COVID-19 in Solid Organ Transplant Recipient: Exploring Cumulative Incidence, Seroprevalence and Risk Factors for Disease Severity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| K | Kidney |
| IQR | inter-quartile range |
| ITA | Islet transplant alone |
| ISS | Istituto Superiore di Sanità |
| LIPS | luciferase immunoprecipitation system |
| OD | Odd ratio |
| PA | Pancreas alone |
| PK | Pancreas–kidney |
| RBD | Receptor binding domain |
| RT-PCR | reverse transcriptase-polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SOT | Solid organ transplantation |
References
- Unim, B.; Palmieri, L.; Lo Noce, C.; Brusaferro, S.; Onder, G. Prevalence of COVID-19-related symptoms by age group. Aging Clin. Exp. Res. 2021, 33, 1145–1147. [Google Scholar] [CrossRef]
- Palmieri, L.; Vanacore, N.; Donfrancesco, C.; Lo Noce, C.; Canevelli, M.; Punzo, O.; Raparelli, V.; Pezzotti, P.; Riccardo, F.; Bella, A. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J. Gerontol. Ser. A 2020, 75, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Wingert, A.; Pillay, J.; Gates, M.; Guitard, S.; Rahman, S.; Beck, A.; Vandermeer, B.; Hartling, L. Risk factors for severity of COVID-19: A rapid review to inform vaccine prioritisation in Canada. BMJ Open 2021, 11, e044684. [Google Scholar] [CrossRef] [PubMed]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P. Akalin E COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Mohan, S.; Cohen, D.J.; Husain, S.A.; Dube, G.K.; Ratner, L.E.; Arcasoy, S.; Aversa, M.M.; Benvenuto, L.J.; Dadhania, D.M.; et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am. J. Transpl. 2020, 20, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. Covid-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Anglicheau, D.; Matignon, M.; Durrbach, A.; Greze, C.; Frimat, L.; Thaunat, O.; Legris, T.; Moal, V.; Westeel, P.F.; et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020, 98, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.B.; Izzy, S.; Tahir, Z.; Al Jarrah, A.; Fishman, J.A.; El Khoury, J. COVID-19 in solid organ transplant recipients: Dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transpl. Infect. Dis. 2020, 22, e13407. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. The Immunocompromised Transplant Recipient and SARS-CoV-2 Infection. J. Am. Soc. Nephrol. 2020, 31, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, M.; Andres, A.; Loinaz, C.; Delgado, J.F.; Lopez-Medrano, F.; San Juan, R.; Gonzalez, E.; Polanco, N.; Folgueira, M.D.; Lalueza, A.; et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am. J. Transpl. 2020, 20, 1849–1858. [Google Scholar] [CrossRef]
