In Vivo Hepatoprotective and Nephroprotective Activity of Acylated Iridoid Glycosides from Scrophularia hepericifolia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Biological Evaluation
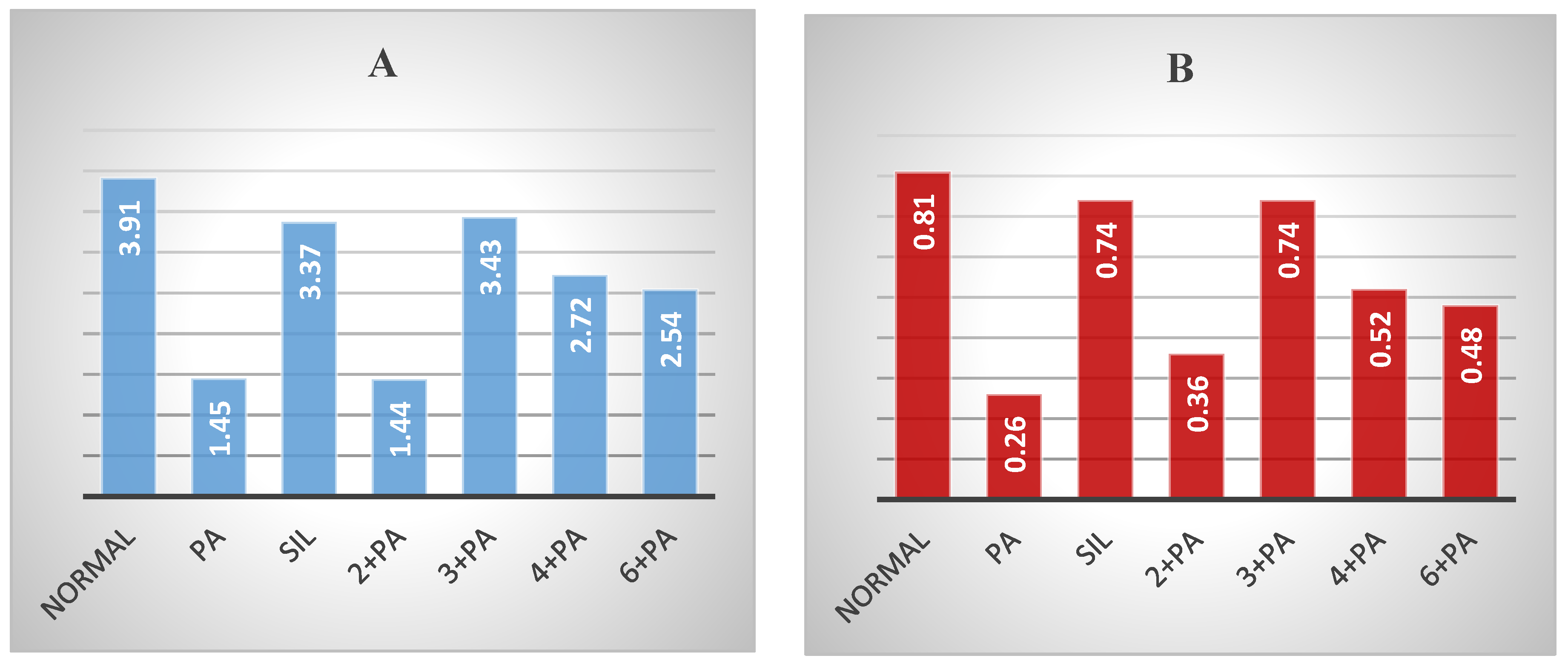
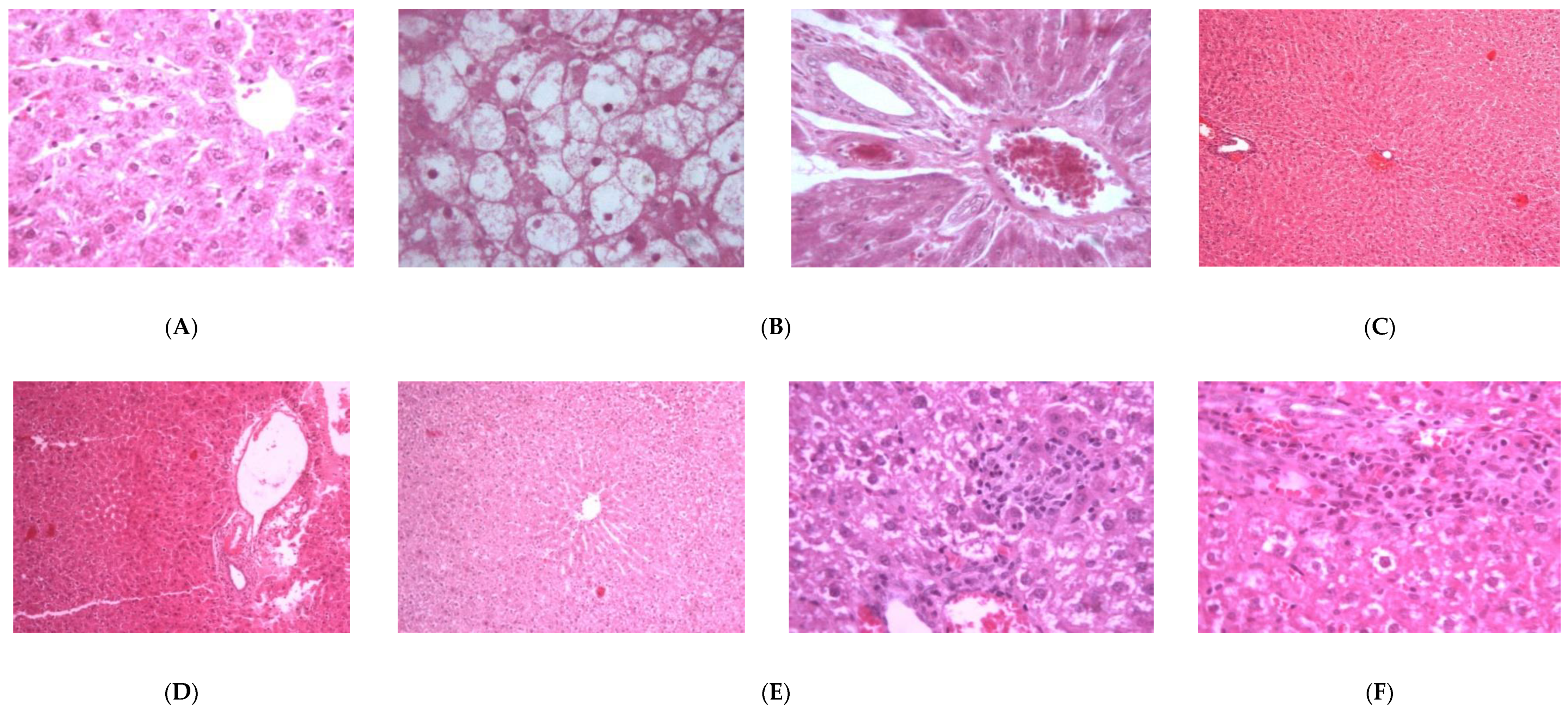
2.2.1. Hepatoprotective Effect
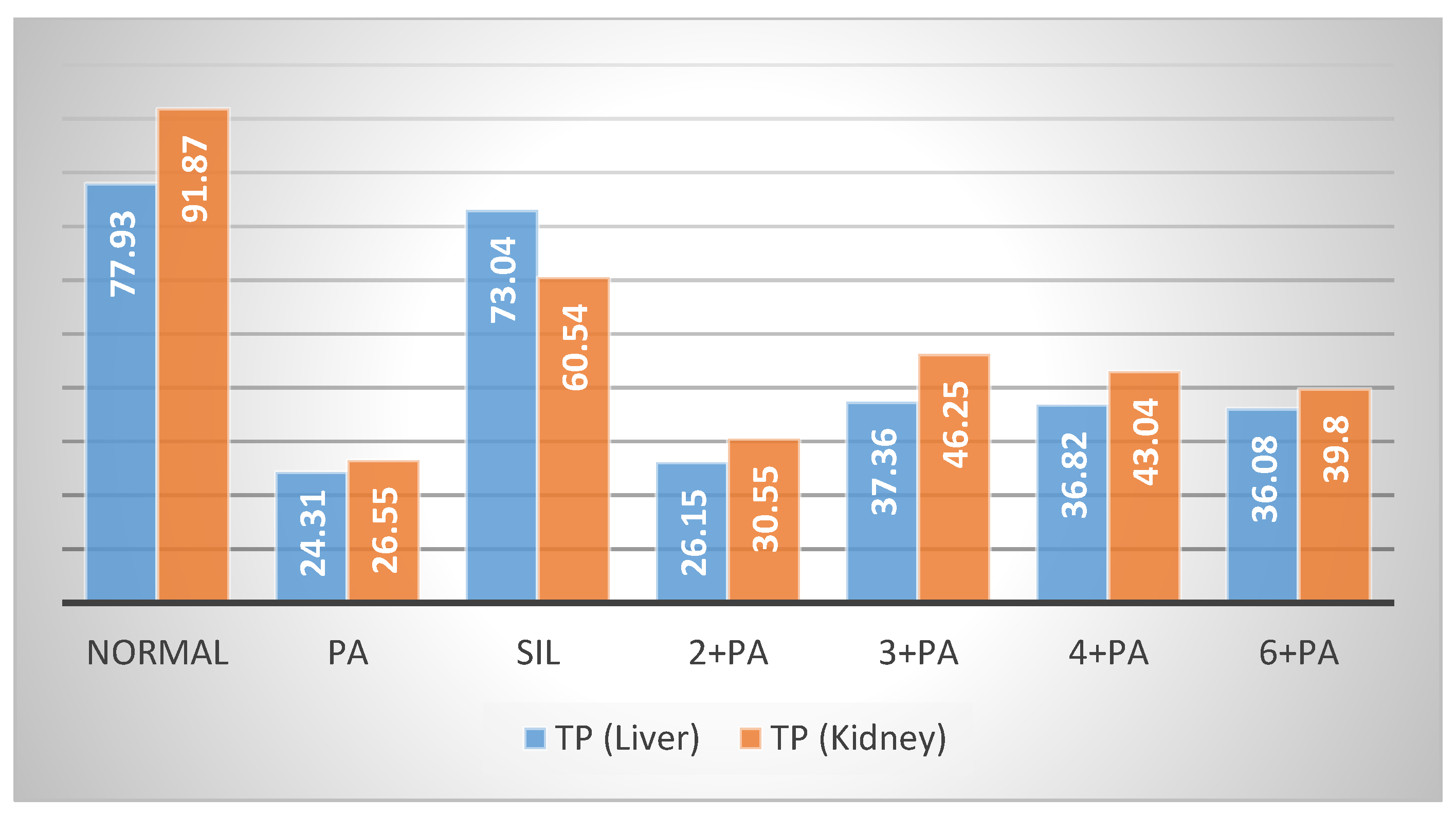
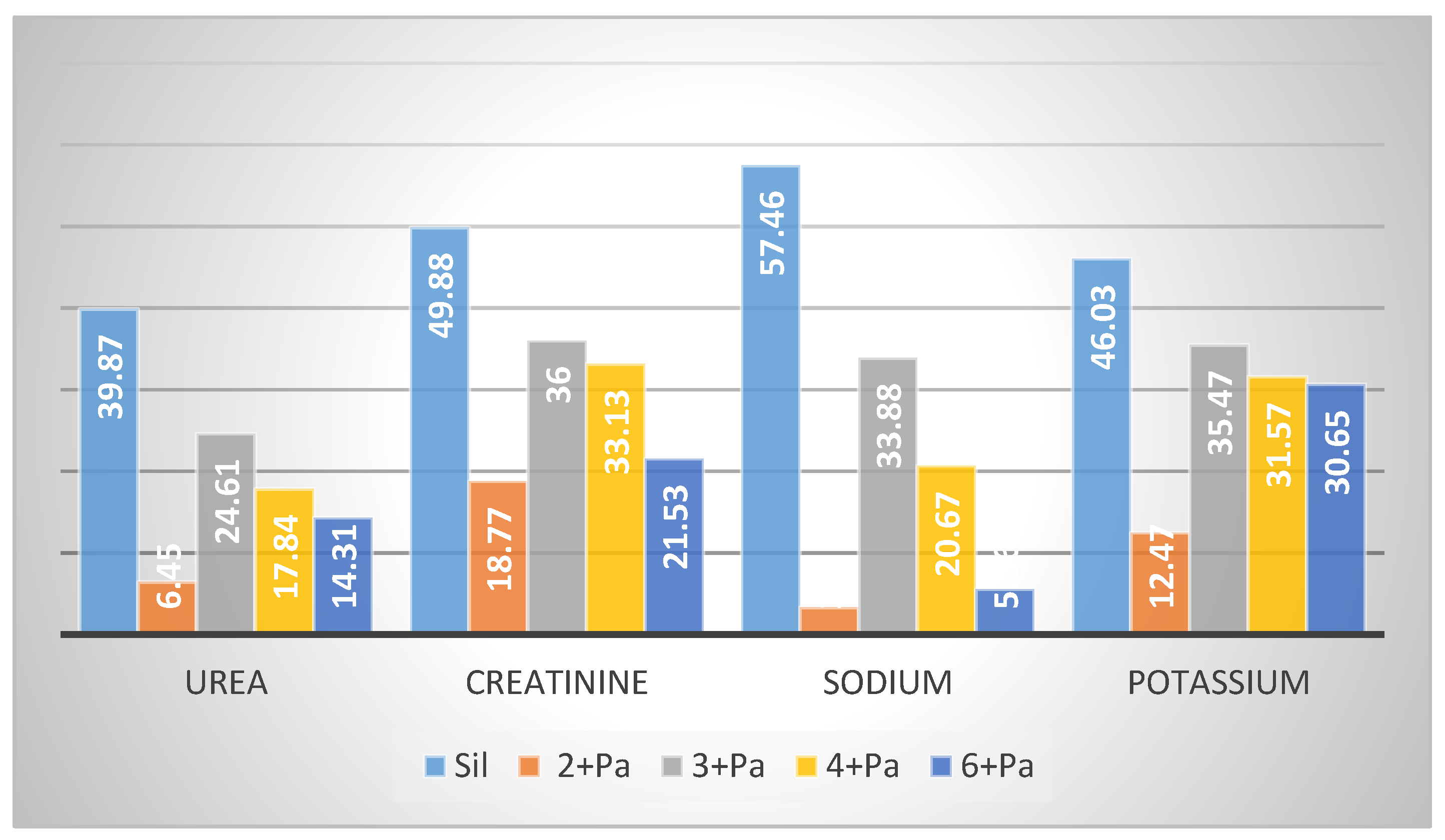
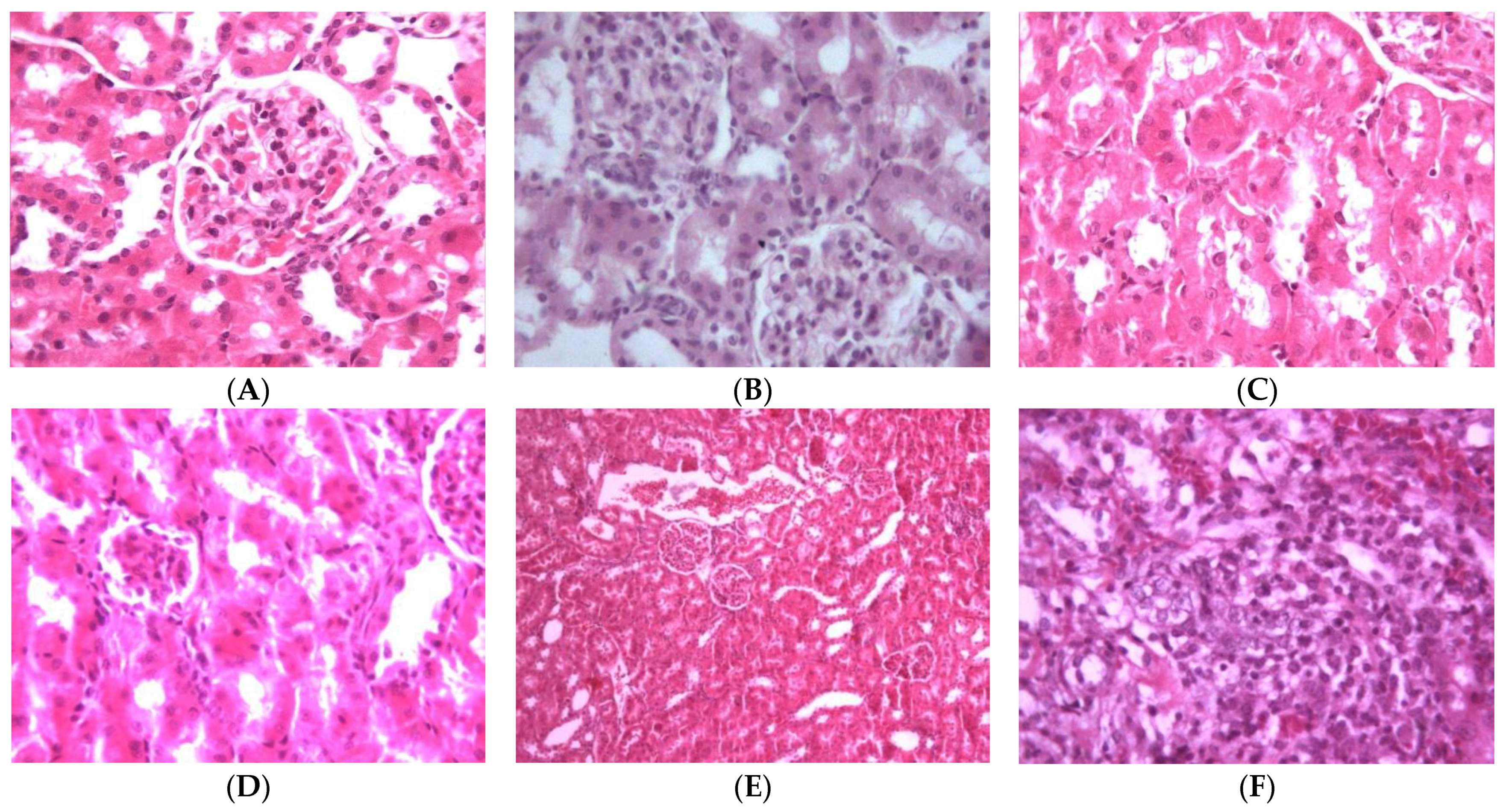
2.2.2. Nephroprotective Effect
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization
3.4.1. 6′-Acetyl hypericifolioside A (1)
3.4.2. 6′-Acetyl hypericifolioside B (2)
3.4.3. Hypericifolioside A (3)
3.4.4. Hypericifolioside B (4)
3.5. Animals
3.6. Hepatoprotective and Nephroprotective Activity
3.7. Determination of Biochemical Serum Parameters
3.8. Determination of Non-Protein Sulfhydryl Groups and Total Protein
3.9. Statistical Analysis
3.10. Histopathology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheunert, A.; Heubl, G. Phylogenetic relationships among New World Scrophularia L. (Scrophulariaceae): New insights inferred from DNA sequence data. Plant Syst. Ecol. 2011, 291, 69–89. [Google Scholar] [CrossRef]
- Collenette, S. Wild Flowers of Saudi Arabia; East Anglian Engraving, Co.: Norwich, UK, 1999. [Google Scholar]
- Flora of Saudi Arabia-Checklist. Available online: http://plantdiversityofsaudiarabia.info/Biodiversity-Saudi-Arabia/Flora/Checklist/Cheklist.htm (accessed on 9 February 2021).
- Zhu, Y.P. Chinese Materia Medica: Chemistry, Pharmacology and Applications; Amsterdam Harwood Academic Publishers: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Marty, A.T. The complete German commission E monographs: Therapeutic guide to herbal medicines. JAMA 1999, 281, 1852–1853. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Lucia, L.M. Ethnobotanical remarks on central and southern Italy. J. Ethnobiol. Ethnomed. 2007, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieroni, A.; Quave, C.L.; Santoro, R.F. Folk pharmaceutical knowledge in the territory of the Dolomiti Lucane, inland southern Italy. J. Ethnopharmacol. 2004, 95, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Pasdaran, A.; Delazar, A.; Nazemiyeh, H.; Nahar, L.; Sarker, S.D. Chemical composition, and antibacterial (against Staphylococcus aureus) and free radical-scavenging activities of the essential oils of Scrophularia amplexicaulis Benth. Rec. Nat. Prod. 2012, 6, 350–355. [Google Scholar]
