The Overall Efficacy and Outcomes of Metronomic Tegafur-Uracil Chemotherapy on Locally Advanced Head and Neck Squamous Cell Carcinoma: A Real-World Cohort Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Treatment
2.3. Treatment Response and Safety Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
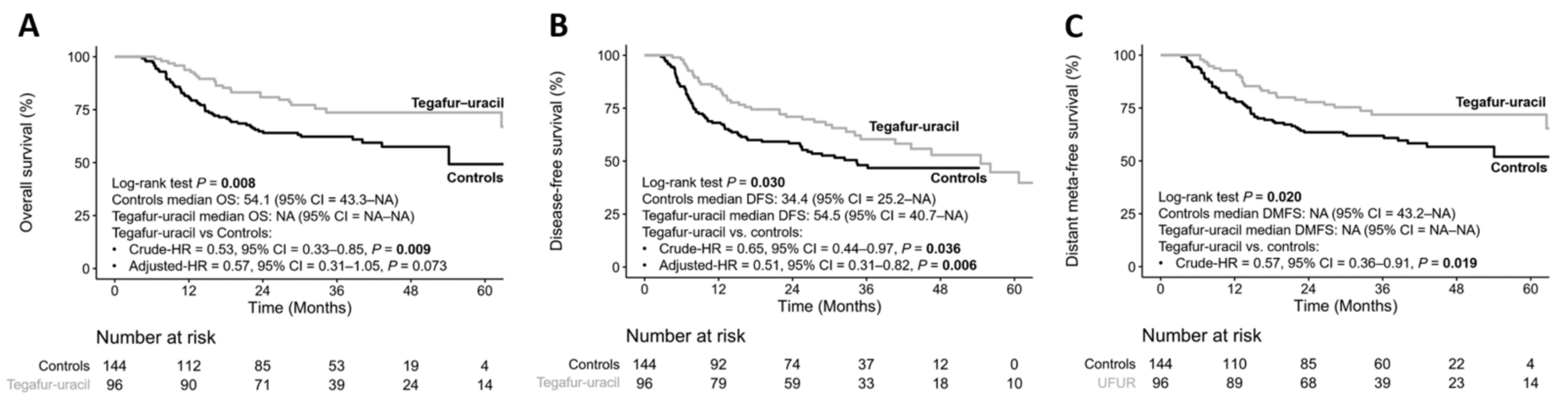
3.2. Treatment Outcomes
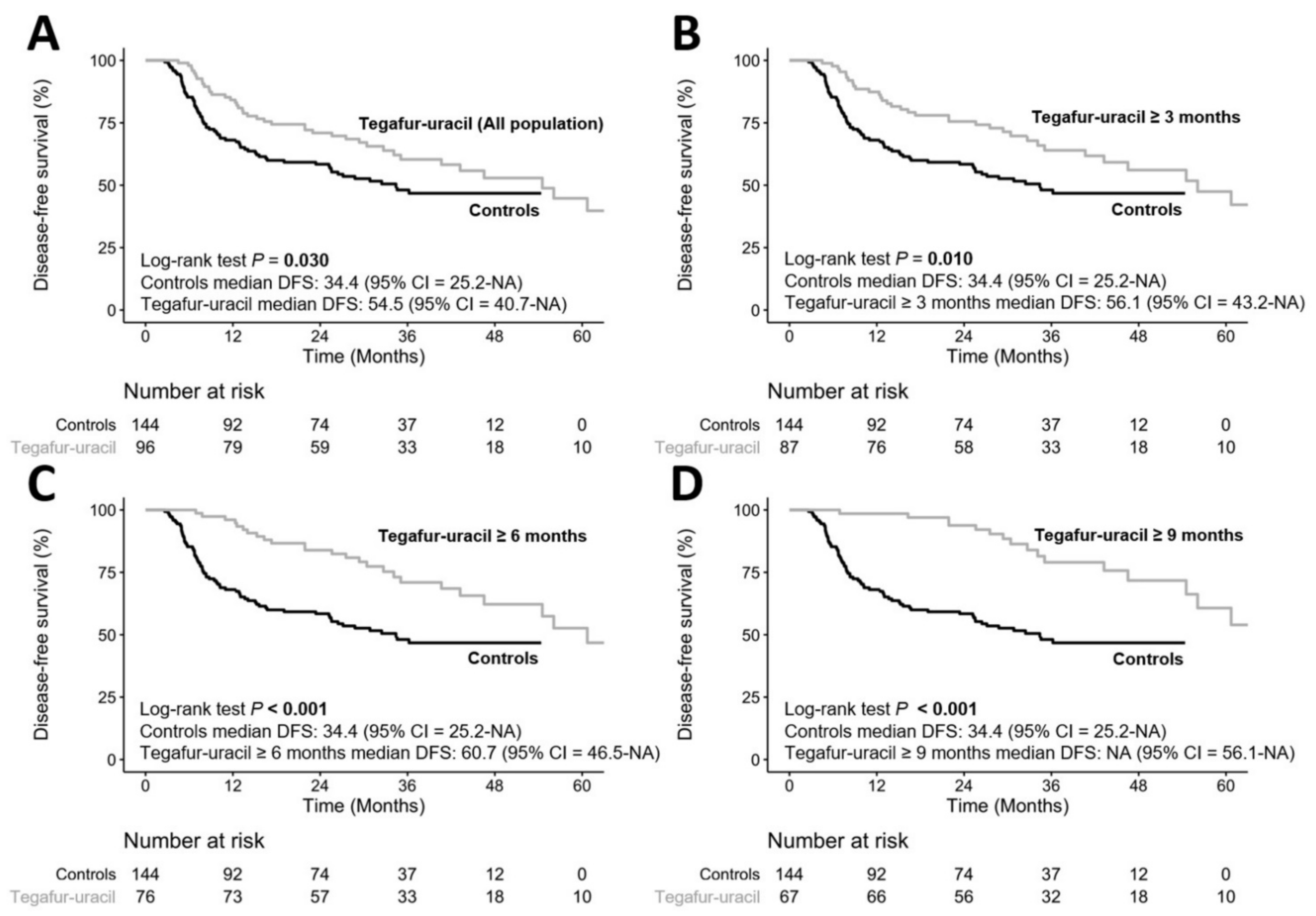
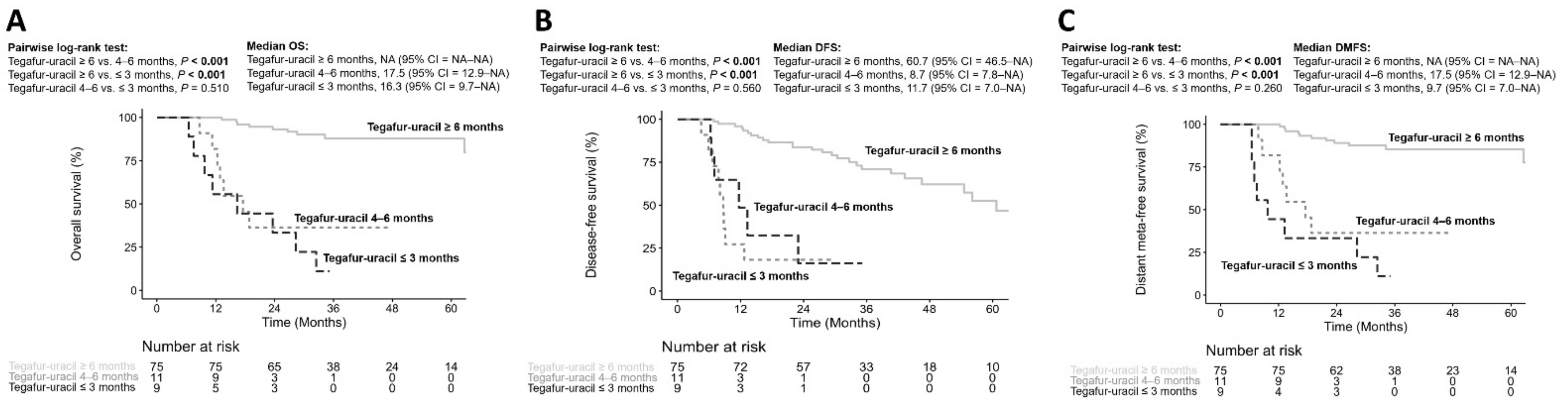
3.3. Durations of Tegafur–Uracil Administration Affected the Clinical Outcomes of HNSCC Patients
3.4. Safety and Analysis of the Treatment Failure
3.5. Risk Factor Evaluation for Disease Progression
3.6. Determining the Risk Factor for Poorer Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Yu, K.J.; Chiang, C.-J.; Chen, T.-C.; Wang, C.-P. Head and neck cancer incidence trends in Taiwan, 1980~2014. Int. J. Head Neck Sci. 2017, 1, 180–189. [Google Scholar]
- Pfister, D.G.; Spencer, S.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; Colevas, A.D.; Dunphy, F.; Eisele, D.W.; et al. Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2014, 12, 1454–1487. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Brakenhoff, R.H.; Leemans, C.R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann. Oncol. 2012, 23 (Suppl. S10), x173–x177. [Google Scholar] [CrossRef]
- Vives, M.; Ginesta, M.M.; Gracova, K.; Graupera, M.; Casanovas, O.; Capella, G.; Serrano, T.; Laquente, B.; Vinals, F. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int. J. Cancer 2013, 133, 2464–2472. [Google Scholar] [CrossRef]
- Simsek, C.; Esin, E.; Yalcin, S. Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience. J. Oncol. 2019, 2019, 5483791. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Benevento, I.; Musella, A.; Musio, D.; Tombolini, V. Metronomic chemotherapy in head and neck cancer. Cancer Lett. 2017, 400, 219–222. [Google Scholar] [CrossRef]
- Kareva, I. A Combination of Immune Checkpoint Inhibition with Metronomic Chemotherapy as a Way of Targeting Therapy-Resistant Cancer Cells. Int. J. Mol. Sci. 2017, 18, 2134. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Bocci, G. Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett. 2018, 432, 28–37. [Google Scholar] [CrossRef]
