
| Ifb-Fwd (A01d3) | Cholinergic feedback neurons | SS02065-Gal4 (R32C05-p65AD, R38E10- GDBD) | Inter-segmental coordination | Forward locomotion | [22] |
| Ifb-Bwd (A27K) | Cholinergic feedback neurons | SS026694-Gal4 (VT020818-AD R91E03-GDBD) | Inter-segmental coordination | Backward locomotion | [22] |
| Wave neurons (A02o) | Cholinergic and glutamatergic | VT25803-Gal4 (#v202269) | Inter-segmental coordination | Backward Locomotion, Escape behaviour | [24] |
| MDNS | Cholinergic descending neurons | MDNS- Gal4 (R49F02-AD; R53F07-DBD) | Inter-segmental coordination | Backward locomotion | [23] |
| iIN1 | GABAergic inhibitory interneurons | R83H09-Gal4 (#41311) SS01411-Gal4 | Intrasegmental phase difference | Forward locomotion | [27] |
| PMSIs (A02b and A02m) | Glutamatergic inhibitory interneurons | Per-Gal4 | Intrasegmental coordination | Speed of locomotion | [38] |
| PDM-DN | Cholinergic | PDM-DN-Gal4 | N/A | Chemotaxis | [32] |
| Odd neurons | Cholinergic | odd-Gal4 | N/A | Chemotaxis | [80] |
| SEZ neurons | NP4820-Gal4 | N/A | Chemotaxis | [81] | |
| SEZ-Subset neurons | R23F01-Gal4 | N/A | Chemotaxis | [81] | |
| Pair1/SEZ- DN1 | GABAergic | R75C02-lexA lexAop-myr:GFP (#54365) | Intrasegmental coordination | Backward locomotion/chemotaxis | [23] |
| ELs | EL-Gal4 | Bilateral motor coordination | C-bends and Wavy body postures | [82] | |
| Griddle-2 neurons | GABAergic | JRC-SS0918- split-Gal4 | N/A | Hunch behaviour | [30] |
| fbLN-Hb | GABAergic | JRC-SS0888- split-Gal4 | N/A | Bend behaviour | [30] |
| Vda/Vdc | Somatosensory neurons | tutl-GAL4 | N/A | [25] | |
| Late-born ELs | R11F02-Gal4 (#49828) | Bilateral motor coordination | C-bends and Wavy body postures | [82] | |
| Brain and SOG region | BL-GAL4 | Exploratory Behaviour | [40,41,55,68,69,70,71,72,73] |
| Neurons/Genes | Drivers (#BDSC Stock) | Class of Sensory Neurons Expressed | Thermo-Nociception | Chemo-Nociception | Mechano-Nociception | References |
|---|---|---|---|---|---|---|
| Md-1V sensory neurons | PPK-Gal4,R38A10-LexA (#54106) MD-IV Gal4 | Md-1V-multidendritic neurons | + | + | + | [105] |
| Chordotonal neurons | Ch-Gal4 | Mechanosensory | + | + | [24,29] | |
| mSCIS | R94B10- Gal4 (#41325) | Md-1V-multidendritic neurons | + | [105] | ||
| Bar-H1 (SNa) motor neuron | BarH1- Gal4 | Md-1V-multidendritic neurons | + | [105] | ||
| Basin Neurons | R72F11-Gal4 (#39786) | Md-1V-multidendritic neurons | + | + | [29] | |
| Goro Neurons | R69F06-Gal4 and LexA | Md-1V-multidendritic neurons and class-II and Class-III sensory neurons | + | [29] | ||
| Down and back Neurons (DNB) | 412 -Gal4 | Md-1V-multidendritic neurons and class-II and Class-III sensory neurons | + | [31] | ||
| Foraging (for) gene | pr1-Gal4 | Tracheal multidendritic neurons on larval body wall | + | [106] | ||
| A08n | R82E12 -Gal4 (#40183) | Md-1V-multidendritic neurons | + | [107] | ||
| Dp-ilp7 | ilp7- Gal4 | Md-1V-multidendritic neurons and class-II and Class-III sensory neurons | + | [107] | ||
| Basin-1 | R20B01-Gal4 (#48877) | Md-1V-multidendritic neurons | + | [24,29] | ||
| Chordotonal neurons | iav-Gal4 (#52273) | Mechano-sensory | + | + | [24,29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowda, S.B.M.; Salim, S.; Mohammad, F. Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva. Biology 2021, 10, 90. https://doi.org/10.3390/biology10020090
Gowda SBM, Salim S, Mohammad F. Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva. Biology. 2021; 10(2):90. https://doi.org/10.3390/biology10020090
Chicago/Turabian StyleGowda, Swetha B. M., Safa Salim, and Farhan Mohammad. 2021. "Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva" Biology 10, no. 2: 90. https://doi.org/10.3390/biology10020090
APA StyleGowda, S. B. M., Salim, S., & Mohammad, F. (2021). Anatomy and Neural Pathways Modulating Distinct Locomotor Behaviors in Drosophila Larva. Biology, 10(2), 90. https://doi.org/10.3390/biology10020090







