A Link between Intrahepatic Cholestasis and Genetic Variations in Intracellular Trafficking Regulators
Abstract
Simple Summary
Abstract
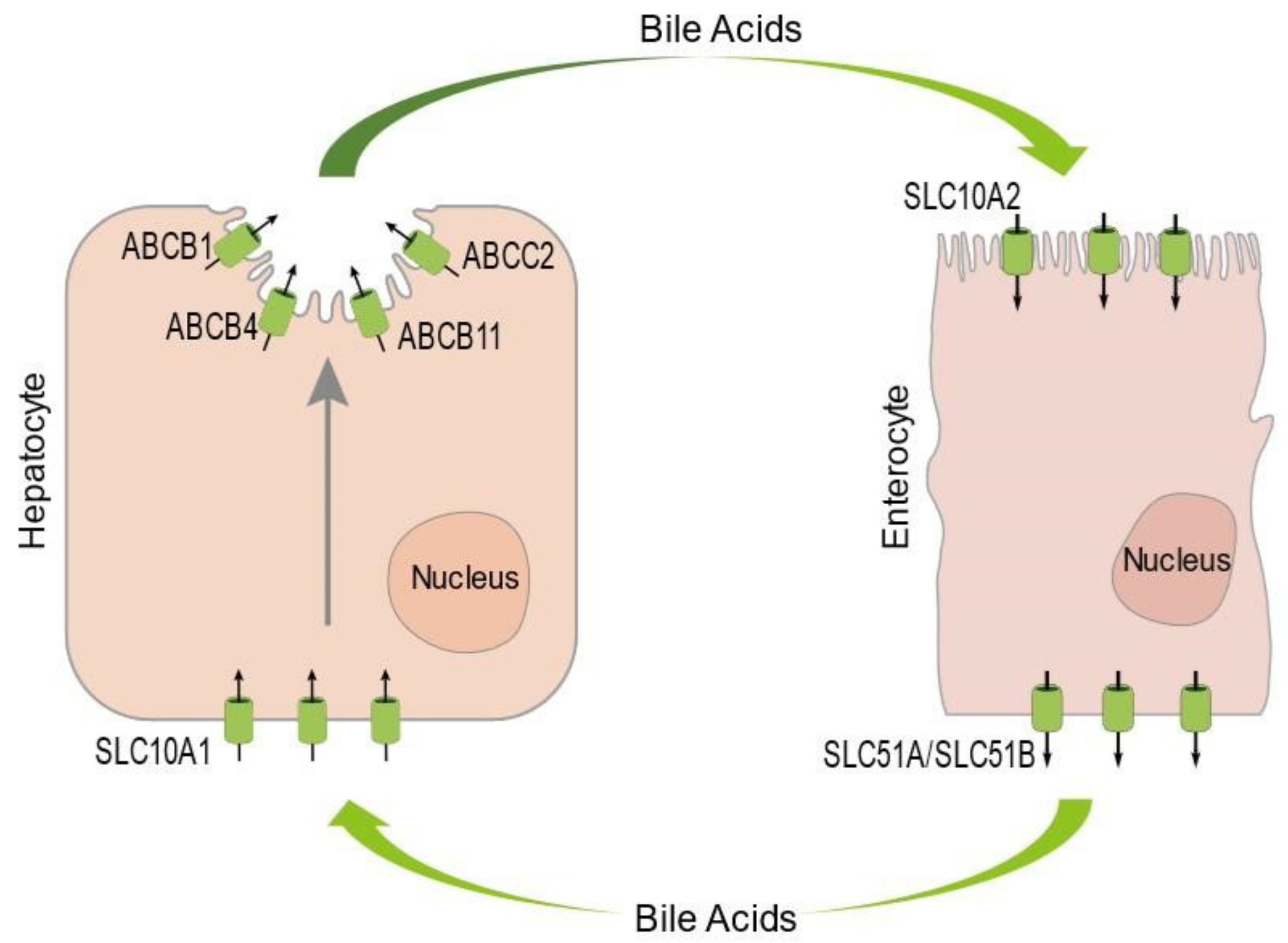
1. An Introduction to the Enterohepatic Circulation of Bile Acids and Familial Intrahepatic Cholestasis
2. An increasing Number of FIC-Associated Genes Are Involved in the Regulation of Intracellular Protein Trafficking
2.1. Intracellular Trafficking of ABCB11 and Other Bile Canalicular Proteins
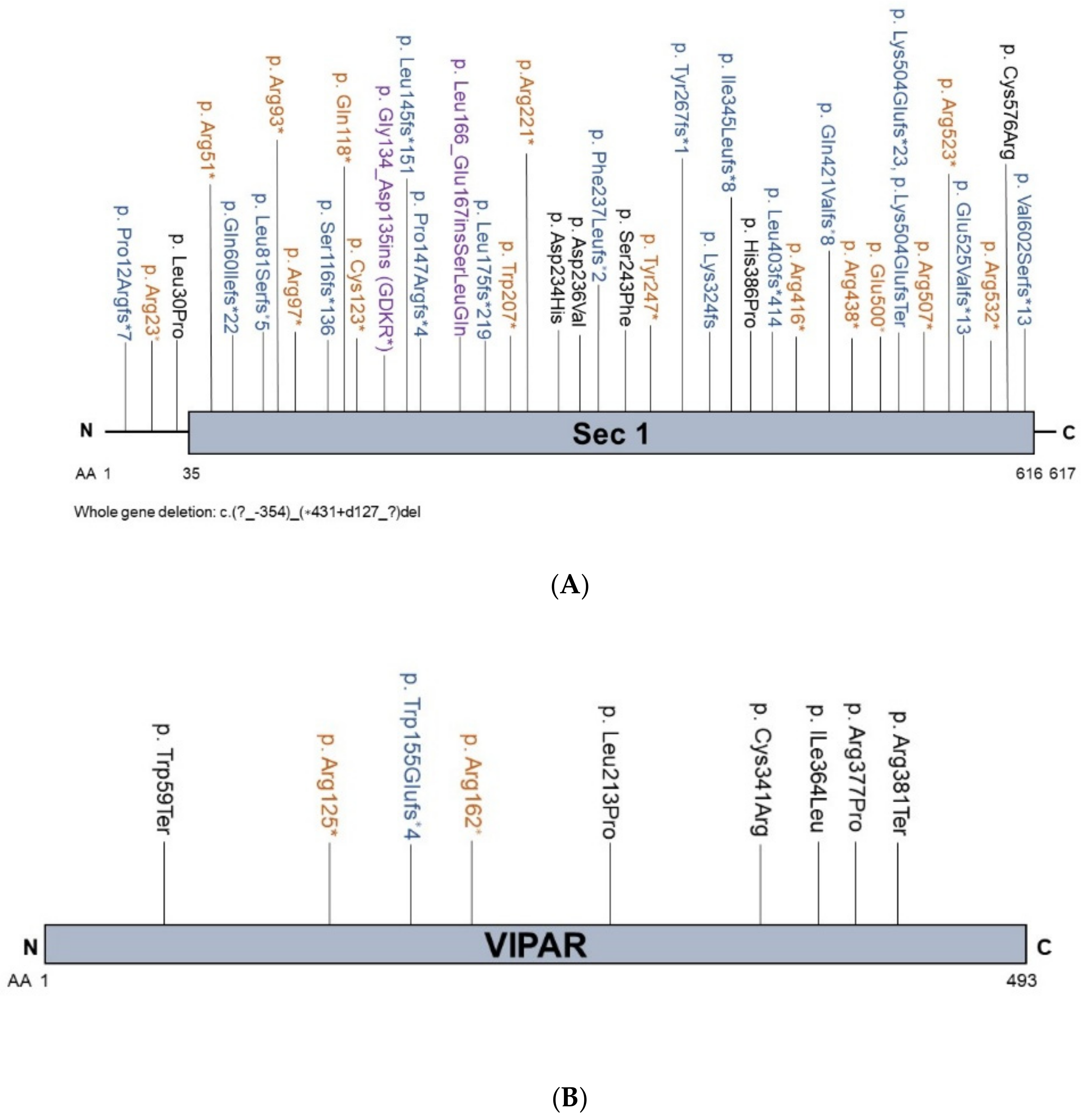
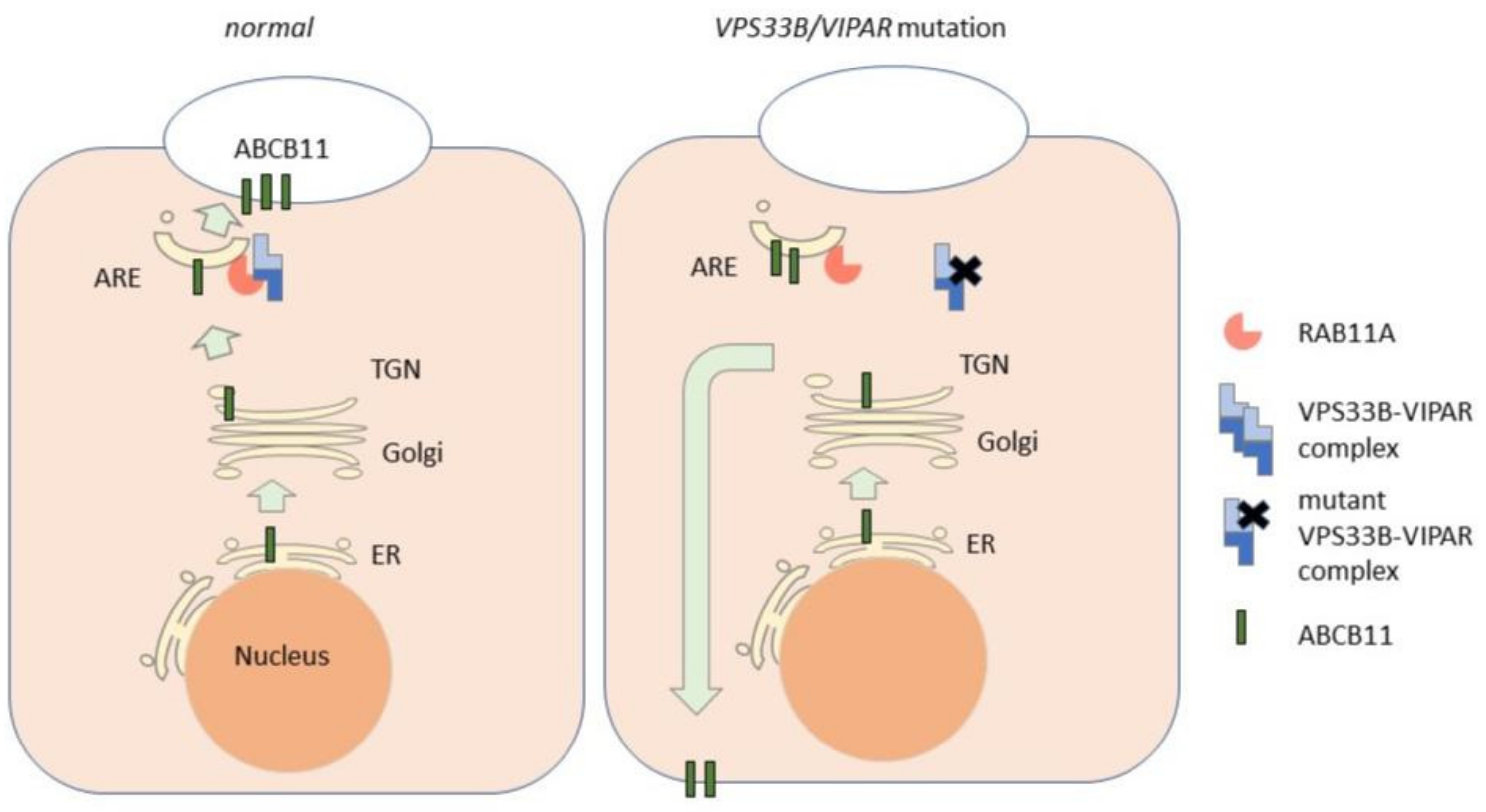
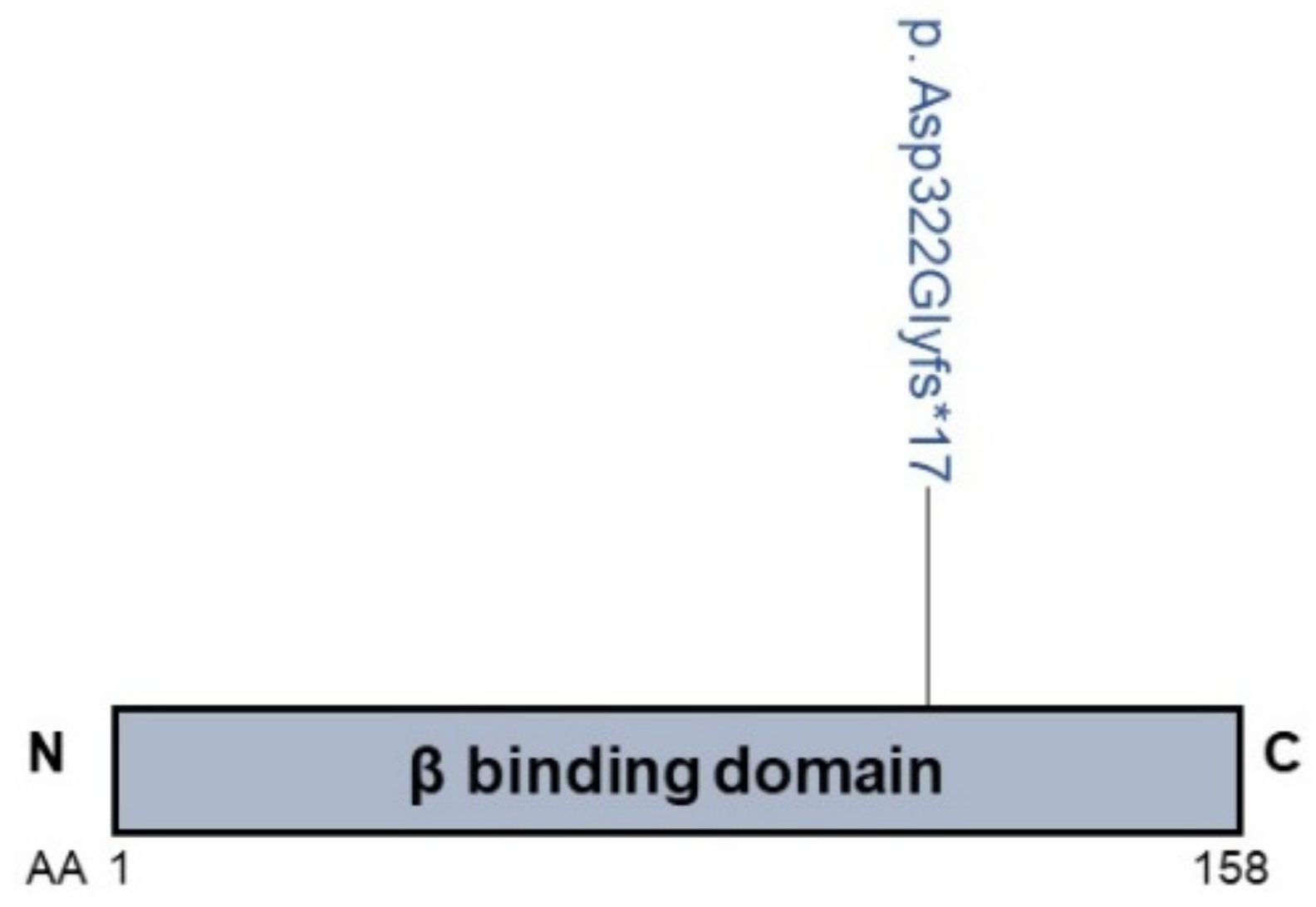
2.2. VPS33B and VIPAS39
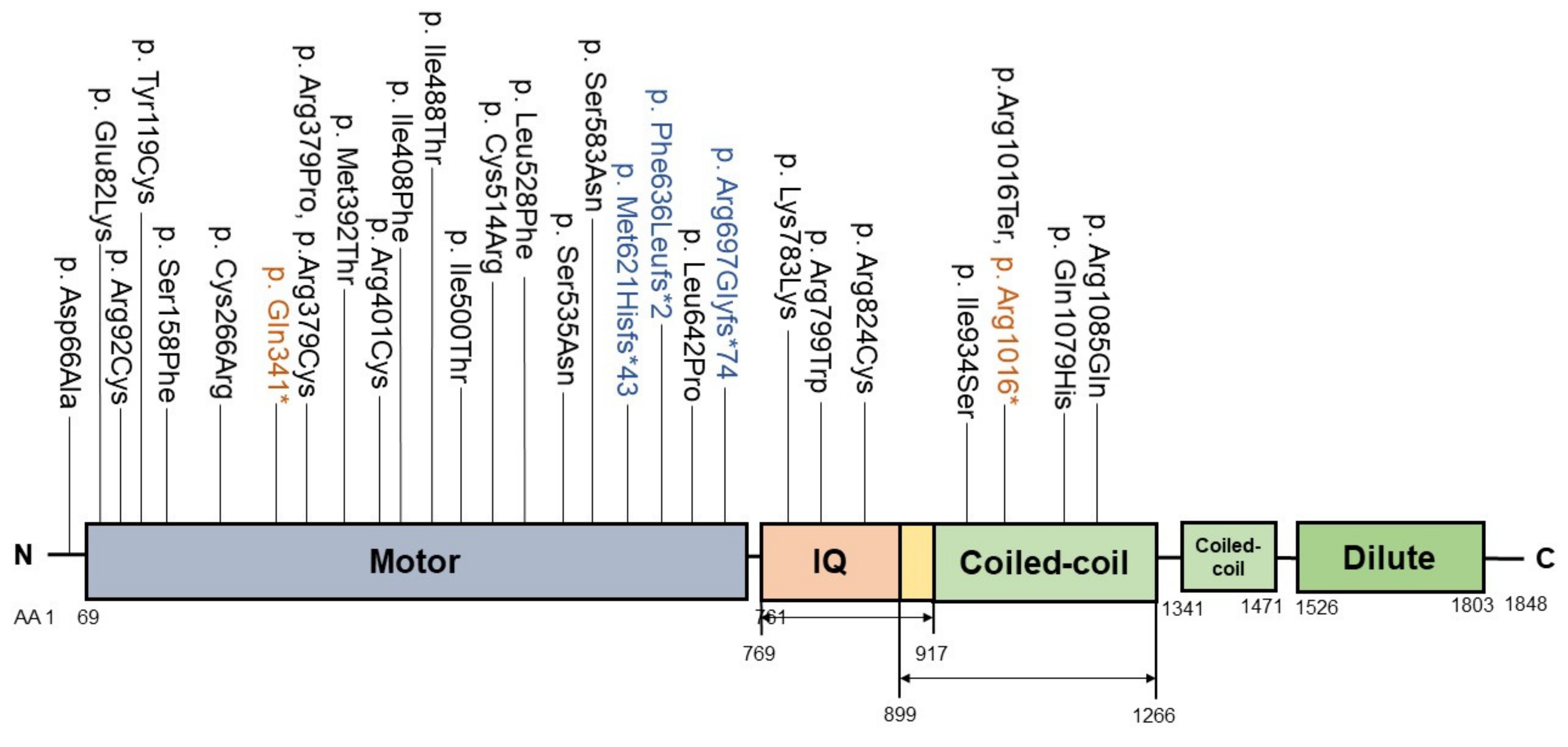
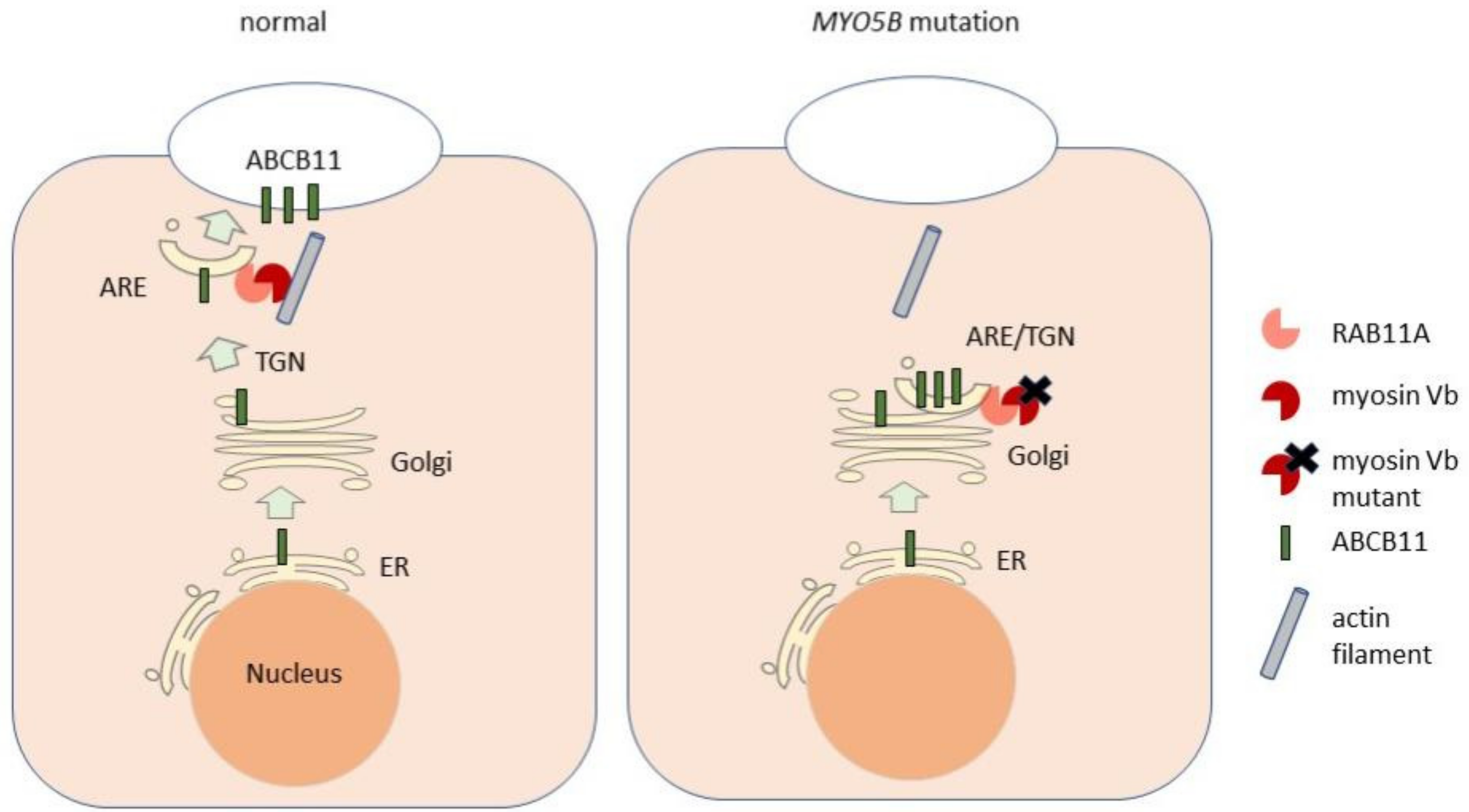
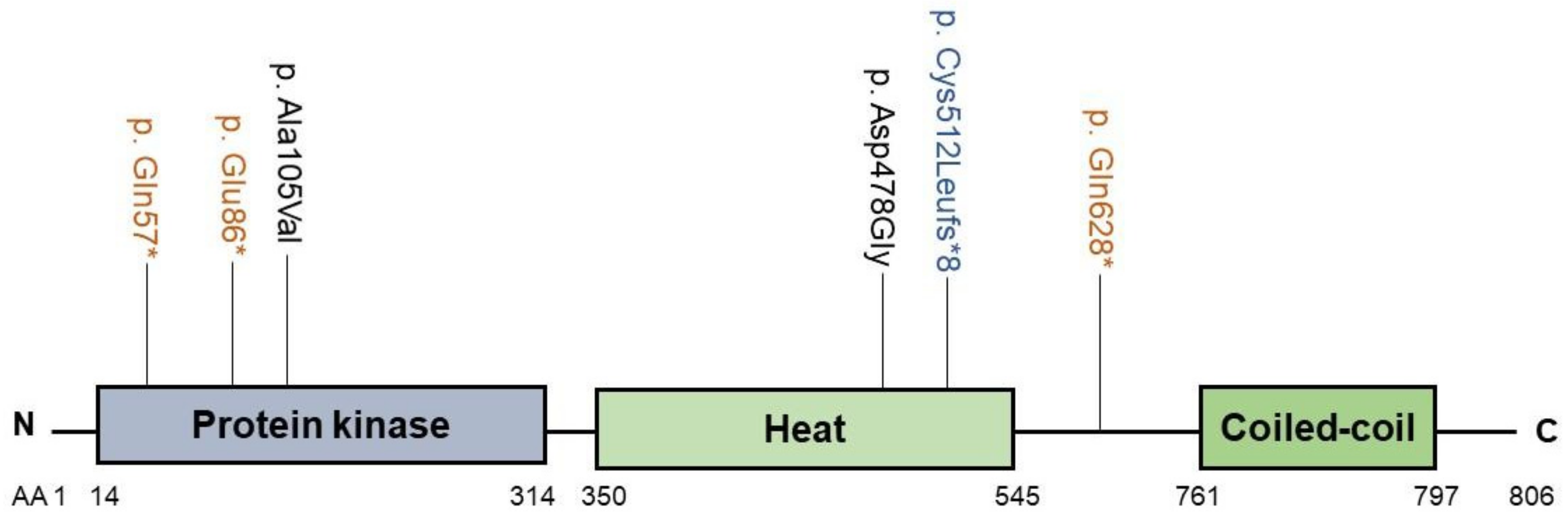
2.3. MYO5B
2.4. AP1S1
2.5. SCYL1
3. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP Binding Cassette |
| ARC | arthrogryposis–renal dysfunction–cholestasis |
| ASBT | apical sodium bile acid trans |
| BSEP | bile salt export pump |
| CALFAN | low gamma-glutamyltransferase cholestasis, acute liver failure and neurodegeneration |
| COPI | coatomer-I |
| E17G | estradiol-17beta-d-glucuronide |
| FIC | familial intrahepatic cholestasis |
| FXR | farnesoid X receptor |
| GDP | guanosine diphosphate |
| GGT | gamma glutamyltransferase |
| GPI | glycosylphosphatidylinositol |
| GTP | guanosine triphosphate |
| MDR | multidrug resistance |
| MEDNIK | mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, keratodermia) syndrome |
| MRP | multidrug-resistance protein |
| MVID | microvillus inclusion disease |
| NTCP | sodium taurocholate cotransporting polypeptide |
| OST | organic solute transporter |
| PFIC | progressive familial intrahepatic cholestasis |
| SLC | solute carrier |
| SNARE | soluble N-ethylmaleimide–sensitive factor activating protein receptor |
| TGN | trans-Golgi network |
| TUDC | tauroursodeoxycholate |
References
- Jetter, A.; Kullak-Ublick, G.A. Drugs and hepatic transporters: A review. Pharmacol Res. 2020, 154, 104234. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Bloks, V.W.; Verkade, E.; Heiner-Fokkema, M.R.; Kuipers, F. New insights in the multiple roles of bile acids and their signaling pathways in metabolic control. Curr. Opin. Lipidol. 2018, 29, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Pan, G. An important intestinal transporter that regulates the enterohepatic circulation of bile acids and cholesterol homeostasis: The apical sodium-dependent bile acid transporter (SLC10A2/ASBT). Clin. Res. Hepatol. Gastroenterol. 2017, 41, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, L.; Shan, W.; Kong, L.; Chen, N.; Lou, Y.; Zeng, S.; Shan, W. The Role of the Sodium-taurocholate Co-transporting Polypeptide (NTCP) and Bile Salt Export Pump (BSEP) in Related Liver Disease. Curr. Drug Metab. 2019, 20, 377–389. [Google Scholar] [CrossRef]