- Ahmed, B.; Al-Rehaily, A.J.; Al-Howiriny, T.A.; El-Sayed, K.A.; Ahmad, M.S. Scropolioside-D2 and harpagoside-B: Two new iridoid glycosides from Scrophularia deserti and their antidiabetic and antiinflammatory activity. Biol. Pharm. Bull. 2003, 26, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.J.; Hou, Y.L.; Shen, X.Y.; Pan, X.D.; Zhang, X.; Yao, X.S. Monoterpene pyridine alkaloids and phenolics from Scrophularia ningpoensis and their cardioprotective effect. Fitoterapia 2013, 88, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, S.; Kim, J.; Kim, Y. Hepatoprotective phenylpropanoids from Scrophularia buergeriana roots against CCl4-induced toxicity: Action mechanism and structure–activity relationship. Planta Med. 2003, 68, 407–411. [Google Scholar] [CrossRef]
- Garg, H.; Bhandari, S.; Tripathi, S.; Patnaik, G.; Puri, A.; Saxena, R.; Saxena, R. Antihepatotoxic and immunostimulant properties of iridoid glycosides of Scrophularia koelzii. Phytother. Res. 1994, 8, 224–228. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Simmonds, M.S.; Sampson, J.; Houghton, P.J.; Grice, P. Wound healing activity of acylated iridoid glycosides from Scrophularia nodosa. Phytother. Res. 2002, 16, 33–35. [Google Scholar] [CrossRef]
- Germinara, G.S.; Frontera, A.M.; De Cristofaro, A.; Rotundo, G. Insecticidal activity of different extracts from Scrophularia canina L. against Culex pipiens molestus Forskal (Diptera, Culicidae). J. Environ. Sci. Health B 2011, 46, 473–479. [Google Scholar] [PubMed]
- Han, M.-F.; Zhang, X.; Zhang, L.-Q.; Li, Y.-M. Iridoid and phenylethanol glycosides from Scrophularia umbrosa with inhibitory activity on nitric oxide production. Phytochem. Lett. 2018, 28, 37–41. [Google Scholar] [CrossRef]
- Renda, G.; Korkmaz, B.; Kılıç, M.; Duman, M.K.; Kırmızıbekmez, H. Evaluation of in vivo analgesic activity of Scrophularia kotscyhana and isolation of bioactive compounds through activity-guided fractionation. Nat. Prod. Res. 2018, 32, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Townsend, S.A.; Newsome, P.N. Non-alcoholic fatty liver disease in 2016. Br. Med. Bull. 2016, 119, 43–56. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bellentani, S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): The available clinical evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Alqasoumi, S.I. Evaluation of the hepatroprotective and nephroprotective activities of Scrophularia hypericifolia growing in Saudi Arabia. Saudi Pharma. J. 2014, 22, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Swiatek, L.; Lehmann, D.; Sticher, O. Iridoid glycosides of Scrophularia lateriflora Trautv. (Scrophulariaceae). Pharm. Acta Helv. 1981, 56, 37–44. [Google Scholar]
- Stavri, M.; Mathew, K.T.; Gibbons, S. Antimicrobial constituents of Scrophularia deserti. Phytochemistry 2006, 67, 1530–1533. [Google Scholar] [CrossRef]
- Nykmukanova, M.M.; Eskalieva, B.K.; Burasheva, G.S.; Iqbal Choudhary, M.; Adhikari, A.; Amadou, D. Iridoids from Verbascum marschallianum. Chem. Nat. Compd. 2017, 53, 580–581. [Google Scholar] [CrossRef]
- Brownstein, K.J.; Gargouri, M.; Folk, W.R.; Gang, D.R. Iridoid and phenylethanoid/phenylpropanoid metabolite profiles of Scrophularia and Verbascum species used medicinally in North America. Metabolomics 2017, 13, 133. [Google Scholar] [CrossRef]
- Ganaie, H.A.; Ali, M.N.; Ganai, B.A.; Meraj, M.; Ahmad, M. Antibacterial activity of 14, 15-dihydroajugapitin and 8-o-acetylharpagide isolated from Ajuga bracteosa Wall ex. Benth against human pathogenic bacteria. Microb. Pathog. 2017, 103, 114–118. [Google Scholar] [CrossRef]
- You, Y.; Wang, J.; Tong, Y.; Hao, Q.; Li, Y.; Yang, H.; Huang, L.; Liao, F. Anti-inflammatory effect of acetylharpagide demonstrated by its influence on leukocyte adhesion and transmigration in endothelial cells under controlled shear stress. Clin. Hemorheol. Microcirc. 2014, 56, 205–217. [Google Scholar] [CrossRef]
- Breschi, M.C.; Martinotti, E.; Catalano, S.; Flamini, G.; Morelli, I.; Pagni, A.M. Vasoconstrictor activity of 8-O-acetylharpagide from Ajuga reptans. J. Nat. Prod. 1992, 55, 1145–1148. [Google Scholar] [CrossRef]
- Konoshima, T.; Takasaki, M.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of an iridoid glycoside, 8-acetylharpagide, from Ajuga decumbens. Cancer Lett. 2000, 157, 87–92. [Google Scholar] [CrossRef]
- Kawamura, F.; Ohara, S. Antifungal activity of iridoid glycosides from the heartwood of Gmelina arborea. Holzforschung 2005, 59, 153–155. [Google Scholar] [CrossRef]