- Crespo, M.; Perez-Saez, M.J.; Redondo-Pachon, D.; Llinas-Mallol, L.; Montero, M.M.; Villar-Garcia, J.; Arias-Cabrales, C.; Buxeda, A.; Burballa, C.; Vazquez, S.; et al. COVID-19 in elderly kidney transplant recipients. Am. J. Transpl. 2020, 20, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Kates, O.S.; Haydel, B.M.; Florman, S.S.; Rana, M.M.; Chaudhry, Z.S.; Ramesh, M.S.; Safa, K.; Kotton, C.N.; Blumberg, E.A.; Besharatian, B.D.; et al. COVID-19 in solid organ transplant: A multi-center cohort study. Clin. Infect. Dis. 2020, 73, 4090–4099. [Google Scholar] [CrossRef] [PubMed]
- Aversa, M.; Benvenuto, L.; Anderson, M.; Shah, L.; Robbins, H.; Pereira, M.; Scheffert, J.; Carroll, M.; Hum, J.; Nolan, M.; et al. COVID-19 in lung transplant recipients: A single center case series from New York City. Am. J. Transpl. 2020, 20, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Mothi, S.S.; Azzi, Y.; Haverly, M.; Farouk, S.S.; Perez-Saez, M.J.; Redondo-Pachon, M.D.; Murphy, B.; Florman, S.; Cyrino, L.G.; et al. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am. J. Transpl. 2020, 20, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Chiang, T.P.; Marr, K.A.; Brennan, D.C.; Sait, A.S.; Garibaldi, B.T.; Shah, P.; Ostrander, D.; Steinke, S.M.; Permpalung, N.; et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: A retrospective cohort. Am. J. Transpl. 2021, 21, 2498–2508. [Google Scholar] [CrossRef]
- Bossini, N.; Alberici, F.; Delbarba, E.; Valerio, F.; Manenti, C.; Possenti, S.; Econimo, L.; Maffei, C.; Pola, A.; Terlizzi, V.; et al. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am. J. Transpl. 2020, 20, 3019–3029. [Google Scholar] [CrossRef]
- Fava, A.; Cucchiari, D.; Montero, N.; Toapanta, N.; Centellas, F.J.; Vila-Santandreu, A.; Coloma, A.; Meneghini, M.; Manonelles, A.; Sellares, J.; et al. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am. J. Transpl. 2020, 20, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Bhalla, A.; Azhar, A.; Tsujita, M.; Talwar, M.; Balaraman, V.; Sodhi, A.; Kadaria, D.; Eason, J.D.; Hayek, S.S.; et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am. J. Transpl. 2020, 20, 3061–3071. [Google Scholar] [CrossRef]