- Slijepcevic, D.; Abbing, R.L.P.R.; Katafuchi, T.; Blank, A.; Donkers, J.M.; Van Hoppe, S.; De Waart, D.R.; Tolenaars, D.; Van Der Meer, J.H.M.; Wildenberg, M.; et al. Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology 2017, 66, 1631–1643. [Google Scholar] [CrossRef]
- Slijepcevic, D.; van de Graaf, S.F.J. Bile Acid Uptake Transporters as Targets for Therapy. Dig. Dis. 2017, 35, 251–258. [Google Scholar] [CrossRef]
- Kunst, R.F.; Verkade, H.J.; Elferink, R.P.O.; van de Graaf, S.F.J. Targeting the four pillars of enterohepatic bile salt cycling; lessons from genetics and pharmacology. Hepatology 2020. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Van Mil, S.W.; Oldenburg, B.; Siersema, P.D.; Klomp, L.W.; Van Erpecum, K.J. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim. Biophys. Acta 2010, 1801, 683–692. [Google Scholar] [CrossRef]
- Neimark, E.; Chen, F.; Li, X.; Shneider, B.L. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology 2004, 40, 149–156. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Denson, L.A.; Sturm, E.; Echevarria, W.; Zimmerman, T.L.; Makishima, M.; Mangelsdorf, D.J.; Karpen, S.J. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 2001, 121, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Suchy, F.J.; Ananthanarayanan, M. Bile salt excretory pump: Biology and pathobiology. J. Pediatr. Gastroenterol. Nutr. 2006, 43 (Suppl. 1), S10–S16. [Google Scholar] [CrossRef]
- Bull, L.N.; Thompson, R.J. Progressive Familial Intrahepatic Cholestasis. Clin. Liver Dis. 2018, 22, 657–669. [Google Scholar] [CrossRef]
- Jacquemin, E. Progressive familial intrahepatic cholestasis. Clin. Res. Hepatol. Gastroenterol. 2012, 36 (Suppl. 1), S26–S35. [Google Scholar] [CrossRef]
- Strautnieks, S.S.; Bull, L.N.; Knisely, A.S.; Kocoshis, S.A.; Dahl, N.; Arnell, H.; Sokal, E.; Dahan, K.; Childs, S.; Ling, V.; et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat. Genet. 1998, 20, 233–238. [Google Scholar] [CrossRef]
- Wang, L.; Dong, H.; Soroka, C.J.; Wei, N.; Boyer, J.L.; Hochstrasser, M. Degradation of the bile salt export pump at endoplasmic reticulum in progressive familial intrahepatic cholestasis type II. Hepatology 2008, 48, 1558–1569. [Google Scholar] [CrossRef]
- Hayashi, H.; Takada, T.; Suzuki, H.; Akita, H.; Sugiyama, Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology 2005, 41, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Bull, L.N.; Van Eijk, M.J.T.; Pawlikowska, L.; Deyoung, J.A.; Juijn, J.A.; Liao, M.; Klomp, L.W.J.; Lomri, N.; Berger, R.; Scharschmidt, B.R.; et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 1998, 18, 219–224. [Google Scholar] [CrossRef]