- Gourd, E. Metronomic chemotherapy option for advanced oral cancer. Lancet Oncol. 2019, 20, e614. [Google Scholar] [CrossRef]
- Wellington, K.; Goa, K.L. Oral tegafur/uracil. Drugs Aging 2001, 18, 935–948, discussion 949–950. [Google Scholar] [CrossRef]
- Brockstein, B.E.; Votes, E.E. Oral Chemotherapy in Head and Neck Cancer. Drugs 1999, 58, 91–97. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, W.Y.; Ho, C.L.; Chao, T.Y.; Lee, J.C. Evaluation of oral tegafur-uracil as metronomic therapy following concurrent chemoradiotherapy in patients with non-distant metastatic TNM stage IV nasopharyngeal carcinoma. Head Neck 2019, 41, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.Y.; Chen, G.; Chang, D.C.; Chien, S.Y.; Chen, M.K. The Impact of Metronomic Adjuvant Chemotherapy in Patients with Advanced Oral Cancer. Ann. Surg. Oncol. 2018, 25, 2091–2097. [Google Scholar] [CrossRef]
- Hsieh, M.Y.; Chen, M.K. ASO Author Reflections: Tegafur-Uracil in Advanced Oral Cancer. Ann. Surg. Oncol. 2018, 25, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Takes, R.P.; Rinaldo, A.; Silver, C.E.; Haigentz, M., Jr.; Woolgar, J.A.; Triantafyllou, A.; Mondin, V.; Paccagnella, D.; de Bree, R.; Shaha, A.R.; et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012, 48, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Duprez, F.; Berwouts, D.; De Neve, W.; Bonte, K.; Boterberg, T.; Deron, P.; Huvenne, W.; Rottey, S.; Mareel, M. Distant metastases in head and neck cancer. Head Neck 2017, 39, 1733–1743. [Google Scholar] [CrossRef]
- Mascitti, M.; Tempesta, A.; Togni, L.; Capodiferro, S.; Troiano, G.; Rubini, C.; Maiorano, E.; Santarelli, A.; Favia, G.; Limongelli, L. Histological features and survival in young patients with HPV-negative oral squamous cell carcinoma. Oral Dis. 2020, 26, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Lailler, C.; Clatot, F.; Galmiche, A. Perineural invasion in head and neck squamous cell carcinoma: Background, mechanisms, and prognostic implications. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Jardim, J.F.; Francisco, A.L.; Gondak, R.; Damascena, A.; Kowalski, L.P. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2015, 44, 23–28. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [PubMed]
