Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application
Abstract
:Simple Summary
Abstract
1. Introduction to Stress Biology and Biomarkers
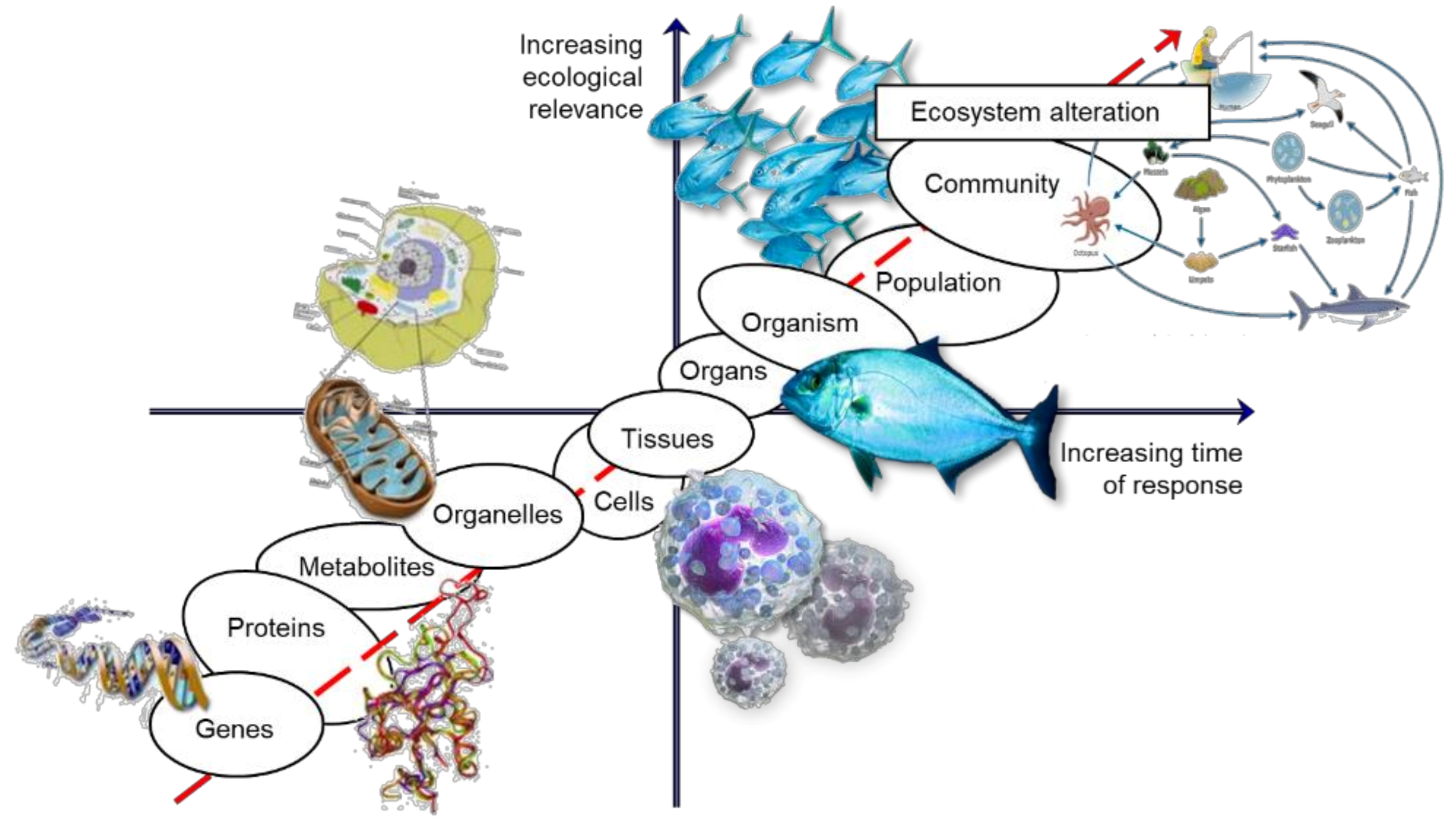
2. Linking Levels of Biological Organization
3. The Use of Biomarkers
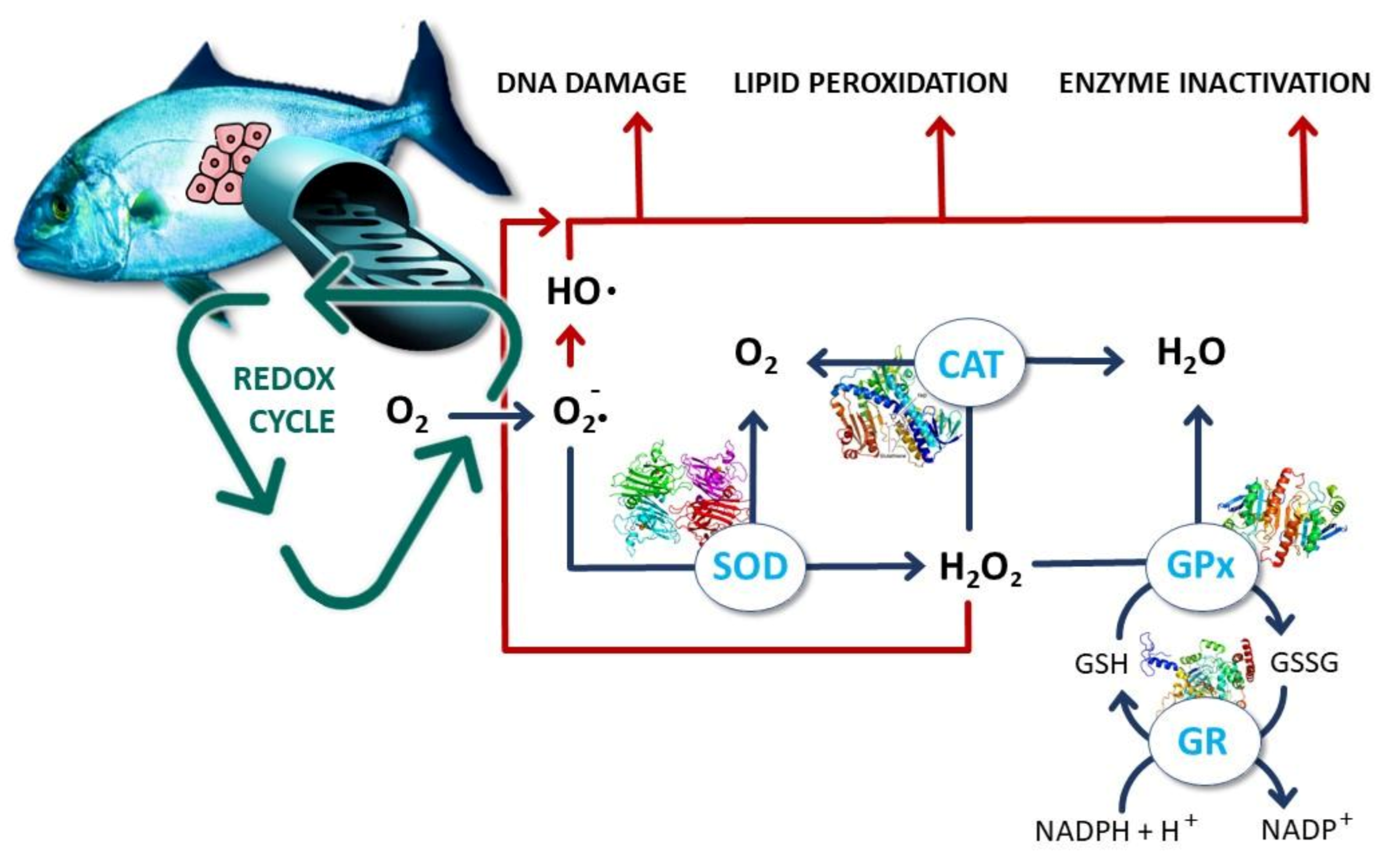
4. Oxidative Stress as a Cornerstone Example
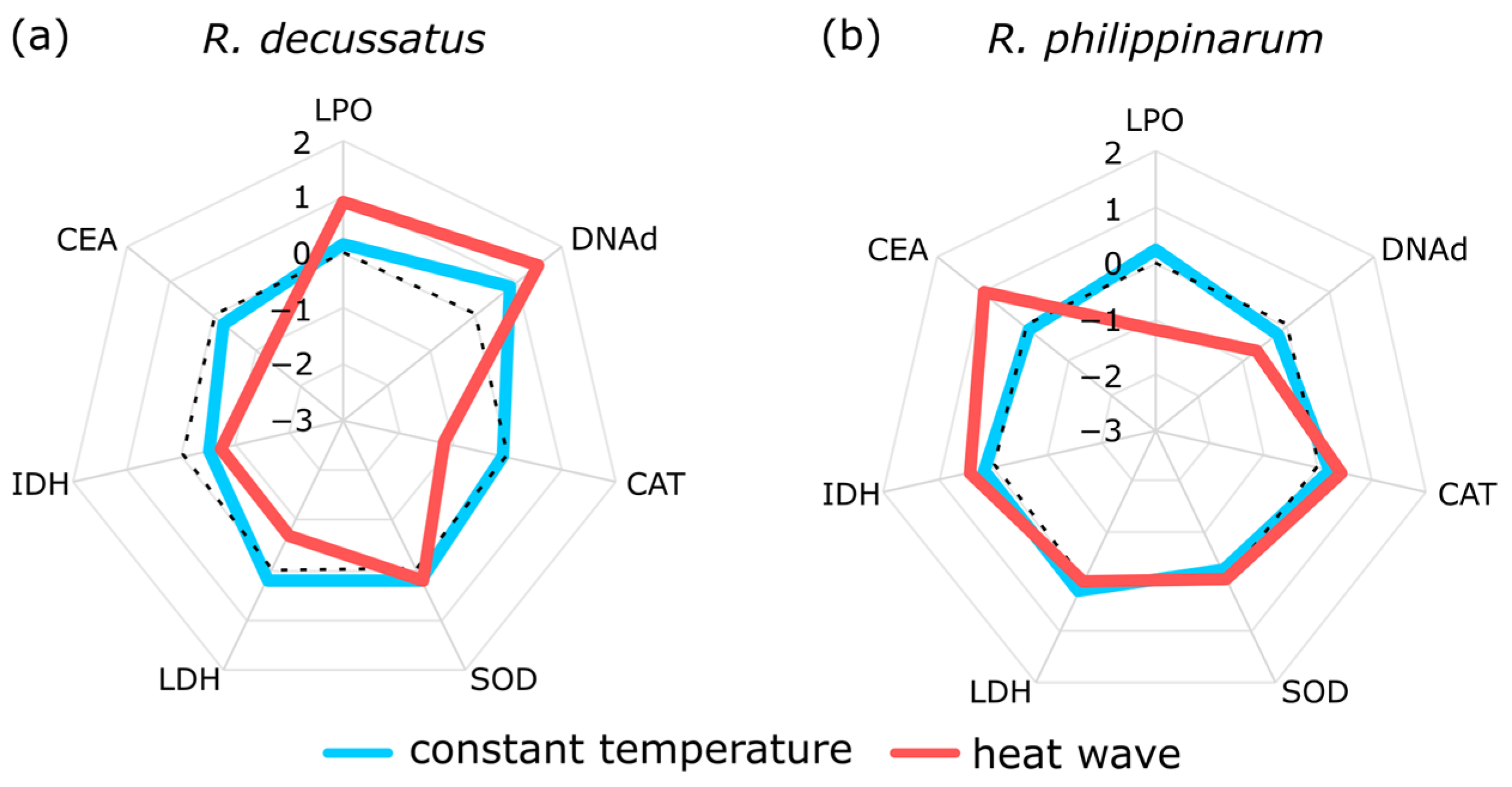