- Avery, R.K. COVID-19 Therapeutics for Solid Organ Transplant Recipients; 6 Months Into the Pandemic: Where Are We Now? Transplantation 2021, 105, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Hadi, Y.B.; Naqvi, S.F.Z.; Kupec, J.T.; Sofka, S.; Sarwari, A. Outcomes of COVID-19 in Solid Organ Transplant Recipients: A Propensity-matched Analysis of a Large Research Network. Transplantation 2021, 105, 1365–1371. [Google Scholar] [CrossRef]
- Secchi, M.; Bazzigaluppi, E.; Brigatti, C.; Marzinotto, I.; Tresoldi, C.; Rovere-Querini, P.; Poli, A.; Castagna, A.; Scarlatti, G.; Zangrillo, A.; et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J. Clin. Investig. 2020, 130, 6366–6378. [Google Scholar] [CrossRef]
- Hippich, M.; Holthaus, L.; Assfalg, R.; Zapardiel-Gonzalo, J.; Kapfelsperger, H.; Heigermoser, M.; Haupt, F.; Ewald, D.A.; Welzhofer, T.C.; Marcus, B.A.; et al. A Public Health Antibody Screening Indicates a 6-Fold Higher SARS-CoV-2 Exposure Rate than Reported Cases in Children. Medicine 2021, 2, 149–163. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, 192–197. [Google Scholar] [CrossRef]
- Vistoli, F.; Furian, L.; Maggiore, U.; Caldara, R.; Cantaluppi, V.; Ferraresso, M.; Zaza, G.; Cardillo, M.; Biancofiore, G.; Menichetti, F.; et al. COVID-19 and kidney transplantation: An Italian Survey and Consensus. J. Nephrol. 2020, 33, 667–680. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; di Ruffano, L.F. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Raja, M.A.; Mendoza, M.A.; Villavicencio, A.; Anjan, S.; Reynolds, J.M.; Kittipibul, V.; Fernandez, A.; Guerra, G.; Camargo, J.F.; Simkins, J.; et al. COVID-19 in solid organ transplant recipients: A systematic review and meta-analysis of current literature. Transplant. Rev. 2021, 35, 100588. [Google Scholar] [CrossRef]
- Wu, K.S.; Lin, P.C.; Chen, Y.S.; Pan, T.C.; Tang, P.L. The use of statins was associated with reduced COVID-19 mortality: A systematic review and meta-analysis. Ann. Med. 2021, 53, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Statins and clinical outcomes with COVID-19: Meta-analyses of observational studies. Diabetes Metab. 2020, 47, 101220. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Statin and outcomes of coronavirus disease 2019 (COVID-19): A systematic review, meta-analysis, and meta-regression. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Coll, E.; Fernandez-Ruiz, M.; Padilla, M.; Moreso, F.; Hernandez-Vicente, A.; Yanez, I.; Molina, M.; Vazquez-Sanchez, T.; Crespo, M.; Facundo, C.; et al. COVID-19 in Solid Organ Transplant Recipients in Spain Throughout 2020: Catching the Wave? Transplantation 2021, 105, 2146. [Google Scholar] [CrossRef]
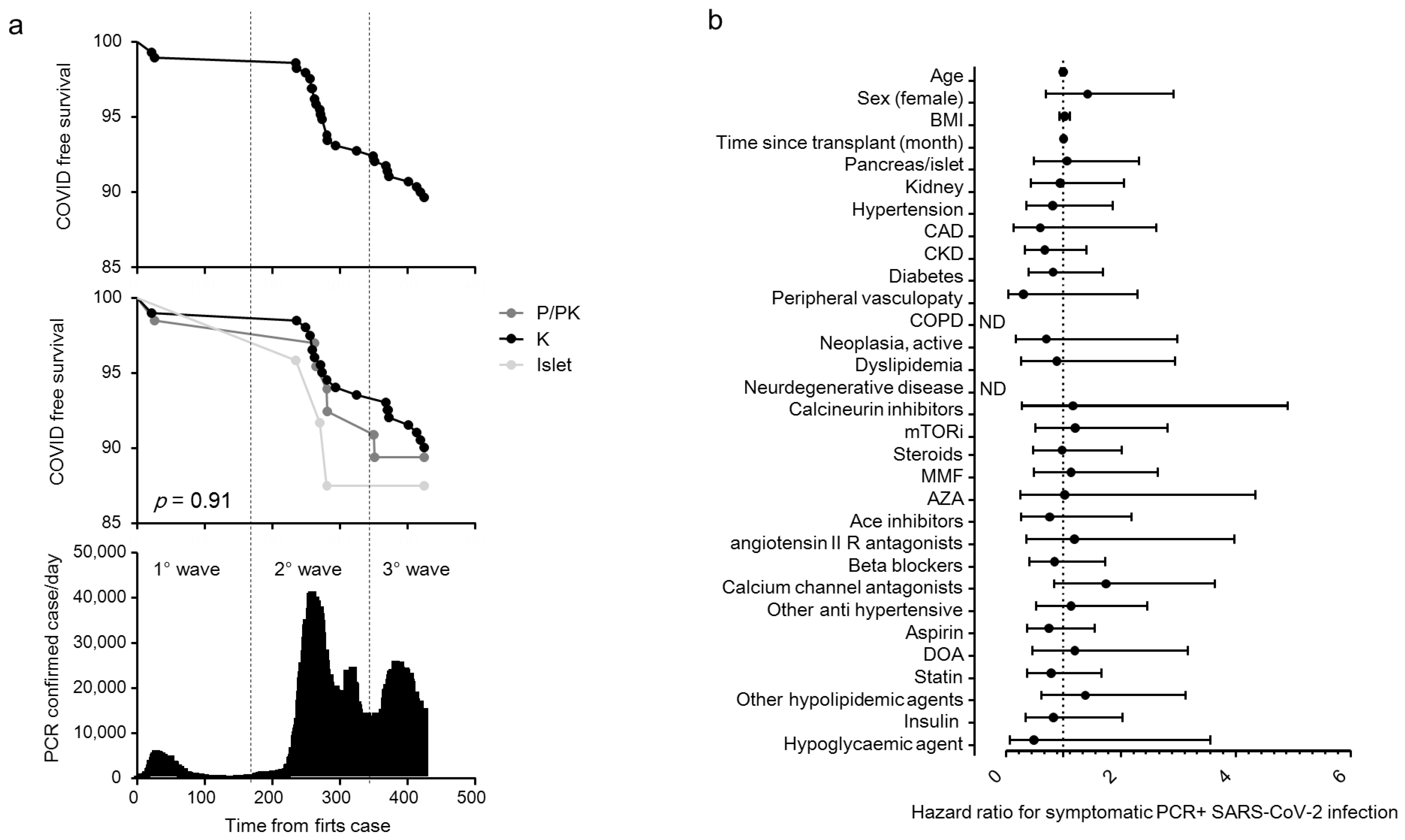
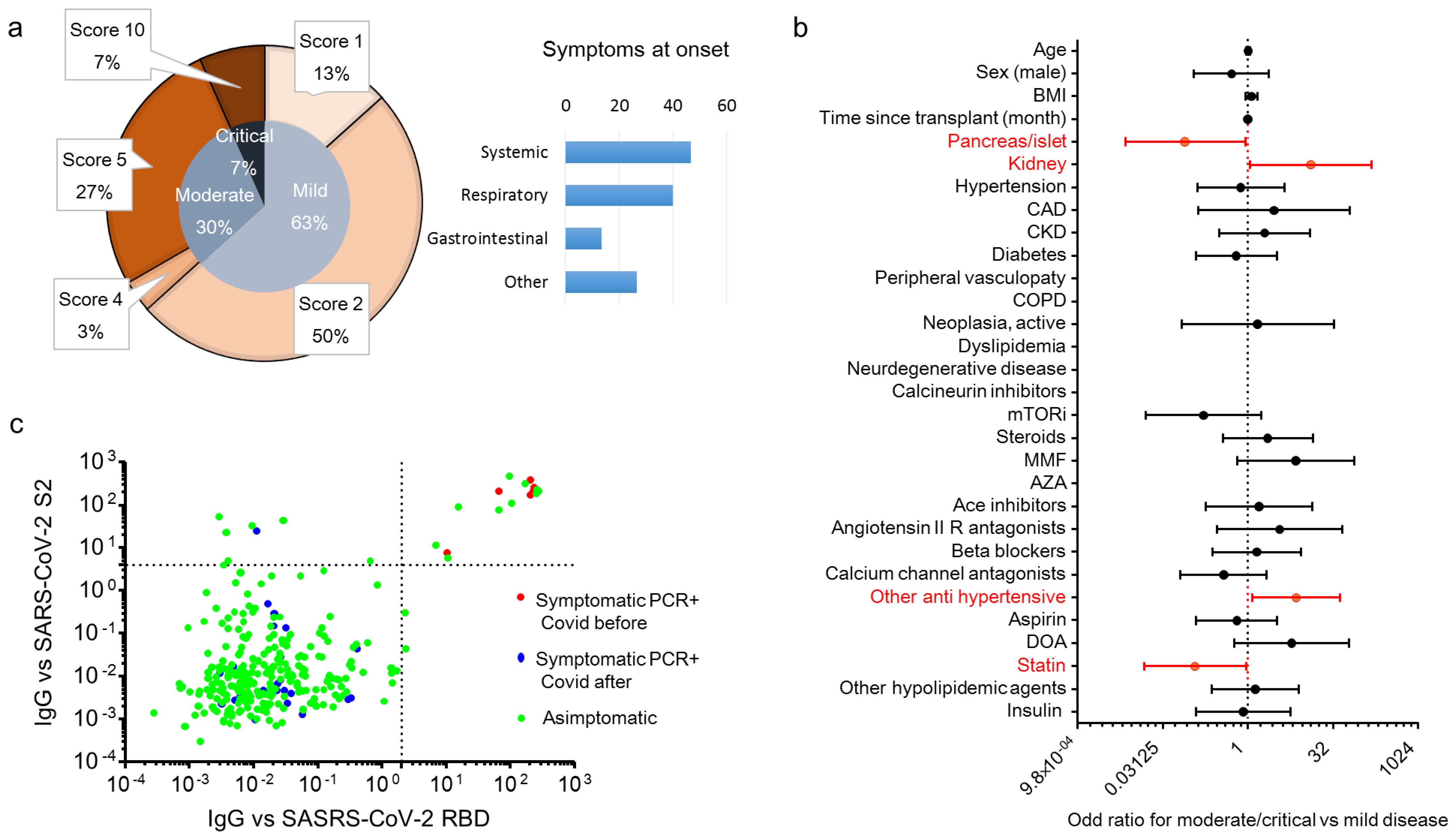
| Items | All | Islet | Pancreas ± Kidney | Kidney | p |
|---|---|---|---|---|---|
| N | 291 | 24 | 66 | 201 | |
| Age in years, median (IQR) | 56 (47–65) | 51 (36–60) | 54 (47–59) | 57 (49–66) | 0.001 |
| Sex M/F | 179/112 | 11/13 | 41/25 | 127/74 | 0.264 |
| Race Caucasian (N (%)) | 284 (97.6) | 24 (100) | 64 (97) | 196 (97.5) | 0.631 |
| Body mass index (kg/m2) | 24.2 (21.8–26.6) | 22.2 (17.8–23.5) | 23.2 (20–26.7) | 25 (22.5–27) | <0.001 |
| Months since transplant, median (IQR) | 53.4 (17–121) | 79 (34–131) | 75 (24–160) | 48 (12–106) | 0.005 |
| Comorbidities (N (%)) | |||||
| Hypertension | 227 (78) | 11 (45.8) | 42 (63.6) | 174 (86.6) | <0.001 |
| Coronary artery disease | 35 (12) | 2 (8.3) | 11 (16.7) | 22 (10.9) | 0.392 |
| Chronic kidney disease | 156 (53.6) | 2 (8.3) | 29 (43.9) | 125 (62.2) | <0.001 |
| Diabetes | 140 (48.1) | 24 (100) | 66 (100) | 50 (24.9) | <0.001 |
| Peripheral vasculopathy | 30 (10.3) | 1 (4.2) | 7 (10.6) | 22 (10.9) | 0.585 |
| Chronic obstructive pulmonary disease | 1 (0.3) | 0 (0) | 0 (0) | 1 (0.5) | 0.799 |
| Neoplasia active | 27 (9.3) | 1 (4.2) | 3 (4.5) | 23 (11.4) | 0.164 |
| Dyslipidemia | 35 (12) | 2 (8.3) | 5 (7.6) | 28 (13.9) | 0.327 |
| Neuro degenerative disease | 2 (0.7) | 1 (4.2) | 1 (1.5) | 0 (0) | 0.043 |
| Baseline therapy | |||||
| Calcineurin inhibitor (CNI) | 270 (92.8) | 21 (87.5) | 64 (97) | 185 (92) | 0.235 |
| Mammalian target of rapamycin inhibitors (mTORi) | 58 (19.9) | 9 (37.5) | 3 (4.5) | 46 (22.9) | <0.001 |
| Steroids | 133 (45.7) | 1 (4.2) | 31 (47) | 101 (50.2) | <0.001 |
| Mycophenolate mofetil | 218 (74.9) | 11 (45.8) | 59 (89.4) | 148 (73.6) | <0.001 |
| Azathioprine | 18 (6.2) | 6 (25) | 4 (6.1) | 8 (4) | <0.001 |
| “Intensity” of immunosuppression | |||||
| - Triple regimen | 122 (41.9) | 1 (4.2) | 30 (45.5) | 91 (45.3) | |
| ○ CNI+antimetabolite+steroid | 102 (83.6) | 0 (0) | 28 (93.3) | 74 (81.3) | |