- Tsukahara, K.; Kubota, A.; Hasegawa, Y.; Takemura, H.; Terada, T.; Taguchi, T.; Nagahara, K.; Nakatani, H.; Yoshino, K.; Higaki, Y. Randomized phase III trial of adjuvant chemotherapy with S-1 after curative treatment in patients with squamous-cell carcinoma of the head and neck (ACTS-HNC). PLoS ONE 2015, 10, e0116965. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Berrier, A.; Robinson, M.; Remenar, E.; Housset, M.; De Mendoza, F.H.; Fayette, J.; Mehanna, H.; El-Hariry, I.; Compton, N. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: Rationale for future randomised trials in human papilloma virus-negative disease. Eur. J. Cancer 2013, 49, 1609–1618. [Google Scholar] [CrossRef]
- Pai, P.S.; Vaidya, A.D.; Prabhash, K.; Banavali, S.D. Oral metronomic scheduling of anticancer therapy-based treatment compared to existing standard of care in locally advanced oral squamous cell cancers: A matched-pair analysis. Indian J. Cancer 2013, 50, 135–141. [Google Scholar] [PubMed]
- Pandey, A.; Desai, A.; Ostwal, V.; Patil, V.; Kulkarni, A.; Kulkarni, R.; Patil, N.; Chaukar, D.; Prabhash, K.; Banavali, S.D. Outcome of operable oral cavity cancer and impact of maintenance metronomic chemotherapy: A retrospective study from rural India. South. Asian J. Cancer 2016, 5, 52–55. [Google Scholar]
- Patil, V.; Noronha, V.; Dhumal, S.B.; Joshi, A.; Menon, N.; Bhattacharjee, A.; Kulkarni, S.; Ankathi, S.K.; Mahajan, A.; Sable, N.; et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: An open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob. Health 2020, 8, e1213–e1222. [Google Scholar] [CrossRef]
- Harrington, K.; Temam, S.; Mehanna, H.; D’Cruz, A.; Jain, M.; D’Onofrio, I.; Manikhas, G.; Horvath, Z.; Sun, Y.; Dietzsch, S. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: A phase III, randomized, double-blind, placebo-controlled study. J. Clin. Oncol. 2015, 33, 4202–4209. [Google Scholar]
- Burtness, B.; Haddad, R.; Dinis, J.; Trigo, J.; Yokota, T.; de Souza Viana, L.; Romanov, I.; Vermorken, J.; Bourhis, J.; Tahara, M. Afatinib vs placebo as adjuvant therapy after chemoradiotherapy in squamous cell carcinoma of the head and neck: A randomized clinical trial. JAMA Oncol. 2019, 5, 1170–1180. [Google Scholar] [CrossRef]