- Al Ati, H.Y.; Fawzy, G.A.; El Gamal, A.A.; Khalil, A.T.; El Tahir, K.; Abdel-Kader, M.S.; Gilani, A.H. Phytochemical and biological evaluation of Buddleja polystachya growing in Saudi Arabia. Pak. J. Pharm. Sci. 2015, 28, 1533–1540. [Google Scholar] [PubMed]
- Yang, X.D.; Yang, L.J.; Yang, S.; Zhao, J.F.; Zhang, H.B.; Li, L. Two new iridoid glycosides from Lagotis yunnanensis. Z Nat. B 2007, 62, 749–752. [Google Scholar]
- Zhang, L.; Zhu, T.; Qian, F.; Xu, J.; Dorje, G.; Zhao, Z.; Guo, F.; Li, Y. Iridoid glycosides isolated from Scrophularia dentata Royle ex Benth. and their anti-inflammatory activity. Fitoterapia 2014, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Jiang, S.H.; Gao, W.Y.; Zhu, D.Y. Iridoid glycosides from Scrophularia ningpoensis. Phytochemistry 1999, 50, 101–104. [Google Scholar] [CrossRef]
- Tunmann, P.; Lux, R. Zur Kenntniss der Inhaltsstoffe aus der Wurzel von H.p Dtsch. Apoth. Ztg. 1962, 102, 1274–1275. [Google Scholar]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Saracoglu, I.; Oztunca, F.H.; Nagatsu, A.; Harput, U.S. Iridoid content and biological activities of Veronica cuneifolia subsp. cuneifolia and V. cymbalaria. Pharm. Biol. 2011, 49, 1150–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillete, J.R.; Brrodle, B.N. Acetaminophen induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- Remirez, D.; Commandeur, J.N.M.; Ed Groot, E.; Vermeulen, P.E. Mechanism of protection of Lobenzarti against paracetamol-induced toxicity in rat hepatocytes. Eur. J. Pharmacol. 1995, 293, 301–308. [Google Scholar] [CrossRef]
- McConnachie, L.A.; Mohar, I.; Hudson, F.N.; Ware, C.B.; Ladiges, W.C.; Fernandez, C.; Chatterton-Kirchmeier, S.; White, C.C.; Pierce, R.H.; Kavanagh, T.J. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol. Sci. 2007, 99, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, H.J.; Seeff, L.B. Enzymes in hepatic disease. In Diagnostic Enzymology; Goodly, E.I., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1970. [Google Scholar]
- Lin, C.C.; Shieh, D.E.; Yen, M.N. Hepatoprotective effects of fractions Ban-zhi-lian of experimental liver injuries in rats. J. Ethnopharmacol. 1997, 56, 193–200. [Google Scholar] [CrossRef]
- Dehmlow, C.; Murawski, N.; de Groot, H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silybinin in human cells. Life Sci. 1996, 52, 1591–1600. [Google Scholar] [CrossRef]