| ○ CNI+mTORi+steroid | 14 (11.5) | 0 (0) | 1 (3.3) | 13 (14.3) | |
| ○ mTORi+antimetabolite+steroid | 5 (4.1) | 0 (0) | 1 (3.3) | 4 (4.4) | |
| ○ mTORi+CNI+antimetabolite | 1 (0.8) | 1 (100) | 0 (0) | 0 (0) | |
| - Double regimen | 162 (55.7) | 22 (91.7) | 36 (54.5) | 104 (51.7) | |
| ○ CNI+antimetabolite | 118 (72.8) | 13 (59.1) | 34 (94.4) | 71 (68.3) | |
| ○ CNI+mTORi | 28 (17.3) | 6 (27.3) | 0 (0) | 22 (21.2) | |
| ○ CNI+steroid | 5 (3.1) | 1 (4.5) | 1 (2.8) | 3 (2.9) | |
| ○ mTORi+steroid | 5 (3.1) | 0 (0) | 0 (0) | 5 (4.8) | |
| ○ mTORi+antimetabolite | 4 (2.5) | 2 (9.1) | 1 (2.8) | 1 (1) | |
| ○ antimetabolite+steroid | 2 (1.2) | 0 (0) | 0 (0) | 2 (1.9) | |
| - Single regimen | 7 (2.4) | 1 (4.2) | 0 (0) | 6 (3) | |
| ○ Antimetabolite | 4 (57.1) | 1 (100) | 0 (0) | 3 (50) | |
| ○ CNI | 2 (28.6) | 0 (0) | 0 (0) | 2 (33.3) | |
| ○ mTORi | 1 (14.3) | 0 (0) | 0 (0) | 1 (16.7) | |
| Ace inhibitors | 51 (17.5) | 6 (25) | 7 (10.6) | 38 (18.9) | 0.185 |
| Angiotensin II receptor type 1 antagonists | 26 (8.9) | 1 (4.2) | 5 (7.6) | 20 (10) | 0.584 |
| Beta blockers | 157 (54) | 6 (25) | 33 (50) | 118 (58.7) | 0.006 |
| Calcium channel antagonists | 125 (43) | 3 (12.5) | 26 (39.4) | 96 (47.8) | 0.003 |
| Other anti-hypertensive | 106 (36.4) | 2 (8.3) | 20 (30.3) | 84 (41.8) | 0.003 |
| Aspirin | 185 (63.6) | 6 (25) | 45 (68.2) | 134 (66.7) | <0.001 |
| Direct oral anticoagulant | 44 (15.1) | 4 (16.7) | 12 (18.2) | 28 (13.9) | 0.688 |
| Statin | 134 (46) | 7 (29.2) | 27 (40.9) | 100 (49.8) | 0.102 |
| Other hypolipidemic agents | 61 (21) | 2 (8.3) | 8 (12.1) | 51 (25.4) | 0.02 |
| Insulin | 69 (23.7) | 18 (75) | 19 (28.8) | 32 (15.9) | <0.001 |
| Hypoglycemic agent | 19 (6.5) | 0 (0) | 5 (7.6) | 14 (7) | 0.395 |
| Items | SARS-CoV-2 RT-PCR Negative | SARS-Cov-2 RT-PCR Positive | p |
|---|---|---|---|
| N | 261 | 30 | |
| Age in years, median (IQR) | 56 (47–65) | 52 (48–61) | 0.341 |
| Sex M/F | 163/98 | 16/14 | 0.331 |
| Race Caucasian (N (%)) | 256 (98.1) | 28 (93.3) | 0.156 |
| Body mass index (kg/m2) | 24.2 (21.8–26.6) | 24 (21.9–26.9) | 0.817 |
| Type of transplant | |||
| - Kidney | 181 (69.3) | 20 (66.7) | 0.924 |
| - Pancreas ± kidney | 59 (22.6) | 7 (23.3) | |
| - Islets | 21 (8) | 3 (10) | |
| Comorbidities (N (%)) | |||
| - Hypertension | 205 (78.5) | 22 (73.3) | 0.492 |
| - Coronary artery disease | 33 (12.6) | 2 (6.7) | 0.552 |