- Cohen, E.; Ferris, R.; Psyrri, A.; Haddad, R.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.-H.; Lin, J.; Razaq, M. 910O Primary results of the phase III JAVELIN head & neck 100 trial: Avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). Ann. Oncol. 2020, 31, S658. [Google Scholar]
- Basaki, Y.; Chikahisa, L.; Aoyagi, K.; Miyadera, K.; Yonekura, K.; Hashimoto, A.; Okabe, S.; Wierzba, K.; Yamada, Y. γ-Hydroxybutyric acid and 5-fluorouracil, metabolites of UFT, inhibit the angiogenesis induced by vascular endothelial growth factor. Angiogenesis 2001, 4, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, M.; Miyake, H. Maintenance chemotherapy with UFT for head and neck carcinoma. Oncol. N. Y. 2000, 14, 93–95. [Google Scholar]
| Total | Tegafur–Uracil | Controls | p | |
|---|---|---|---|---|
| Cases, row % | 240 | 96 (40%) | 144 (60%) | |
| Age, years (mean ± SD) | 55 ± 10 | 56 ± 10 | 54 ± 10 | 0.196 |
| Sex, male | 234 (97.5%) | 94 (97.9%) | 140 (97.2%) | 0.036 |
| Risk behavior | ||||
| Alcohol | 172 (71.7%) | 77 (80.2%) | 95 (66.0%) | 0.024 |
| Betel | 178 (74.2%) | 76 (79.2%) | 102 (70.8%) | 0.196 |
| Cigarette | 210 (87.5%) | 87 (90.6%) | 123 (85.4%) | 0.319 |
| Comorbidity group (1) | 0.499 | |||
| No comorbidity | 99 (41.2%) | 35 (36.5%) | 64 (44.4%) | |
| At least one or more | 173 (72.1%) | 72 (75.0%) | 101(70.1%) | |
| Comorbidity group (2) | 0.474 | |||
| No comorbidity | 99 (41.2%) | 35 (36.5%) | 64 (44.4%) | |
| 1–3 | 117 (48.8%) | 49 (51.0%) | 68 (47.2%) | |
| >3 | 24 (10.0%) | 12 (12.5%) | 12 (8.3%) | |
| Comorbidities (details) | ||||
| Hypertension | 93 (38.8%) | 45 (46.9%) | 48 (33.3%) | 0.048 |
| Diabetes mellitus | 51 (21.2%) | 21 (21.9%) | 30 (20.8%) | 0.974 |
| Coronary heart disease | 5 (2.1%) | 2 (2.1%) | 3 (2.1%) | 1.000 |
| Chronic kidney disease | 12 (5%) | 5 (5.2%) | 7 (4.9%) | 1.000 |
| Chronic lung diseases (ex. COPD) | 2 (0.8%) | 1 (1.0%) | 1 (0.7%) | 1.000 |
| Cerebrovascular disease | 9 (3.8%) | 7 (7.3%) | 2 (1.4%) | 0.032 |
| Peptic ulcer disease/GERD | 25 (10.4%) | 7 (7.3%) | 18 (12.5%) | 0.281 |
| Hepatitis B/C | 40 (16.7%) | 12 (12.5%) | 28 (19.4%) | 0.216 |
| Gout | 11 (4.6%) | 5 (5.2%) | 6 (4.2%) | 0.950 |
| Tumor location | 0.001 | |||