5. Two Is Better Than One, but the More the Merrier
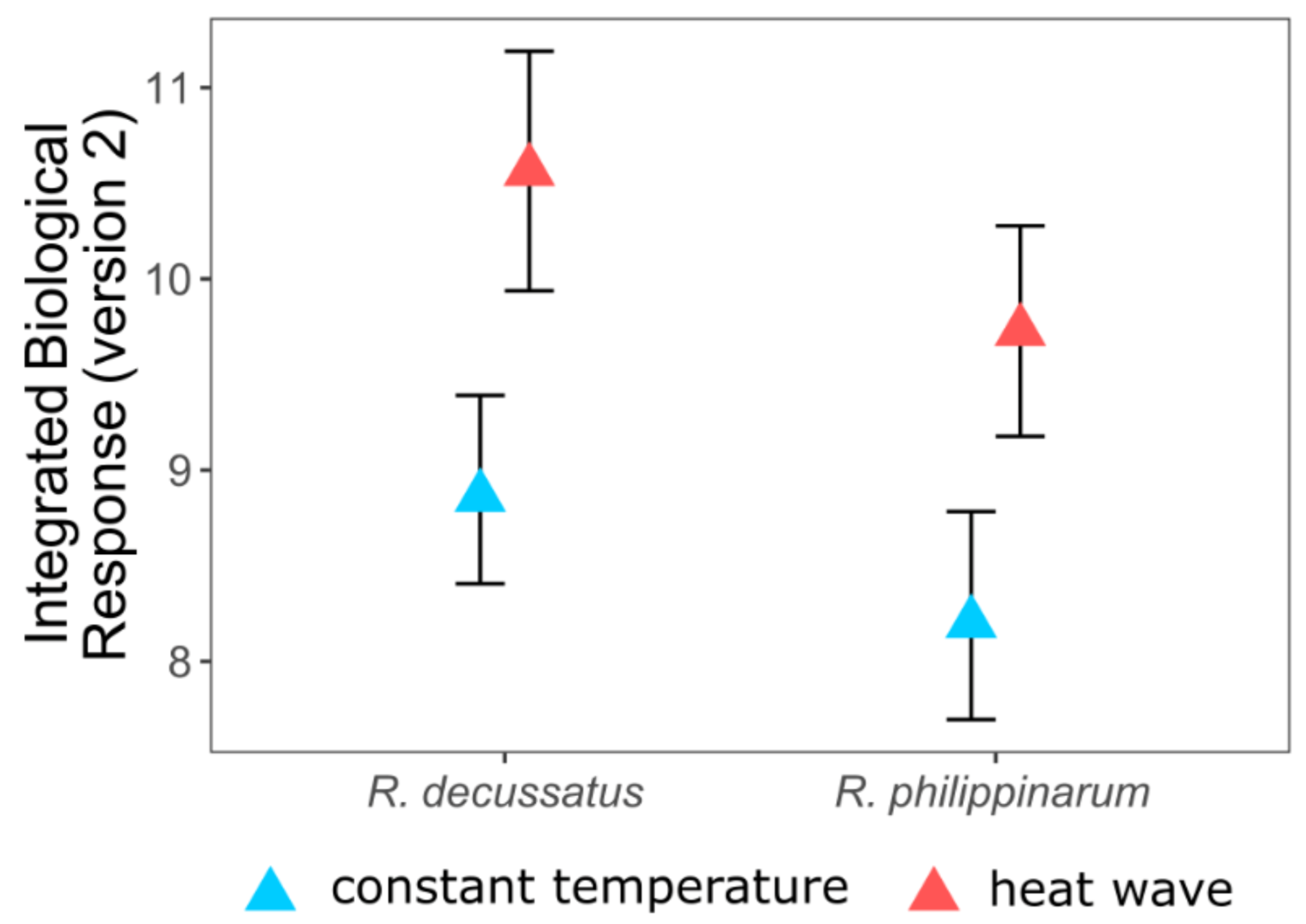
6. Understanding Biomarker Communication
7. Applications and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, P.K. Use of biomarkers in environmental monitoring. Ocean Coast. Manag. 2009, 52, 348–354. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Small, A.M.; Adey, W.H.; Lutz, S.M.; Reese, E.G.; Roberts, D.L. A macrophyte-based rapid biosurvey of stream water quality: Restoration at the watershed scale. Restor. Ecol. 1996, 4, 124–145. [Google Scholar] [CrossRef]
- Lavoie, I.; Campeau, S.; Grenier, M.; Dillon, P.J. A diatom-based index for the biological assessment of eastern Canadian rivers: An application of correspondence analysis (CA). Can. J. Fish. Aquat. Sci. 2006, 63, 1793–1811. [Google Scholar] [CrossRef]
- Johnson, R.K.; Hering, D. Response of taxonomic groups in streams to gradients in resource and habitat characteristics. J. Appl. Ecol. 2009, 46, 175–186. [Google Scholar] [CrossRef]
- United States Clean Water Act (PL 92–500) 1972. p. 92e500. Available online: https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg816.pdf (accessed on 1 November 2021).