- Saller, R.; Melzer, J.; Rechling, J.; Brignoli, R.; Meier, R. An updated systematic review of the pharmacology of silymarin. Forschende Komplementärmedizin 2007, 14, 70–80. [Google Scholar] [CrossRef]
- Pocock, G.; Richards, C.D. Human Physiology: The Basis of Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Adelman, R.D.; Spangler, W.L.; Beasom, F.; Ishizaki, G.; Conzelman, G.M. Frusemide enhancement of neltimicin nephrotoxicity in dogs. J. Antimicrob. Chemother. 1981, 7, 431–435. [Google Scholar] [CrossRef]
- Adam, G.O.; Rahman, M.M.; Lee, S.-J.; Kim, G.-B.; Kang, H.-S.; Kim, J.-S.; Kim, S.-J. Hepatoprotective effects of Nigella sativa seed extract against acetaminophen-induced oxidative stress. Asian Pac. J. Trop. Med. 2016, 9, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Ogunleyea, G.S.; Fagbohunb, O.F.; Babalola, O.O. Chenopodium ambrosioides var. ambrosioides leaf extracts possess regenerative and ameliorative effects against mercury-induced hepatotoxicity and nephrotoxicity. Ind. Crops Prod. 2020, 154, 112723. [Google Scholar] [CrossRef]
- Nandy, S.; Mukherjee, A.; Pandey, D.K.; Ray, P.; Dey, A. Indian Sarsaparilla (Hemidesmus indicus): Recent progress in research on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112609. [Google Scholar] [CrossRef]
- Latif, A.; Ashiq, K.; Qayyum, M.; Ashiq, S.; Ali, E.; Anwer, I. Phytochemical and pharmacological profile of the medicinal herb: Bryophyllum pinnatum. J. Anim. Plant Sci. 2019, 29, 1528–1534. [Google Scholar]
- Panchal, M.A.; Murti, K.; Lambole, V. Pharmacological properties of Verbascum Thapsus—A review. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 15. [Google Scholar]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [Green Version]
- Dai, K.; Yi, X.-J.; Huang, X.-J.; Muhammad, A.; Li, M.; Li, J.; Yanga, G.-Z.; Gao, Y. Hepatoprotective activity of iridoids, seco-iridoids and analog glycosides from Gentianaceae on HepG2 cells via CYP3A4 induction and mitochondrial pathway. Food Funct. 2018, 9, 2673. [Google Scholar] [CrossRef]
- Edwards, C.R.W.; Bouchier, I.A.D. Davidson’s Principles and Practice Medicine; Churchill Livingstone Press: London, UK, 1991. [Google Scholar]
- Varley, H.; Alan, H.G. Tests in renal disease. In Practical Clinical Biochemistry; William Heinemann Medical Book Ltd.: London, UK, 1984; Volume 1123. [Google Scholar]