| Oral cavity | 145 (60.4%) | 46 (47.9%) | 99 (68.8%) | |
| Oropharynx | 45 (18.8%) | 19 (19.8%) | 26 (18.1%) | |
| Hypopharynx and larynx, others | 50 (20.8%) | 31 (32.3%) | 19 (13.2%) | |
| Grade | 0.078 | |||
| Well-differentiated | 73 (30.4%) | 24 (25.0%) | 49 (34.0%) | |
| Moderately differentiated | 130 (54.2%) | 52 (54.2%) | 78 (54.2%) | |
| Poorly differentiated | 34 (14.2%) | 19 (19.8%) | 15 (10.4%) | |
| Unknown | 3 | 1 | 2 | |
| Stage | 0.350 | |||
| III | 51 (21.2%) | 17 (17.7%) | 34 (23.6%) | |
| IV | 189 (78.8%) | 79 (82.3%) | 110 (76.4%) | |
| LVI | 55 (22.9%) | 29 (30.2%) | 26 (18.1%) | 0.018 |
| PNI | 60 (25.0%) | 25 (26.0%) | 35 (24.3%) | 0.693 |
| ENE | 77 (32.1%) | 46 (47.9%) | 31 (21.5%) | <0.001 |
| Margin positive | 42 (17.5%) | 23 (24.0%) | 19 (13.2%) | 0.025 |
| Treatment before tegafur–uracil | ||||
| Surgery alone | 38 (15.8%) | 8 (8.3%) | 30 (20.8%) | 0.027 |
| Surgery and CRT | 151 (62.9%) | 67 (69.8%) | 84 (58.3%) | |
| CRT alone | 45 (18.8%) | 17 (17.7%) | 28 (19.4%) | |
| Surgery | 189 (78.8%) | 75 (78.1%) | 114(79.2%) | 0.974 |
| PF | 55 (22.9%) | 19 (19.8%) | 36 (25.0%) | 0.433 |
| CRT | 196 (81.7%) | 84 (87.5%) | 112 (77.8%) | 0.082 |
| Adverse Events | Grade 1 | Grade 2 |
|---|---|---|
| Nausea | 9 (3.8%) | |
| Vomiting | 8 (3.3%) | |
| Mucositis | 5 (2.1%) | |
| Neutropenia | 4 (1.7%) | 3 (1.2%) |
| Anemia | 2 (0.8%) | 1 (0.4%) |
| Thrombocytopenia | 1 (0.4%) | |
| Chronic kidney disease, acute exacerbation | 1 (0.4%) | |
| Diarrhea | 3 (1.2%) | |
| Epigastralgia | 1 (0.4%) | |
| Poor appetite | 1 (0.4%) | |
| Skin itch | 2 (0.8%) | |
| Skin rash | 2 (0.8%) |
| Total | Tegafur–Uracil | Controls | p | |
|---|---|---|---|---|
| Treatment failure | 0.669 | |||
| Primary tumor recurrence | 68 (28.3%) | 26 (27.1%) | 42 (29.2%) | |
| Regional lymph nodes metastasis | 19 (7.9%) | 7 (7.3%) | 12 (8.3%) | |
| Distant metastasis | 23 (9.6%) | 7 (7.3%) | 16 (11.1%) |
| Variable | Comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| Crude-HR (95% CI) | p | Adjusted-HR (95% CI) | p | ||
| Group | Tegafur–uracil vs. controls | 0.65 (0.44–0.97) | 0.036 | 0.51 (0.31–0.82) | 0.006 |
| Age | ≥ 55 vs. < 55 years | 0.63 (0.43–0.92) | 0.016 | 0.82 (0.52–1.29) | 0.393 |