- The EU Water Framework Directive. European parliament council directive 2000/60/EC of the european parliament and of the council of 23 October 2000, establishing a framework for community action in the field of water policy. Off. J. Eur. Union 2000, 327, 1–73. [Google Scholar]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV Cellular energy allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Lemos, M.F.L.; Soares, A.M.V.M.; Correia, A.; Esteves, A.C. Proteins in ecotoxicology—How, why and why not? Proteomics 2010, 10, 873–887. [Google Scholar] [CrossRef]
- Maltby, L. Studying stress: The importance of organism-level responses. Ecol. Appl. 1999, 9, 431–440. [Google Scholar] [CrossRef]
- Lam, P.K.S.; Gray, J.S. The use of biomarkers in environmental monitoring programmes. Mar. Pollut. Bull. 2003, 46, 182–186. [Google Scholar] [CrossRef]
- National Institutes of Health. Biomarkers definitions working group biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- World Health Organization International Programme on Chemical Safety. Biomarkers and Risk Assessment: Concepts and Principles/Published under the Joint Sponsorship of the United Nations Environment Programme; The International Labour Organisation; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Van Gestel, C.A.M.; van Brummelen, T.C. Incorporation of the biomarker concept in ecotoxicology calls for a redefinition of terms. Ecotoxicology 1996, 5, 217–225. [Google Scholar] [CrossRef]
- Depledge, M.H. The rational basis for the use of biomarkers as ecotoxicological tools. In Nondestructive Biomarkers in Vertebrates; Fossi, M.C., Leonzio, C., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 261–285. [Google Scholar]
- Adams, S.M.; Giesy, J.P.; Tremblay, L.A.; Eason, C.T. The use of biomarkers in ecological risk assessment: Recommendations from the Christchurch conference on Biomarkers in Ecotoxicology. Biomarkers 2001, 6, 1–6. [Google Scholar] [CrossRef]
- Venkateswara Rao, J.; Kavitha, P.; Jakka, N.M.; Sridhar, V.; Usman, P.K. Toxicity of organophosphates on morphology and locomotor behavior in brine shrimp, Artemia salina. Arch. Environ. Contam. Toxicol. 2007, 53, 227–232. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, X.; Yan, S.; Zha, J.; Qin, J. Environmentally relevant concentrations of bifenthrin induce changes in behaviour, biomarkers, histological characteristics, and the transcriptome in Corbicula fluminea. Sci. Total Environ. 2020, 7281, 138821. [Google Scholar] [CrossRef]
- Oliveira, C.; Almeida, J.; Guilhermino, L.; Soares, A.M.V.M.; Gravato, C. Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat. Toxicol. 2012, 124, 209–216. [Google Scholar] [CrossRef]
- Ferrario, C.; Parolini, M.; de Felice, B.; Villa, S.; Finizio, A. Linking sub-individual and supra-individual effects in Daphnia magna exposed to sub-lethal concentration of chlorpyrifos. Environ. Pollut. 2018, 235, 411–418. [Google Scholar] [CrossRef]
- Sandahl, J.F.; Baldwin, D.H.; Jenkins, J.J.; Scholz, N.L. Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ. Toxicol. Chem. 2005, 24, 136–145. [Google Scholar] [CrossRef]
- Lammertyn, S.; Masín, C.E.; Zalazar, C.S.; Fernandez, M.E. Biomarkers response and population biological parameters in the earthworm Eisenia fetida after short term exposure to atrazine herbicide. Ecol. Indic. 2021, 121, 107173. [Google Scholar] [CrossRef]
- Jensen, C.S.; Garsdal, L.; Baatrup, E. Acetylcholinesterase inhibition and altered locomotor behavior in the carabid beetle Pterostichus cupreus. A linkage between biomarkers at two levels of biological complexity. Environ. Toxicol. Chem. 1997, 16, 1727–1732. [Google Scholar] [CrossRef]
- Dell’Omo, G.; Turk, A.; Shore, R.F. Secondary poisoning in the common shrew (Sorex araneus) fed earthworms exposed to an organophosphate pesticide. Environ. Toxicol. Chem. 2009, 18, 237–240. [Google Scholar] [CrossRef]