- De Vree, J.M.; Jacquemin, E.; Sturm, E.; Cresteil, D.; Bosma, P.J.; Aten, J.; Deleuze, J.F.; Desrochers, M.; Burdelski, M.; Bernard, O.; et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA 1998, 95, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, E.; Cresteil, D.; Manouvrier, S.; Boute, O.; Hadchouel, M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 1999, 353, 210–211. [Google Scholar] [CrossRef]
- Sambrotta, M.; Strautnieks, S.; Papouli, E.; Rushton, P.; Clark, B.E.; Parry, D.A.; Logan, C.V.; Newbury, L.J.; Kamath, B.M.; Ling, S.; et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat. Genet. 2014, 46, 326–328. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Gong, J.; Hao, C.; Qiu, Y.; Lu, Y.; Feng, J.; Li, J.; Li, Z.; Wang, M.; et al. TJP2 hepatobiliary disorders: Novel variants and clinical diversity. Hum. Mutat. 2020, 41, 502–511. [Google Scholar] [CrossRef]
- Gomez-Ospina, N.; Potter, C.J.; Xiao, R.; Manickam, K.; Kim, M.-S.; Kim, K.H.; Shneider, B.L.; Picarsic, J.L.; Jacobson, C.J.P.K.M.T.A.; Zhang, J.; et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat. Commun. 2016, 7, 10713. [Google Scholar] [CrossRef]
- Gonzales, E.; Taylor, S.A.; Davit-Spraul, A.; Thébaut, A.; Thomassin, N.; Guettier, C.; Whitington, P.F.; Jacquemin, E. MYO5B mutations cause cholestasis with normal serum gamma-glutamyl transferase activity in children without microvillous inclusion disease. Hepatology 2017, 65, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.-L.; Gong, J.-Y.; Feng, J.-Y.; Wang, R.-X.; Han, J.; Liu, T.; Lu, Y.; Li, L.-T.; Zhang, M.-H.; Sheps, J.A.; et al. Defects in myosin VB are associated with a spectrum of previously undiagnosed low γ-glutamyltransferase cholestasis. Hepatology 2017, 65, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Dhekne, H.S.; Pylypenko, O.; Overeem, A.W.; Ferreira, R.J.; Van Der Velde, K.J.; Rings, E.H.; Posovszky, C.; Swertz, M.A.; Houdusse, A.; van Ijzendoorn, S.C.D. MYO5B, STX3, and STXBP2 mutations reveal a common disease mechanism that unifies a subset of congenital diarrheal disorders: A mutation update. Hum. Mutat. 2018, 39, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, M.; Harris, S.L.; Kazmierczak, P.; Shah, P.; Starovoytov, V.; Ohlemiller, K.K.; Schwander, M. Progressive Hearing Loss in Mice Carrying a Mutation in Usp53. J. Neurosci. 2015, 35, 15582–15598. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Gong, J.; Li, L.; Li, J.; Zhang, M.-H.; Lu, Y.; Xie, X.-B.; Hong, Y.-R.; Yu, Z.; et al. Low-GGT intrahepatic cholestasis associated with biallelic USP53 variants: Clinical, histological and ultrastructural characterization. Liver Int. 2020, 40, 1142–1150. [Google Scholar] [CrossRef]
- Gordo-Gilart, R.; Andueza, S.; Hierro, L.; Martínez-Fernández, P.; D’Agostino, D.; Jara, P.; Alvarez, L. Functional analysis of ABCB4 mutations relates clinical outcomes of progressive familial intrahepatic cholestasis type 3 to the degree of MDR3 floppase activity. Gut 2015, 64, 147–155. [Google Scholar] [CrossRef]