- Henry, R.J.; Cannon, D.C.; Winkelman, J.W. Clinical Chemistry, Principles and Techniques, 2nd ed.; Harper Row: New York, NY, USA, 1974. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Woolson, R.F.; Clarke, W.R. Statistical Methods for the Analysis of Biomedical Data, 2nd ed.; John Wiley & Sons. Inc.: New York, NY, USA, 2002. [Google Scholar]
- Prophet, E.P.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histology, 2nd ed.; American Registry of Pathology: Washington, DC, USA, 1994. [Google Scholar]


| Pos. | 1 | 2 | 3 | 4 | 7 | |||
|---|---|---|---|---|---|---|---|---|
| CD3OD | DMSO d6 | Pyridine d6 | CD3OD | Pyridine d6 | CD3OD | Pyridine d6 | CD3OD | |
| 1 | 4.95 (d, 9.8) | 4.83 (d, 9.2) | 5.45 ** | 5.14 ** | 5.51 (d, 8.8) | 5.11 ** | 5.48 ** | 5.11 ** |
| 3 | 6.43 (d, 5.8) | 6.44 (d, 1.5) | 6.47 (d, 5.2) | 6.43 (bd, 5.5) | 6.48 (d, 5.7) | 6.42 (bd, 6.0) | 6.45 (d, 4.7) | 6.41 (d, 5.7) |
| 4 | 5.16 (t, 5.4) | 5.04 ** | 5.45 ** | 5.14 ** | 5.19 (t, 5.0) | 5.11 ** | 5.12 ** | 5.11 ** |
| 5 | 2.54 (bq, 4.5) | 2.39 (bs) | 2.76 (bs) | 2.55 (bq, 4.3) | 2.76 (bq, 3.9) | 2.51 (bq, 4.6) | 2.73 (bs) | 2.45 (bq, 4.4) |
| 6 | 4.03 (d, 8.0) | 3.93 ** | 4.25 ** | 4.11 (d, 8.0) | 4.12 ** | 4.09 (d, 8.4) | 4.07 (d, 7.6) | 4.05 (d, 8.1) |
| 7 | 3.71 (s) | 3.69 (s) | 3.93 (s) | 3.72 (s) | 3.80 (s) | 3.70 (s) | 3.77 (s) | 3.69 (s) |
| 9 | 2.65 (t, 9.0) | 2.46 (bt, 8.0) | 2.85 (t, 7.7) | 2.64 (t, 9.4) | 2.84 (t, 8.9) | 2.62 (bt, 9.3) | 2.82 (bt, 8.0) | 2.60 (t, 8.0) |
| 10 | 3.78 (d, 13.0) | 3.63 (d, 12.4) | 4.39 (d, 13.0) | 3.87 (d, 13.2) | 4.48 ** | 3.85 (d, 13.2) | 4.47 (s) | 3.85 ** |
| 4.18 ** | 3.93 ** | 4.50 (d, 13.0) | 4.16 (m) | 4.18 (d, 13.2) | 4.18 (d, 13.2) | |||
| 1′ | 4.83 (d, 7.9) | 4.66 (d, 7.0) | 5.45 (Overl.) | 4.83 (d, 7.9) | 5.51 (d, 8.8) | 4.80 (d, 7.9) | 5.48 ** | 4.80 (d, 7.9) |
| 2′ | 3.32 (m) | 3.11 (bt, 7.5) | 4.10 (m) | 3.32 (m) | 4.12 ** | 3.28 (m) | 4.14 (t, 7.8) | 3.30 (m) |
| 3′ | 3.43 (m) | 3.25 (bt, 8.4) | 4.25 ** | 3.46 (t, 9.0) | 4.30 ** | 3.44 (bt,9.0) | 4.07 (d, 7.6) | 3.43 (q, 8.9) |
| 4′ | 3.43 (m) | 3.19 (bt, 8.9) | 4.10 (m) | 3.32 (m) | 4.19 (bt, 9.2) | 3.28 (m) | 4.20 (t, 8.7) | 3.30 (m) |
| 5′ | 3.52 (m) | 3.42 (m) | 3.99 (m) | 3.37 (m) | 4.01 (bt, 6.7) | 3.33 (m) | 4.00 (bs) | 3.30 (m) |