- Forbes, V.E.; Palmqvist, A.; Bach, L. The use and misuse of biomarkers in ecotoxicology. Environ. Toxicol. Chem. 2006, 25, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, D. Necessity of defining biomarkers for use in ecological risk assessments. Mar. Pollut. Bull. 1999, 39, 48–53. [Google Scholar] [CrossRef]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M. (Eds.) Principles of Ecotoxicology, 2nd ed.; Taylor & Francis: London, UK, 2004; 309p. [Google Scholar]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the in vitro potential of natural extracts to protect lipids from oxidative damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Di Mateo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The Ascorbate-glutathione-α-tocopherol Triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.P. [11] Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar]
- Muruzabal, D.; Collins, A.; Azqueta, A. The enzyme-modified comet assay: Past, present and future. Food Chem. Toxicol. 2021, 147, 111865. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.M.V.M.; Barata, C.; Novais, S.C.; Lemos, M.F.L. Asparagopsis armata exudate cocktail: The quest for the mechanisms of toxic action of an invasive seaweed on marine invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef]
- Monteiro, H.R.; Pestana, J.L.T.; Novais, S.C.; Leston, S.; Ramos, F.; Soares, A.M.V.M.; Devreese, B.; Lemos, M.F.L. Assessment of fipronil toxicity to the freshwater midge Chironomus riparius: Molecular, biochemical, and organismal responses. Aquat. Toxicol. 2019, 216, 105192. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Agathokleous, E. Hormesis: Transforming disciplines that rely on the dose response. IUBMB Life 2021, 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.D.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.F.; Leandro, S.M. Homarus gammarus larvae under an ocean acidification scenario: Responses across different levels of biological organization. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 203, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Calow, P. Physiological costs of combating chemical toxicants: Ecological implications. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 1991, 100, 3–6. [Google Scholar] [CrossRef]
- Meng, S.; Tran, T.T.; van Dinh, K.; Delnat, V.; Stoks, R. Acute warming increases pesticide toxicity more than transgenerational warming by reducing the energy budget. Sci. Total Environ. 2022, 805, 150373. [Google Scholar] [CrossRef]
- Silva, C.O.; Novais, S.C.; Soares, A.M.V.M.; Barata, C.; Lemos, M.F.L. Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins 2021, 13, 15. [Google Scholar] [CrossRef]
- Louis, F.; Devin, S.; Giambérini, L.; Potet, M.; David, E.; Pain-Devin, S. Energy allocation in two dreissenid species under metal stress. Environ. Pollut. 2019, 245, 889–897. [Google Scholar] [CrossRef]
- Paul, N.; Novais, S.C.; Silva, C.S.E.; Mendes, S.; Kunzmann, A.; Lemos, M.F.L. Global warming overrides physiological anti-predatory mechanisms in intertidal rock pool fish Gobius paganellus. Sci. Total Environ. 2021, 776, 145736. [Google Scholar] [CrossRef]
- Lozano, C.; Lee, C.; Wattiez, R.; Lebaron, P.; Matallana-Surget, S. Unraveling the molecular effects of oxybenzone on the proteome of an environmentally relevant marine bacterium. Sci. Total Environ. 2021, 793, 148431. [Google Scholar] [CrossRef]
- Gauthier, L.; Tison-Rosebery, J.; Morin, S.; Mazzella, N. Metabolome response to anthropogenic contamination on microalgae: A review. Metabolomics 2020, 16, 8. [Google Scholar] [CrossRef]
- Dumas, T.; Courant, F.; Almunia, C.; Boccard, J.; Rosain, D.; Duporté, G.; Armengaud, J.; Fenet, H.; Gomez, E. An integrated metabolomics and proteogenomics approach reveals molecular alterations following carbamazepine exposure in the male mussel Mytilus galloprovincialis. Chemosphere 2022, 286, 131793. [Google Scholar] [CrossRef]
- Simões, T.; Fonseca, S.B.; Augusto, A.; Granada, L.; Ozório, R.O.A.; Gonçalves, J.F.M.; Pascoal, L.A.F.; Silva, J.H.V.; Lemos, M.F.L. Changes in fatty acid profile and chemical composition of meagre (Argyrosomus regius) fed with different lipid and selenium diets. Eur. J. Lipid Sci. Technol. 2017, 119, e201600016. [Google Scholar] [CrossRef]
- Silva, C.S.E.; Lemos, M.F.L.; Faria, A.M.; Lopes, A.F.; Mendes, S.; Gonçalves, E.J.; Novais, S.C. Sand smelt ability to cope and recover from ocean’s elevated CO2 levels. Ecotoxicol. Environ. Saf. 2018, 154, 302–310. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.; Feijão, E.; Reis-Santos, P.; Caçador, I.; Matos, A.R.; Fonseca, V.F. Fatty acid profiles of estuarine macroalgae are biomarkers of anthropogenic pressures: Development and application of a multivariate pressure index. Sci. Total Environ. 2021, 788, 147817. [Google Scholar] [CrossRef]