- Gissen, P.; Johnson, C.A.; Morgan, N.V.; Stapelbroek, J.M.; Forshew, T.; Cooper, W.N.; McKiernan, P.J.; Klomp, L.W.J.; Morris, A.A.M.; Wraith, J.E.; et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat. Genet. 2004, 36, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.R.; Straatman-Iwanowska, A.; Zaucker, A.; Wakabayashi, Y.; Bruce, C.K.; Luo, G.; Rahman, F.; Gürakan, F.; Utine, E.; Özkan, T.B.; et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat. Genet. 2010, 42, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Travaglini, L.; Drouin, C.A.; Ceballos-Picot, I.; Rizza, T.; Bertini, E.; Carrozzo, R.; Petrini, S.; De Lonlay, P.; El Hachem, M.; et al. MEDNIK syndrome: A novel defect of copper metabolism treatable by zinc acetate therapy. Brain 2013, 136, 872–881. [Google Scholar] [CrossRef]
- Lenz, D.; McClean, P.; Kansu, A.; Bonnen, P.E.; Ranucci, G.; Thiel, C.; Straub, B.K.; Harting, I.; Alhaddad, B.; Dimitrov, B.; et al. SCYL1 variants cause a syndrome with low γ- glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration (CALFAN). Genet. Med. 2018, 20, 1255–1265. [Google Scholar] [CrossRef]
- Treyer, A.; Müsch, A. Hepatocyte polarity. Compr. Physiol. 2013, 3, 243–287. [Google Scholar]
- Aït-Slimane, T.; Galmes, R.; Trugnan, G.; Maurice, M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol. Biol. Cell. 2009, 20, 3792–3800. [Google Scholar] [CrossRef]
- Bartles, J.R.; Hubbard, A.L. Plasma membrane protein sorting in epithelial cells: Do secretory pathways hold the key? Trends Biochem. Sci. 1988, 13, 181–184. [Google Scholar] [CrossRef]
- Schell, M.J.; Maurice, M.; Stieger, B.; Hubbard, A.L. 5′nucleotidase is sorted to the apical domain of hepatocytes via an indirect route. J. Cell Biol. 1992, 119, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Hemery, I.; Durand-Schneider, A.M.; Feldmann, G.; Vaerman, J.P.; Maurice, M. The transcytotic pathway of an apical plasma membrane protein (B10) in hepatocytes is similar to that of IgA and occurs via a tubular pericentriolar compartment. J. Cell Sci. 1996, 109 Pt 6, 1215–1227. [Google Scholar]
- Ihrke, G.; Martin, G.V.; Shanks, M.R.; Schrader, M.; Schroer, T.A.; Hubbard, A.L. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J. Cell Biol. 1998, 141, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Nyasae, L.K.; Hubbard, A.L.; Tuma, P.L. Transcytotic efflux from early endosomes is dependent on cholesterol and glycosphingolipids in polarized hepatic cells. Mol. Biol. Cell. 2003, 14, 2689–2705. [Google Scholar] [CrossRef]
- Slimane, T.A.; Trugnan, G.; van IJzendoorn, S.C.D.; Hoekstra, D. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: Role of distinct lipid microdomains. Mol. Biol. Cell. 2003, 14, 611–624. [Google Scholar] [CrossRef]