| Risk behavior | Yes vs. no | 1.69 (0.74–3.86) | 0.200 | - | |
| Alcohol | Yes vs. no | 1.31 (0.85–2.03) | 0.200 | - | |
| Betel | Yes vs. no | 2.33 (1.37–3.96) | 0.002 | 1.87 (0.94–3.71) | 0.075 |
| Cigarette | Yes vs. no | 2.05 (1.00–4.22) | 0.051 | - | |
| Comorbidity status (1) | Yes vs. no | 0.82 (0.55–1.23) | 0.300 | - | |
| Comorbidity status (2) | 1–3 vs. 0 | 0.80 (0.52–1.21) | 0.300 | - | |
| > 3 vs. 0 | 1.002 (0.52–1.94) | 0.994 | - | ||
| Tumor location | Oropharynx vs. oral cavity | 0.92 (0.57–1.50) | 0.700 | - | |
| Others vs. oral cavity | 0.68 (0.41–1.14) | 0.150 | - | ||
| Grade | Moderately vs. well | 1.46 (0.96–2.23) | 0.075 | - | |
| Poorly vs. well | 0.62 (0.30–1.30) | 0.200 | - | ||
| LVI | Yes vs. no | 1.42 (0.89–2.26) | 0.140 | - | |
| PNI | Yes vs. no | 1.77 (1.13–2.76) | 0.012 | 1.35 (0.85–2.15) | 0.204 |
| ENE | Yes vs. no | 1.62 (1.05–2.51) | 0.031 | 1.88 (1.17–3.01) | 0.009 |
| Margin positivity | Yes vs. no | 1.09 (0.66–1.79) | 0.700 | - | |
| Treatment before tegafur–uracil | Surgery alone vs. none | 0.30 (0.08–1.14) | 0.078 | - | |
| Surgery and CRT vs. none | 0.77 (0.24–2.45) | 0.700 | - | ||
| CRT alone vs. none | 0.99 (0.30–3.28) | 0.984 | - | ||
| Surgery | Yes vs. no | 0.68 (0.44–1.04) | 0.076 | - | |
| CRT | Yes vs. no | 2.18 (1.17–4.07) | 0.014 | 2.46 (1.10–5.51) | 0.028 |
| Variable | Comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| Crude-HR (95% CI) | p | Adjusted-HR (95% CI) | p | ||
| Group | Tegafur–uracil vs. controls | 0.53 (0.33–0.85) | 0.009 | 0.57 (0.31–1.05) | 0.073 |
| Age | ≥ 55 vs. < 55 years | 0.78 (0.51–1.22) | 0.300 | - | |
| Risk behavior | Yes vs. no | 2.40 (0.76–7.59) | 0.140 | - | |
| Alcohol | Yes vs. no | 1.69 (0.98–2.93) | 0.060 | ||
| Betel | Yes vs. no | 2.30 (1.22–4.35) | 0.010 | 2.14 (0.90–5.09) | 0.084 |
| Cigarette | Yes vs. no | 2.30 (0.93–5.69) | 0.072 | - | |
| Comorbidity status (1) | Yes vs. no | 1.10 (0.67–1.81) | 0.700 | - | |
| Comorbidity status (2) | 1–3 vs. 0 | 1.02 (0.61–1.71) | 0.928 | - | |
| >3 vs. 0 | 1.61 (0.78–3.34) | 0.200 | - | ||
| Tumor location | Oropharynx vs. oral cavity | 0.98 (0.56–1.72) | 0.943 | - | |
| Others vs. oral cavity | 0.75 (0.41–1.35) | 0.300 | - | ||
| Grade | Moderately vs. well | 2.18 (1.28–3.72) | 0.004 | 2.43 (1.16–5.10) | 0.019 |