- Silva, C.S.E.; Novais, S.N.; Simões, T.; Caramalho, M.; Gravato, C.; Rodrigues, M.J.; Maranhão, P.; Lemos, M.F.L. Using biomarkers to address the impacts of pollution on limpets (Patella depressa) and their mechanisms to cope with stress. Ecol. Indic. 2018, 95, 1077–1086. [Google Scholar] [CrossRef]
- Pires, V.L.; Novais, S.C.; Lemos, M.F.L.; Fonseca, F.F.; Duarte, B. Evaluation of multivariate biomarker indexes application in ecotoxicity tests with marine diatoms exposed to emerging contaminants. Appl. Sci. 2021, 11, 3878. [Google Scholar] [CrossRef]
- Borja, A.; Dauer, D.M. Assessing the environmental quality status in estuarine and coastal systems: Comparing methodologies and indices. Ecol. Indic. 2008, 8, 331–337. [Google Scholar] [CrossRef]
- Beck, M.W.; O’Hara, C.; Lowndes, J.S.S.; Ma-Zor, R.D.; Theroux, S.; Gillett, D.J.; Lane, B.; Gearheart, G. The importance of open science for biological assessment of aquatic environments. PeerJ 2020, 8, 1–27. [Google Scholar] [CrossRef]
- Broeg, K.; Westernhagen, H.V.; Zander, S.; Körting, W.; Koehler, A. The “bioeffect assessment index” (BAI): A concept for the quantification of effects of marine pollution by an integrated biomarker approach. Mar. Pollut. Bull. 2005, 50, 495–503. [Google Scholar] [CrossRef]
- Narbonne, J.F.; Daubeze, M.; Clerandeau, C.; Garrigues, P. Scale of classification based on biochemical markers in mussels: Application to pollution monitoring in European coasts. Biomarkers 1999, 4, 415–424. [Google Scholar]
- Hagger, J.A.; Jones, M.B.; Lowe, D.; Leonard, D.R.P.; Owen, R.; Galloway, T.S. Application of biomarkers for improving risk assessments of chemicals under the Water Framework Directive: A case study. Mar. Pollut. Bull. 2008, 56, 1111–1118. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. Int. J. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Crespo, D.; Leston, S.; Rato, L.; Martinho, F.; Novais, S.C.; Pardal, M.; Lemos, M.F.L. (submitted) Do invasive bivalve species outperform their native congener in heat wave scenarios? A laboratorial study case with Ruditapes spps. Biology 2021, 10, 1284. [Google Scholar] [CrossRef]
- Sanchez, W.; Burgeot, T.; Porcher, J.-M. A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ. Sci. Pollut. Res. 2012, 20, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.M.; Lopes, A.; Silva, C.S.E.; Novais, S.C.; Lemos, M.F.L.; Gonçalves, E. Reproductive trade-offs in a temperate reef fish under high pCO2 levels. Mar. Environ. Res. 2018, 137, 8–15. [Google Scholar] [CrossRef]
- Silva, C.S.E.; Novais, S.C.; Lemos, M.F.L.; Mendes, S.; Oliveira, A.P.; Gonçalves, E.J.; Faria, A.M. Effects of ocean acidification on the swimming ability, development and biochemical responses of sand smelt larvae. Sci. Total Environ. 2016, 563, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kang, M.; Cha, S.-Y.; Byun, J.; Kim, J.; Baek, J.W.; Park, J.J.; Shin, S.R.; Kim, H.J.; Lee, J.S.; et al. Usefulness of clustering blood biochemical markers to assess thermal stress and acclimation in red seabream, Pagrus major. Aquaculture 2021, 545, 737197. [Google Scholar] [CrossRef]
- Johansen, J.L.; Nadler, L.E.; Habary, A.; Bowden, A.J.; Rummer, J. Thermal acclimation of tropical coral reef fishes to global heat waves. eLife 2021, 10, e59162. [Google Scholar] [CrossRef]
- Somero, G.N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Rato, L.D.; Crespo, D.D.; Lemos, M.F.L. Mechanisms of bioinvasions by coastal crabs using integrative approaches—A conceptual review. Ecol. Indic. 2021, 125, 107578. [Google Scholar] [CrossRef]