- De Marco, M.C.; Martín-Belmonte, F.; Kremer, L.; Albar, J.P.; Correas, I.; Vaerman, J.P.; Marazuela, M.; Byrne, J.A.; Alonso, M.A. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J. Cell Biol. 2002, 159, 37–44. [Google Scholar] [CrossRef]
- Larkin, J.M.; Woo, B.; Balan, V.; Marks, D.L.; Oswald, B.J.; LaRusso, N.F.; McNiven, M.A. Rab3D, a small GTP-binding protein implicated in regulated secretion, is associated with the transcytotic pathway in rat hepatocytes. Hepatology 2000, 32, 348–356. [Google Scholar] [CrossRef]
- Striz, A.C.; Stephan, A.P.; López-Coral, A.; Tuma, P.L. Rab17 regulates apical delivery of hepatic transcytotic vesicles. Mol. Biol. Cell. 2018, 29, 2887–2897. [Google Scholar] [CrossRef]
- Striz, A.C.; Tuma, P.L. The GTP-bound and Sumoylated Form of the rab17 Small Molecular Weight GTPase Selectively Binds Syntaxin 2 in Polarized Hepatic WIF-B Cells. J. Biol. Chem. 2016, 291, 9721–9732. [Google Scholar] [CrossRef] [PubMed]
- Kipp, H.; Arias, I.M. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J. Biol. Chem. 2000, 275, 15917–15925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tian, X.; Chandra, P.; Brouwer, K.L.R. Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol. Pharmacol. 2005, 67, 1334–1341. [Google Scholar] [CrossRef]
- Mochizuki, K.; Kagawa, T.; Numari, A.; Harris, M.J.; Itoh, J.; Watanabe, N.; Mine, T.; Arias, I.M. Two N-linked glycans are required to maintain the transport activity of the bile salt export pump (ABCB11) in MDCK II cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G818–G828. [Google Scholar] [CrossRef] [PubMed]
- Plass, J.R.; Mol, O.; Heegsma, J.; Geuken, M.; De Bruin, J.; Elling, G.; Müller, M.; Faber, K.N.; Jansen, P.L. A progressive familial intrahepatic cholestasis type 2 mutation causes an unstable, temperature-sensitive bile salt export pump. J. Hepatol. 2004, 40, 24–30. [Google Scholar] [CrossRef]
- Kubitz, R.; Sütfels, G.; Kühlkamp, T.; Kölling, R.; Häussinger, D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology 2004, 126, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Wojtal, K.A.; de Vries, E.; Hoekstra, D.; van IJzendoorn, S.C.D. Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol. Biol. Cell. 2006, 17, 3638–3650. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Lippincott-Schwartz, J.; Arias, I.M. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: Constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol. Biol. Cell. 2004, 15, 3485–3496. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Dutt, P.; Lippincott-Schwartz, J.; Arias, I.M. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl. Acad. Sci. USA 2005, 102, 15087–15092. [Google Scholar] [CrossRef]
- Kipp, H.; Pichetshote, N.; Arias, I.M. Transporters on demand: Intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J. Biol. Chem. 2001, 276, 7218–7224. [Google Scholar] [CrossRef]