| 6′ | 4.28 (dd, 5.0, 12.0) | 4.14 (m) | 4.69 (dd, 3.7, 11.4) | 3.67 (dd, 6.5, 11.9) | 4.30 ** | 3.65 (dd, 6.8, 12.0) | 4.30 ** | 3.67 ** |
| 4.47 (d, 6.8) | 4.31 (m) | 4.88 (d, 11.4) | 3.96 (d, 10.9) | 4.53 (d, 11.8) | 3.94 (bd, 12.0) | 4.53 (d, 10.7) | 3.93 ** | |
| 1″ | 5.13 (s) | 5.14 (s) | 5.45 ** | 5.14 ** | 5.43 (s) | 5.11 ** | 5.38 (s) | 5.03 (s) |
| 2″ | 5.43 (bs) | 5.27 (bs) | 5.91(s) | 5.43 (bs) | 5.94 ** | 5.39 (m) | 5.88 (s) | 3.96 (m) |
| 3″ | 5.53 (dd, 2.7,10.0) | 5.27 (bs) | 5.95 (d, 7.7) | 5.55 (dd, 3.2, 10.2) | 6.09 (dd, 2.6, 10.0) | 5.41 (m) | 5.94 (bd, 9.7) | 3.96 (m) |
| 4″ | 5.32 (t, 10.0) | 5.04 ** | 5.71 (t, 9.6) | 5.33 (t, 10.0) | 5.89 ** | 5.17 (t, 9.8) | 5.71 (t, 9.7) | 5.11 ** |
| 5″ | 4.18 ** | 3.93 ** | 4.34 (m) | 4.16 ** | 4.44 (m) | 4.06 (m) | 4.30 ** | 3.67 ** |
| 6″ | 1.27 (d, 6.0) | 1.16 (d, 4.3) | 1.38(d, 5.5) | 1.28 (d, 6.2) | 1.46(d, 6.0) | 1.24 (d, 6.3) | 1.37 (d, 5.4) | 1.20 (d, 6.4) |
| 3‴–5‴ | 7.35 ** | 7.43 (bs), | 7.30 (bs), | 7.34 ** | 7.31 (bs) | 7.43 (m, 3H), | 7.20- 7.57 ** | 7.43 (bs) |
| 2‴, 6‴ | 7.49 (d, 6.6) | 7.69 (bs) | 7.47 (d, 5.8) | 7.47 (d, 6.4) | 7.24 (d, 7.3) | 7.61 (m, 2H) | 7.64 (bs) | |
| 7‴ | 7.61 (d, 16.0) | 7.60 (d, 16.0) | 7.91 (d, 16.0) | 7.60 (d, 16.0) | 7.89 (d, 16.0) | 7.67 (d, 16.0) | 7.92 (d, 16.0) | 7.76 (d, 16.0) |
| 8‴ | 6.40 (d, 16.0) | 6.54 (d, 16.0) | 6.74 (d, 16.0) | 6.39 (d, 16.0) | 6.72 (d, 16.0) | 6.46 (d, 16.0) | 6.74 (d, 16.0) | 6.61 (d, 16.0) |
| 3″″–5″″ | 7.35 ** | - | - | 7.34 ** | 7.57(bs) | - | - | - |
| 2″″, 6″″ | 7.53 (d, 6.6) | 7.51 (d, 6.1) | 7.39 (d, 7.2) | |||||
| 7″″ | 7.70 (d, 16.0) | - | - | 7.71 (d, 16.0) | 7.99 (d, 16.0) | - | - | - |
| 8″″ | 6.50 (d, 16.0) | - | - | 6.49 (d, 16.0) | 6.80 (d, 16.0) | - | - | - |
| Acetyl: | - | |||||||
| 6′ | 2.07 (s) | 2.00 (s) | 1.94 (s) | - | - | - | - | |
| 2″ | 2.19 (s) | 2.02 (s) | 2.06 (s) | 2.19 | 2.10 (s) | 2.06 (s) | 2.07 (s) | |
| 4″ | - | 2.13 (s) | 2.11 (s) | - | - | 2.18 (s) | 2.10 (s) | |
| Pos. | 1 ** | 2 *** | 3 ** | 4 ** | 7 ** | Pos. | 1 ** | 2 *** | 3 ** | 4 ** | 7 ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 93.95 | 94.03 | 93.82 | 93.76 | 93.83 | 3″ | 69.45 | 69.22 | 69.46 | 69.39 | 68.84 |
| 3 | 141.14 | 141.75 | 141.14 | 141.12 | 140.97 | 4″ | 71.05 | 70.70 | 71.07 | 70.90 | 74.15 |
| 4 | 102.06 | 102.46 | 101.95 | 101.80 | 102.09 | 5″ | 66.89 | 65.89 | 66.88 | 66.65 | 66.86 |