- Schmitz, M.; Deutschmann, B.; Markert, N.; Backhaus, T.; Brack, W.; Brauns, M.; Brinkmann, M.; Seiler, T.-B.; Fink, P.; Tang, S.; et al. Demonstration of an aggregated biomarker response approach to assess the impact of point and diffuse contaminant sources in feral fish in a small river case study. Sci. Total Environ. 2022, 804, 150020. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.A.; Marshall, C.D.; Hala, D. Integration of multi-tissue PAH and PCB burdens with biomarker activity in three coastal shark species from the northwestern Gulf of Mexico. Sci. Total Environ. 2019, 650, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, P.; Elia, A.C.; Caldaroni, B.; Menconi, V.; Abete, M.C.; Brizio, P.; Bertoli, M.; Zaccaroni, A.; Gabriele, M.; Dörr, A.J.M.; et al. Oxidative stress ecology in brook trout (Salvelinus fontinalis) from a high-mountain lake (Cottian Alps). Sci. Total Environ. 2020, 715, 136946. [Google Scholar] [CrossRef]
- Poliserpi, M.B.; Cristos, D.; Pérez-Iglesias, J.M.; Brodeur, J.C. Tissue distribution and sublethal effects of imidacloprid in the South American grayish baywing (Agelaioides badius). Chemosphere 2021, 284, 131327. [Google Scholar] [CrossRef]
- Laranjeiro, M.I.; Alves, L.M.F.; da Silva, J.M.; Pereira, J.M.; Norte, A.C.; Paiva, V.H.; Lemos, M.F.L.; Ramos, J.A.; Novais, S.C.; Ceia, F.R. Year-round element quantification of a wide-ranging seabird and their relationships with oxidative stress, trophic ecology and foraging patterns. Environ. Pollut. 2021, 284, 117502. [Google Scholar] [CrossRef]
- Morão, I.F.C.; Lemos, M.F.L.; Felix, R.; Vieira, S.; Barata, C.; Novais, S.C. Stress response markers in blood of São Tomé green sea turtles (Chelonia mydas) and their relation to accumulated metal levels. Environ. Pollut. 2022, 293, 118490. [Google Scholar] [CrossRef]
- Casini, S.; Caliani, I.; Giannetti, M.; Marsili, L.; Maltese, S.; Coppola, D.; Bianchi, N.; Campani, T.; Ancora, S.; Caruso, C.; et al. First ecotoxicological assessment of Caretta caretta (Linnaeus, 1758) in the Mediterranean Sea using an integrated nondestructive protocol. Sci. Total Environ. 2018, 631, 1221–1233. [Google Scholar] [CrossRef]
- Desforges, J.P.; Mikkelsen, B.; Dam, M.; Rigét, F.; Sveegaard, S.; Sonne, C.; Dietz, R.; Basu, N. Mercury and neurochemical biomarkers in multiple brain regions of five Arctic marine mammals. NeuroToxicology 2021, 84, 136–145. [Google Scholar] [CrossRef]
- Eccles, K.M.; Thomas, P.J.; Chan, H.M. Spatial patterns of the exposure-response relationship between mercury and cortisol in the fur of river otter (Lontra canadensis). Chemosphere 2021, 263, 127992. [Google Scholar] [CrossRef]
- Tonn, N.; Novais, S.C.; Silva, C.S.E.; Morais, H.A.; Correia, J.P.S.; Lemos, M.F.L. Stress responses of the sea cucumber Holothuria forskali during aquaculture handling and transportation. Mar. Biol. Res. 2016, 12, 948–957. [Google Scholar] [CrossRef]
- Peixoto, M.J.; Magnoni, L.; Gonçalves, J.F.M.; Twijnstra, R.H.; Kijjoa, A.; Pereira, R.; Palstra, A.P.; Ozório, R.O.A. Effects of dietary supplementation of Gracilaria sp. extracts on fillet quality, oxidative stress, and immune responses in European seabass (Dicentrarchus labrax). J. Appl. Phycol. 2019, 31, 761–770. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Novais, S.C.; Eding, E.; Leugen, I.; Lemos, M.F.L.; Ozorio, R.O.; Geurden, I.; Prunet, P.; Schrama, J.W. Acute stress and an electrolyte-imbalanced diet, but not chronic hypoxia, increase oxidative stress and hamper innate immune status in a rainbow trout (Oncorhynchus mykiss) isogenic line. Front. Physiol. 2019, 10, 453. [Google Scholar] [CrossRef] [Green Version]
- Félix, R.; Félix, C.; Januário, A.P.; Carmona, A.M.; Baptista, T.; Gonçalves, R.A.; Sendão, J.; Novais, S.C.; Lemos, M.F.L. Tailoring shrimp aquafeed to tackle Acute Hepatopancreatic Necrosis Disease by inclusion of industry-friendly seaweed extracts. Aquaculture 2020, 529, 735661. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, M.F.L. Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application. Biology 2021, 10, 1340. https://doi.org/10.3390/biology10121340
Lemos MFL. Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application. Biology. 2021; 10(12):1340. https://doi.org/10.3390/biology10121340
Chicago/Turabian StyleLemos, Marco F. L. 2021. "Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application" Biology 10, no. 12: 1340. https://doi.org/10.3390/biology10121340
APA StyleLemos, M. F. L. (2021). Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application. Biology, 10(12), 1340. https://doi.org/10.3390/biology10121340