- Rahner, C.; Stieger, B.; Landmann, L. Apical endocytosis in rat hepatocytes In situ involves clathrin, traverses a subapical compartment, and leads to lysosomes. Gastroenterology 2000, 119, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.M.; Coleman, H.; Espinosa, A.; Levenson, A.; Park, M.S.; Woo, B.; Zervoudakis, A.; Tinh, V. Intracellular accumulation of pIgA-R and regulators of transcytotic trafficking in cholestatic rat hepatocytes. Hepatology 2003, 38, 1199–1209. [Google Scholar] [CrossRef]
- Zucchetti, A.E.; Barosso, I.R.; Boaglio, A.; Pellegrino, J.M.; Ochoa, E.J.; Roma, M.G.; Crocenzi, F.A.; Sánchez Pozzi, E.J. Prevention of estradiol 17beta-D-glucuronide-induced canalicular transporter internalization by hormonal modulation of cAMP in rat hepatocytes. Mol. Biol. Cell. 2011, 22, 3902–3915. [Google Scholar] [CrossRef] [PubMed]
- Miszczuk, G.S.; Barosso, I.R.; LaRocca, M.C.; Marrone, J.; Marinelli, R.A.; Boaglio, A.C.; Pozzi, E.J.S.; Roma, M.G.; Crocenzi, F.A. Mechanisms of canalicular transporter endocytosis in the cholestatic rat liver. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1072–1085. [Google Scholar] [CrossRef]
- Crocenzi, F.A.; Sánchez Pozzi, E.J.; Ruiz, M.L.; Zucchetti, A.E.; Roma, M.G.; Mottino, A.D.; Vore, M. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology 2008, 48, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.K.; Graf, D.; Schmitt, M.; Vom Dahl, S.; Häussinger, D. Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology 2001, 121, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Schonhoff, C.M.; Webster, C.R.L.; Anwer, M.S. Rab11, but not Rab4, facilitates cyclic AMP- and tauroursodeoxycholate-induced MRP2 translocation to the plasma membrane. Am. J. Physiol. Gastrointest. Liver Physiol 2014, 307, G863–G870. [Google Scholar] [CrossRef]
- Gissen, P.; Tee, L.; Johnson, C.A.; Genin, E.; Caliebe, A.; Chitayat, D.; Clericuzio, C.; Denecke, J.; Di Rocco, M.; Fischler, B.; et al. Clinical and molecular genetic features of ARC syndrome. Hum. Genet. 2006, 120, 396–409. [Google Scholar] [CrossRef]
- Wang, J.-S.; Zhao, J.; Li, L.-T. ARC syndrome with high GGT cholestasis caused by VPS33B mutations. World J. Gastroenterol. 2014, 20, 4830–4834. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, T.; Abuduxikuer, K.; Hao, C.; Gong, J.; Zhang, M.; Li, L.; Yan, Y.; Li, J.; Wang, J.-S. Novel missense mutation in VPS33B is associated with isolated low gamma-glutamyltransferase cholestasis: Attenuated, incomplete phenotype of arthrogryposis, renal dysfunction, and cholestasis syndrome. Hum. Mutat. 2019, 40, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.; Dhar, D.K.; Mazzacuva, F.; Fiadeiro, R.; Burden, J.J.; Lyne, A.-M.; Smith, H.; Straatman-Iwanowska, A.; Banushi, B.; Virasami, A.; et al. Vps33b is crucial for structural and functional hepatocyte polarity. J. Hepatol. 