| 5 | 35.78 | 35.80 | 35.81 | 35.75 | 35.93 | 6″ | 16.53 | 17.62 | 16.54 | 16.35 | 16.54 |
| 6 | 83.74 | 83.21 | 83.61 | 83.47 | 82.77 | 1‴ | 134.00 | 134.11 | 133.97 | 134.07 | 134.32 |
| 7 | 58.02 | 57.56 | 58.14 | 58.07 | 58.13 | 2‴–6‴ | 128.01–130.45 | 129.01–131.26 | 128.00–130.43 | 128.04–130.46 | 127.95–130.26 |
| 8 | 65.08 | 66.62 | 65.21 | 65.19 | 65.25 | 7‴ | 146.28 | 146.07 | 146.26 | 146.04 | 145.39 |
| 9 | 41.85 | 42.14 | 41.92 | 41.87 | 41.91 | 8‴ | 116.50 | 117.41 | 116.51 | 116.47 | 117.42 |
| 10 | 60.17 | 59.22 | 60.07 | 60.01 | 60.06 | 9‴ | 165.93 | 165.75 | 165.92 | 165.89 | 166.98 |
| 1′ | 98.45 | 98.71 | 98.36 | 98.32 | 98.32 | 1″″ | 134.00 | - | 133.97 | - | - |
| 2′ | 73.35 | 73.70 | 73.44 | 73.42 | 73.42 | 2″″–6″″ | 128.01–130.45 | - | 128.00–130.43 | - | - |
| 3′ | 76.02 | 76.51 | 76.28 | 76.27 | 76.25 | 7″″ | 146.07 | - | 146.05 | - | - |
| 4′ | 70.06 | 70.26 | 70.37 | 70.37 | 70.35 | 8″″ | 116.50 | - | 116.51 | - | - |
| 5′ | 74.36 | 74.22 | 77.22 | 77.22 | 77.19 | 9″″ | 166.31 | - | 166.30 | - | - |
| 6′ | 62.74 | 63.27 | 61.58 | 61.55 | 61.52 | Acetyl: 6′ | 19.54, 171.40 | 20.94, 170.72 | - | - | |
| 1″ | 96.41 | 96.11 | 96.41 | 96.28 | 99.06 | 2″ | 19.48, 170.32 | 21.07, 170.23 | 19.49, 170.31 | 19.37, 170.30 | - |
| 2″ | 69.92 | 69.55 | 70.11 | 69.92 | 71.05 | 4″ | - | 21.04, 170.06 | - | 19.39, 170.53 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Kader, M.S.; Alqasoumi, S.I. In Vivo Hepatoprotective and Nephroprotective Activity of Acylated Iridoid Glycosides from Scrophularia hepericifolia. Biology 2021, 10, 145. https://doi.org/10.3390/biology10020145
Abdel-Kader MS, Alqasoumi SI. In Vivo Hepatoprotective and Nephroprotective Activity of Acylated Iridoid Glycosides from Scrophularia hepericifolia. Biology. 2021; 10(2):145. https://doi.org/10.3390/biology10020145
Chicago/Turabian StyleAbdel-Kader, Maged S., and Saleh I. Alqasoumi. 2021. "In Vivo Hepatoprotective and Nephroprotective Activity of Acylated Iridoid Glycosides from Scrophularia hepericifolia" Biology 10, no. 2: 145. https://doi.org/10.3390/biology10020145
APA StyleAbdel-Kader, M. S., & Alqasoumi, S. I. (2021). In Vivo Hepatoprotective and Nephroprotective Activity of Acylated Iridoid Glycosides from Scrophularia hepericifolia. Biology, 10(2), 145. https://doi.org/10.3390/biology10020145