| Poorly vs. well | 0.65 (0.24–1.74) | 0.400 | 0.90 (0.27–3.04) | 0.866 | |
| LVI | Yes vs. no | 1.89 (1.11–3.22) | 0.019 | 1.24 (0.67–2.30) | 0.486 |
| PNI | Yes vs. no | 2.29 (1.35–3.87) | 0.002 | 1.51 (0.84–2.72) | 0.164 |
| ENE | Yes vs. no | 2.07 (1.23–3.50) | 0.006 | 1.81 (1.01–3.23) | 0.047 |
| Margin positivity | Yes vs. no | 0.91 (0.49–1.69) | 0.800 | - | |
| Treatment before tegafur–uracil | Surgery alone vs. none | 0.38 (0.08–1.91) | 0.200 | - | |
| Surgery and CRT vs. none | 0.85 (0.21–3.49) | 0.800 | - | ||
| CRT alone vs. none | 1.19 (0.28–5.11) | 0.800 | - | ||
| Surgery | Yes vs. no | 0.64 (0.39–1.05) | 0.080 | - | |
| CRT | Yes vs. no | 2.03 (0.98–4.22) | 0.058 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-J.; Chan, L.-P.; Tsai, H.-T.; Hsu, C.-M.; Cho, S.-F.; Pan, M.-R.; Liu, Y.-C.; Huang, C.-J.; Wu, C.-W.; Du, J.-S.; et al. The Overall Efficacy and Outcomes of Metronomic Tegafur-Uracil Chemotherapy on Locally Advanced Head and Neck Squamous Cell Carcinoma: A Real-World Cohort Experience. Biology 2021, 10, 168. https://doi.org/10.3390/biology10020168
Yeh T-J, Chan L-P, Tsai H-T, Hsu C-M, Cho S-F, Pan M-R, Liu Y-C, Huang C-J, Wu C-W, Du J-S, et al. The Overall Efficacy and Outcomes of Metronomic Tegafur-Uracil Chemotherapy on Locally Advanced Head and Neck Squamous Cell Carcinoma: A Real-World Cohort Experience. Biology. 2021; 10(2):168. https://doi.org/10.3390/biology10020168
Chicago/Turabian StyleYeh, Tsung-Jang, Leong-Perng Chan, Hui-Ting Tsai, Chin-Mu Hsu, Shih-Feng Cho, Mei-Ren Pan, Yi-Chang Liu, Chih-Jen Huang, Che-Wei Wu, Jeng-Shiun Du, and et al. 2021. "The Overall Efficacy and Outcomes of Metronomic Tegafur-Uracil Chemotherapy on Locally Advanced Head and Neck Squamous Cell Carcinoma: A Real-World Cohort Experience" Biology 10, no. 2: 168. https://doi.org/10.3390/biology10020168
APA StyleYeh, T.-J., Chan, L.-P., Tsai, H.-T., Hsu, C.-M., Cho, S.-F., Pan, M.-R., Liu, Y.-C., Huang, C.-J., Wu, C.-W., Du, J.-S., & Wang, H.-C. (2021). The Overall Efficacy and Outcomes of Metronomic Tegafur-Uracil Chemotherapy on Locally Advanced Head and Neck Squamous Cell Carcinoma: A Real-World Cohort Experience. Biology, 10(2), 168. https://doi.org/10.3390/biology10020168