| - Chronic kidney disease | 143 (54.8) | 13 (43.3) | 0.251 |
| - Diabetes | 127 (48.7) | 13 (43.3) | 0.7 |
| - Peripheral vasculopathy | 29 (11.1) | 1 (3.3) | 0.337 |
| - Chronic obstructive pulmonary disease | 1 (0.4) | 0 (0) | 1 |
| - Neoplasia active | 25 (9.6) | 2 (6.7) | 1 |
| - Dyslipidemia | 32 (12.3) | 3 (10) | 1 |
| - Neuro degenerative disease | 2 (0.8) | 0 (0) | 1 |
| Baseline therapy | |||
| - Calcineurin inhibitor (CNI) | 242 (92.7) | 28 (93.3) | 1 |
| - Mammalian target of rapamycin inhibitors (mTORi) | 51 (19.5) | 7 (23.3) | 0.631 |
| - Steroids | 119 (45.6) | 14 (46.7) | 1 |
| - Antimetabolites | 211 (80.8) | 25 (83.3) | 1 |
| “Intensity” of immunosuppression | |||
| - Triple regimen | 108 (41.4) | 14 (46.7) | 0.421 |
| - Double regimen | 146 (55.9) | 16 (50.2) | |
| - Single regimen | 7 (2.7) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldara, R.; Maffi, P.; Costa, S.; Bazzigaluppi, E.; Brigatti, C.; Lampasona, V.; Magistretti, P.; Manenti, F.; Marzinotto, I.; Pellegrini, S.; et al. COVID-19 in Solid Organ Transplant Recipient: Exploring Cumulative Incidence, Seroprevalence and Risk Factors for Disease Severity. Biology 2021, 10, 1349. https://doi.org/10.3390/biology10121349
Caldara R, Maffi P, Costa S, Bazzigaluppi E, Brigatti C, Lampasona V, Magistretti P, Manenti F, Marzinotto I, Pellegrini S, et al. COVID-19 in Solid Organ Transplant Recipient: Exploring Cumulative Incidence, Seroprevalence and Risk Factors for Disease Severity. Biology. 2021; 10(12):1349. https://doi.org/10.3390/biology10121349
Chicago/Turabian StyleCaldara, Rossana, Paola Maffi, Sabrina Costa, Elena Bazzigaluppi, Cristina Brigatti, Vito Lampasona, Paola Magistretti, Fabio Manenti, Ilaria Marzinotto, Silvia Pellegrini, and et al. 2021. "COVID-19 in Solid Organ Transplant Recipient: Exploring Cumulative Incidence, Seroprevalence and Risk Factors for Disease Severity" Biology 10, no. 12: 1349. https://doi.org/10.3390/biology10121349
APA StyleCaldara, R., Maffi, P., Costa, S., Bazzigaluppi, E., Brigatti, C., Lampasona, V., Magistretti, P., Manenti, F., Marzinotto, I., Pellegrini, S., Scavini, M., Secchi, A., & Piemonti, L. (2021). COVID-19 in Solid Organ Transplant Recipient: Exploring Cumulative Incidence, Seroprevalence and Risk Factors for Disease Severity. Biology, 10(12), 1349. https://doi.org/10.3390/biology10121349