2017, 66, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, C.; Gao, Y.; Fan, S.; Zhang, H.; Sun, J.; Jiang, Y.; Liu, C.; Guan, L.; Liu, J.; et al. Metabolomics and Lipidomics Reveal the Effect of Hepatic Vps33b Deficiency on Bile Acids and Lipids Metabolism. Front. Pharmacol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Kipp, H.; Arias, I.M. Trafficking of canalicular ABC transporters in hepatocytes. Annu. Rev. Physiol. 2002, 64, 595–608. [Google Scholar] [CrossRef]
- Fujita, H.; Tuma, P.L.; Finnegan, C.M.; Locco, L.; Hubbard, AL. Endogenous syntaxins 2, 3 and 4 exhibit distinct but overlapping patterns of expression at the hepatocyte plasma membrane. Biochem. J. 1998, 329, 527–538. [Google Scholar] [CrossRef]
- Low, S.H.; Roche, P.A.; Anderson, H.A.; van Ijzendoorn, S.C.; Zhang, M.; Mostov, K.E.; Weimbs, T. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J. Biol. Chem. 1998, 273, 3422–3430. [Google Scholar] [CrossRef]
- Cutz, E.; Rhoads, J.M.; Drumm, B.; Sherman, P.M.; Durie, P.R.; Forstner, G.G. Microvillus inclusion disease: An inherited defect of brush-border assembly and differentiation. N. Engl. J. Med. 1989, 320, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Lacaille, F.; Verkarre, V.; Mategot, R.; Feldmann, G.; Grodet, A.; Sauvat, F.; Irtan, S.; Davit-Spraul, A.; Jacquemin, E.; et al. MYO5B and bile salt export pump contribute to cholestatic liver disorder in microvillous inclusion disease. Hepatology 2014, 60, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, K.J.; Dhekne, H.S.; Swertz, M.A.; Sirigu, S.; Ropars, V.; Vinke, P.C.; Rengaw, T.; Akker, P.C.; Rings, E.H.; Houdusse, A. An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum. Mutat. 2013, 34, 1597–1605. [Google Scholar] [CrossRef]
- Casanova, J.E.; Wang, X.; Kumar, R.; Bhartur, S.G.; Navarre, J.; Woodrum, J.E.; Altschuler, Y.; Ray, G.S.; Goldenring, J.R. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 1999, 10, 47–61. [Google Scholar] [CrossRef]
- Overeem, A.W.; Li, Q.; Qiu, Y.; Cartón-García, F.; Leng, C.; Klappe, K.; Dronkers, J.; Hsiao, N.; Wang, J.; Arango, D.; et al. A molecular mechanism underlying genotype-specific intrahepatic cholestasis resulting from MYO5B mutations. Hepatology 2019, 72, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Weisz, O.A.; Rodriguez-Boulan, E. Apical trafficking in epithelial cells: Signals, clusters and motors. J. Cell Sci. 2009, 122, 4253–4266. [Google Scholar] [CrossRef]
- Incecik, F.; Bisgin, A.; Yılmaz, M. MEDNIK syndrome with a frame shift causing mutation in AP1S1 gene and literature review of the clinical features. Metab. Brain Dis. 2018, 33, 2065–2068. [Google Scholar] [CrossRef]
- Lam, P.; Xu, S.; Soroka, C.J.; Boyer, J.L. AC-terminal tyrosine-based motif in the bile salt export pump directs clathrin-dependent endocytosis. Hepatology 2012, 55, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Inamura, K.; Aida, K.; Naoi, S.; Horikawa, R.; Nagasaka, H.; Takatani, T.; Fukushima, T.; Hattori, A.; Yabuki, T.; et al. AP2 adaptor complex mediates bile salt export pump internalization and modulates its hepatocanalicular expression and transport function. Hepatology 2012, 55, 1889–1900. [Google Scholar] [CrossRef]
- Wooton-Kee, C.R.; Jain, A.K.; Wagner, M.; Grusak, M.A.; Finegold, M.J.; Lutsenko, S.; Moore, D.D. Elevated copper impairs hepatic nuclear receptor function in Wilson’s disease. J. Clin. Investig. 2015, 125, 3449–3460. [Google Scholar] [CrossRef]
- Burman, J.L.; Bourbonniere, L.; Philie, J.; Stroh, T.; Dejgaard, S.Y.; Presley, J.F.; McPherson, P.S. Scyl1, mutated in a recessive form of spinocerebellar neurodegeneration, regulates COPI-mediated retrograde traffic. J. Biol. Chem. 2008, 283, 22774–22786. [Google Scholar] [CrossRef] [PubMed]
- Witkos, T.M.; Chan, W.L.; Joensuu, M.; Rhiel, M.; Pallister, E.; Thomas-Oates, J.; Mould, A.P.; Mironov, A.A.; Biot, C.; Guérardel, Y.; et al. GORAB scaffolds COPI at the trans-Golgi for efficient enzyme recycling and correct protein glycosylation. Nat. Commun. 2019, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Overeem, A.W.; Klappe, K.; Parisi, S.; Klöters-Planchy, P.; Mataković, L.; Espina, M.D.T.; Drouin, C.A.; Weiss, K.H.; van Ijzendoorn, S.C.D. Pluripotent stem cell-derived bile canaliculi-forming hepatocytes to study genetic liver diseases involving hepatocyte polarity. J. Hepatol. 2019, 71, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Lenz, D.; Staufner, C.; Wächter, S.; Hagedorn, M.; Ebersold, J.; Göhring, G.; Kölker, S.; Hoffmann, G.F.; Jung-Klawitter, S. Generation of an induced pluripotent stem cell (iPSC) line, DHMCi005-A, from a patient with CALFAN syndrome due to mutations in SCYL1. Stem Cell Res. 2019, 37, 101428. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Sun, Y.; van IJzendoorn, S.C.D. A Link between Intrahepatic Cholestasis and Genetic Variations in Intracellular Trafficking Regulators. Biology 2021, 10, 119. https://doi.org/10.3390/biology10020119
Li Q, Sun Y, van IJzendoorn SCD. A Link between Intrahepatic Cholestasis and Genetic Variations in Intracellular Trafficking Regulators. Biology. 2021; 10(2):119. https://doi.org/10.3390/biology10020119
Chicago/Turabian StyleLi, Qinghong, Yue Sun, and Sven C. D. van IJzendoorn. 2021. "A Link between Intrahepatic Cholestasis and Genetic Variations in Intracellular Trafficking Regulators" Biology 10, no. 2: 119. https://doi.org/10.3390/biology10020119
APA StyleLi, Q., Sun, Y., & van IJzendoorn, S. C. D. (2021). A Link between Intrahepatic Cholestasis and Genetic Variations in Intracellular Trafficking Regulators. Biology, 10(2), 119. https://doi.org/10.3390/biology10020119




